Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Conner Chen and Version 4 by Conner Chen.
In animal cells, the centrosome is a membrane-less organelle consisting of two interconnected centrioles, pericentriolar materials (PCM) and some additional structures. DNA replication and centrosome duplication during a cell cycle must share common regulations.
- cell cycle
- centrosome
- centriole
1. What Is the Centrosome?
In animal cells, the centrosome is a membrane-less organelle consisting of two interconnected centrioles, pericentriolar materials (PCM) and some additional structures [1]. The centriole is a cylinder shape structure with a diameter of about 200 nm and a length of about 500 nm made of nine triplets of microtubules (Figure 1).
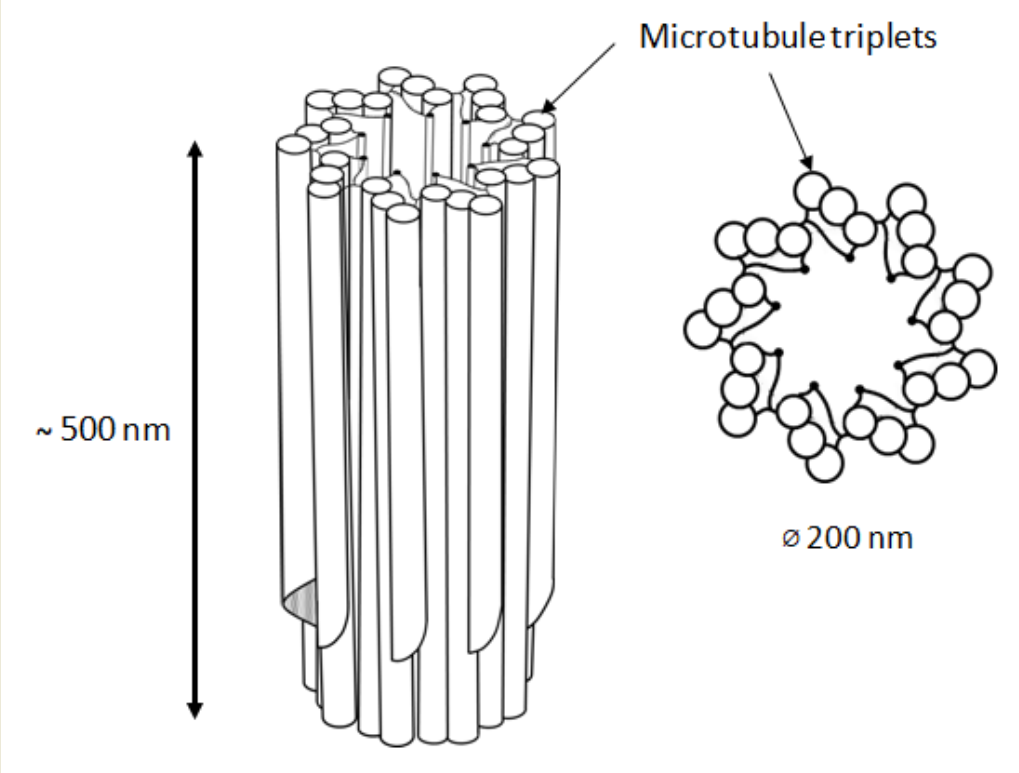
Figure 1. 9 triplets of microtubules to form a centriole.
The triplets of microtubules are always twisted counterclockwise when viewed from the proximal end of the centriole [2]. Each triplet is composed of microtubules A, B and C, with microtubule C being shorter than the other two and ending in a characteristic hook at the distal end of the centriolar cylinder (Figure 2 middle). Three types of centrioles can simultaneously exist in a cell (Figure 2). The older and most mature centriole (at least 2 cell cycles old) is called the mother centriole in opposition to the younger one, called the daughter centriole (which appeared in the previous cell cycle), and possesses two kinds of appendages named distal and subdistal appendages. Each triplet of microtubules possesses one distal appendage which makes nine distal appendages per centriole [3]. During the formation of the primary cilium distal appendages are essential to attach the centriole to the plasma membrane [4]. The daughter centriole has electron-dense ribs at the site of the future formation of distal appendages (Figure 2, middle).
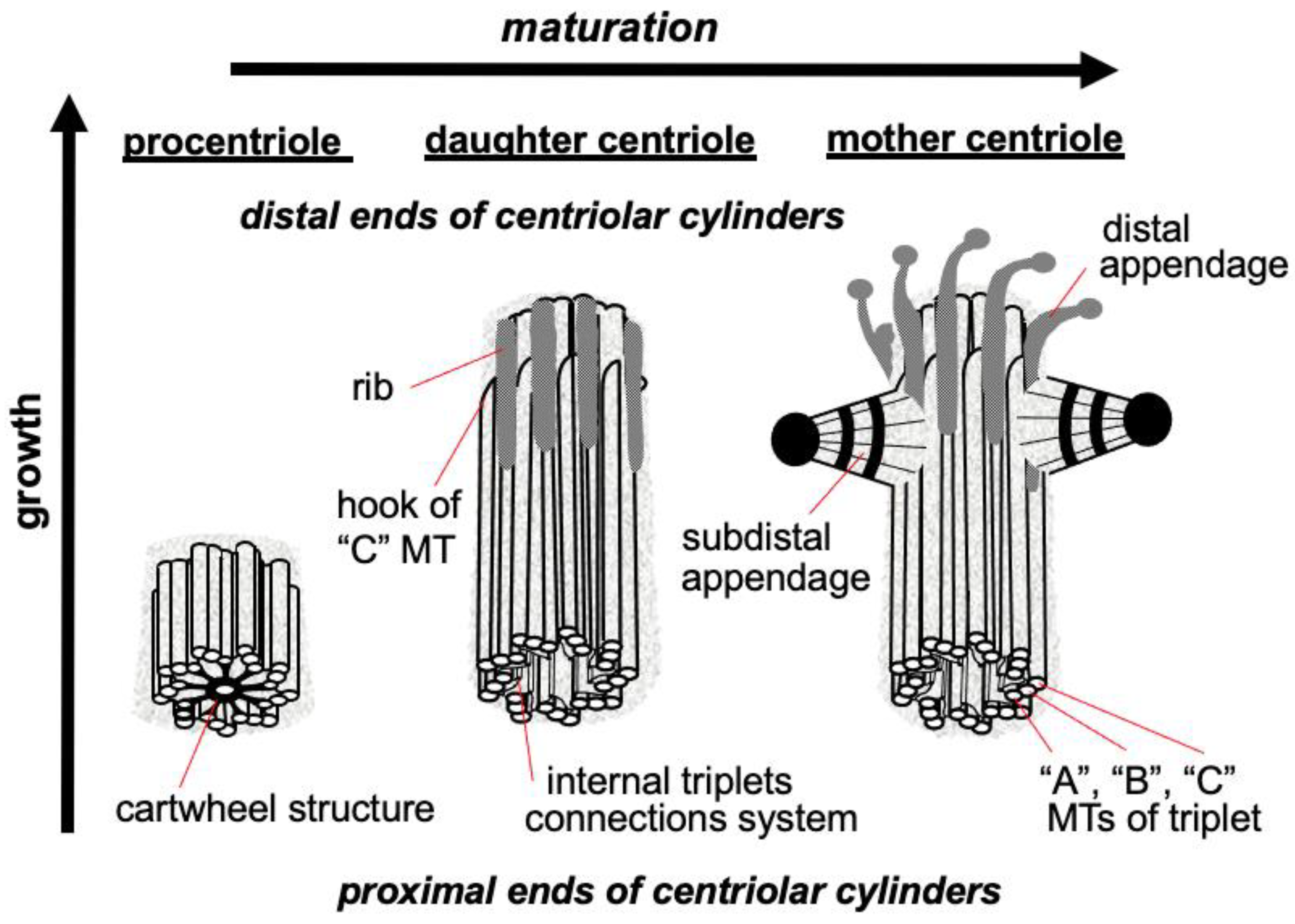
Figure 2. Procentriole, daughter and mother centrioles. Three morphological types of centriolar cylinders that can simultaneously exist in a cell. The mother centriole has an “age” at least 2 cell cycles, the daughter centriole originated in the previous cell cycle, the procentriole appeared in the current cell cycle.
Regarding subdistal appendages their number varies from 0 to 13 depending on the cell types [5]. The number of subdistal appendages depends on the microtubule nucleating activity of the centrosome, in some types of cells subdistal appendages can be arranged in two or even three rows. The base of each subdistal appendage rests on two or three centriole triplets [3].
Proteins responsible for the nucleation and anchoring of microtubules localize on the heads of the subdistal appendages [6]. These appendages are absent in mitotic centrosomes; they disassemble during G2 phase and reappear on the surface of the mother centriole at the beginning of G1 phase [7].
In the proximal part inner lumen of the centriolar cylinder of the mother and daughter of the centrioles, a system of ligaments is located, which connects the triplets from the inside to each other. In the youngest centriole—procentriole, which arose in the current cell cycle, in the inner lumen there is a “cartwheel structure”, which determines the nine-beam symmetry of centrioles during their formation due to the lateral interaction of SAS6 protein dimers, the angle between which is 40 degrees [8][9].
The centrosome includes not only centrioles, but also several additional structures: satellites, striated rootlets, less morphologically pronounced pericentriolar material and a system of ligaments between the proximal ends of the mother and daughter centrioles. The centrosome is the main nucleation center of microtubules in the cell [10]. Connected to the centrosome are the minus ends of microtubules that attach to the heads of the subdistal appendages, the surface of the predominantly mother centriole and the pericentriolar material (Figure 3).
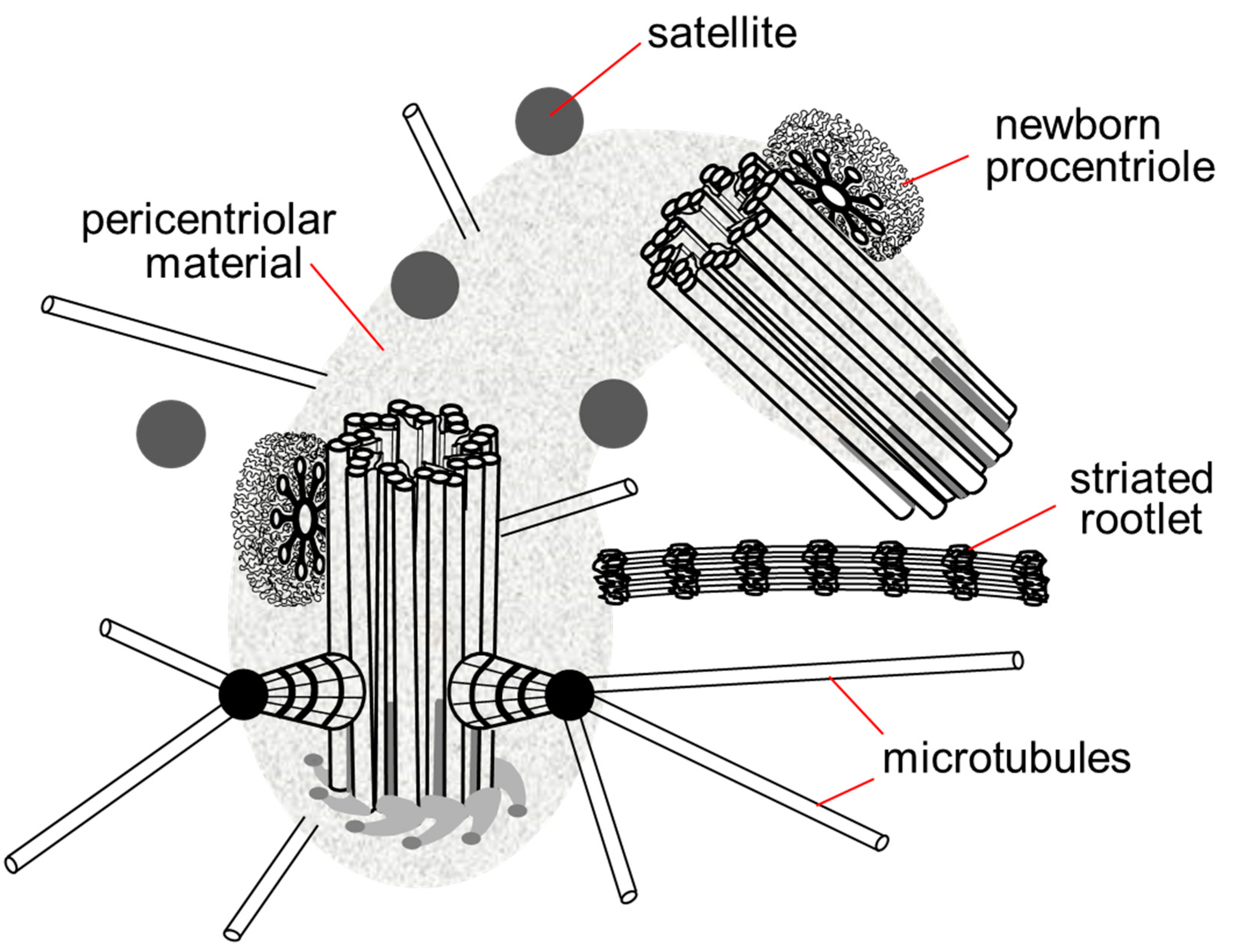
Figure 3. Centrosome in the late G1 phase of the cell cycle. The pericentriolar material completely surrounds the mother centriole and the proximal end of the daughter centriole, the satellites are located around the centrosome, in contact with the pericentriolar material, the striated rootlet is not an obligatory component of the centrosome in non-ciliary cells, its functions are not known.
Finally, the centrosomes nucleate microtubules that participate to four main structures in the cell: the radial MT network in interphase, the bipolar spindle in mitosis, the procentriole and the cilia and flagella [5].
The two centrioles act as a platform to localize and assemble functional protein complexes. For example, the pericentriolar material surrounding the centrosomes consists of proteins that will ensure microtubule nucleation, the main function of centrosomes. Two dynamics of nucleation of microtubules follow each other at the centrosomes during the cell cycle progression. In interphase the nucleated microtubules are not very dynamic and of great length, they will constitute the cytoskeleton of microtubule of the interphase cells and will take part in giving them their shape. In mitosis, the network of interphase microtubules disappears to make room for a much more dynamic network with more numerous and predominantly shorter microtubules that will only ensure one function: the assembly of the bipolar spindle essential to the segregation of chromosomes. In mitosis, the centrosomes recruit more proteins dedicated to intense nucleation of microtubules, the pericentriolar material of centrosomes becomes more extensive and denser: this mechanism is called the maturation of centrosomes. The mitotic spindle is bipolar, so the cell in mitosis must possess two centrosomes, one for each pole of the spindle. The duplication of centrosomes in interphase must therefore be strictly controlled so that it produces only two centrosomes. A higher number of centrosomes prevents the assembly of a bipolar spindle, disrupts chromosome segregation and induces aneuploidy, a situation frequently observed in cancer cells.
2. Common Features between the DNA and the Centrosome Cycle
DNA replication and centrosome duplication during a cell cycle must share common regulations because each of the two new cells formed must inherit only one copy of the genome and one copy of the centrosome from the initial cell [11][12].
The genome of eukaryotic cells is composed of DNA, structured in a double helix that carries the genes. The genome is present in two copies in each cell; each gene is therefore present in the form of two alleles. The cells are therefore diploid. This genome must be replicated (copied) at each cell cycle so that the two daughter cells inherit the same genome as the mother cell. To copy the genome, the double helix is opened and each strand is copied so that each double helix generates two new double helices, each consisting of a mother and a daughter strand [13][14].
The centrosome of higher eukaryotic cells (multicellular organisms) after mitosis consists of two centrioles. The centrosome must be duplicated (copied) during each cell cycle so that the two daughter cells inherit the same centrosome (two centrioles) as the mother cell. During the cell cycle, a new centriole is constructed on the surface of each centriole. Each daughter cell then inherits a centrosome composed of an old centriole (that of the mother cell) and a new one [15].
The temptation was therefore great to consider that, as with DNA, each centriole serves as a model for recreating a new one. However, unlike DNA, which must be copied to preserve genetic sequences and therefore cannot be synthesized de novo, the centriole is not copied. The old centriole only serves as a platform to recruit proteins that will build a new centriole. As evidence, the mechanism can occur de novo in the absence of any centriole [16][17].
The initiation of DNA replication is under the control of the restriction point (R), a checkpoint in G1 phase which when satisfied authorizes the cell to enter the cell cycle which will lead to cell division. As for the DNA, the duplication of the centrosome also depends on this restriction point.
The authorization to enter the cycle results in the production of the E2F family of transcription factors that will transactivate the genes whose products are required either for DNA replication or for centrosome duplication. The start of centrosome duplication precedes DNA replication [18] and the two events are independent of each other. Indeed, the addition of arabinosyl cytosine to cells inhibits DNA replication without affecting centriole duplication [19]. Similarly, centrioles continue to duplicate in sea urchin egg extracts in the presence of aphidicolin, an inhibitor of DNA replication, or even in previously enucleated sea urchin egg extracts [20]. Treatment of CHO cells with hydroxyurea or aphidicolin for a time equivalent to the duration of several cell cycles blocks DNA replication but does not prevent centrioles from continuing to replicate [21].
Another commonality between the DNA and the centrosome is the rule that allows DNA replication and centrosome duplication to occur only once and only once per cell cycle. As replication proceeds, the newly synthesized DNA is no longer allowed to be replicated again, only the passage through a phase of mitosis removes this blockage. The same rule applies to centrosomes which once duplicated are only allowed to replicate once they have passed through mitosis. The modalities of these authorizations are different for the DNA and the centrosome.
References
- Bornens, M. The centrosome in cells and organisms. Science 2012, 335, 422–426.
- Uzbekov, R.; Prigent, C. Clockwise or anticlockwise, turning the centriole triplets in the right direction! FEBS Lett. 2007, 581, 1251–1254.
- Uzbekov, R.; Alieva, I. Who are you, subdistal appendages of centriole? Open Biol. 2018, 8, 180062.
- Tanos, B.E.; Yang, H.J.; Soni, R.; Wang, W.J.; Macaluso, F.P.; Asara, J.M.; Tsou, M.F.B. Centriole distal appendages promote membrane docking, leading to cilia initiation. Genes Dev. 2013, 27, 163–168.
- Uzbekov, R.E.; Avidor-Reiss, T. Principal postulates of centrosomal biology. Cells 2020, 9, 2156.
- Delgehyr, N.; Sillibourne, J.; Bornens, M. Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J. Cell Sci. 2005, 118 Pt 8, 1565–1575.
- Vorobjev, I.A.; Chentsov, Y.S. Centrioles in the Cell Cycle. I Epithelial Cells. J. Cell Biol. 1982, 98, 938–949.
- Kitagawa, D.; Vakonakis, I.; Olieric, N.; Hilbert, M.; Keller, D.; Olieric, V.; Bortfeld, M.; Erat, M.C.; Fluckiger, I.; Gonczy, P.; et al. Structural basis of the 9-fold symmetry of centrioles. Cell 2011, 144, 364–375.
- van Breugel, M.; Hirono, M.; Andreeva, A.; Yanagisawa, H.A.; Yamaguchi, S.; Nakazawa, Y.; Morgner, N.; Petrovich, M.; Ebong, I.O.; Robinson, C.V.; et al. Structures of sas-6 suggest its organization in centrioles. Science 2011, 331, 1196–1199.
- Osborn, M.; Weber, K. Cytoplasmic microtubules in tissue culture cells appear to grow from an organizing structure towards the plasma membrane. Proc. Natl. Acad. Sci. USA 1976, 73, 867–871.
- Diffley, J.F. Once and only once upon a time: Specifying and regulating origins of DNA replication in eukaryotic cells. Genes Dev. 1996, 10, 2819–2830.
- Zitouni, S.; Francia, M.E.; Leal, F.; Montenegro Gouveia, S.; Nabais, C.; Duarte, P.; Gilberto, S.; Brito, D.; Moyer, T.; Kandels-Lewis, S.; et al. CDK1 Prevents Unscheduled PLK4-STIL Complex Assembly in Centriole Biogenesis. Curr. Biol. 2016, 26, 1127–1137.
- Watson, J.D.; Crick, F.H. Genetical implications of the structure of deoxyribonucleic acid. Nature 1953, 171, 964–967.
- Meselson, M.; Stahl, F.W. The replication of DNA in Escherichia coli. Proc. Natl. Acad. Sci. USA 1958, 44, 671–682.
- Gönczy, P.; Hatzopoulos, G.N. Centriole assembly at a glance. J. Cell Sci. 2019, 132, jcs228833.
- Dirksen, E.R. The presence of centrioles in artificially activated sea urchin eggs. J. Biophys. Biochem. Cytol. 1961, 11, 244–247.
- Szöllösi, D.; Calarco, P.; Donahue, R.P. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J. Cell Sci. 1972, 11, 521–541.
- Uzbekov, R.E. Centriole duplication in PE (SPEV) cells starts before beginning of DNA replication. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2007, 1, 206–211.
- Rattner, J.B.; Phillips, S.G. Independence of centriole formation and DNA synthesis. J. Cell Biol. 1973, 57, 359–372.
- Sluder, G. Centrosomes and the cell cycle. J. Cell Sci. Suppl. 1989, 12, 253–275.
- Balczon, R.; Bao, L.; Zimmer, W.E.; Brown, K.; Zinkowski, R.P.; Brinkley, B.R. Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells. J. Cell Biol. 1995, 130, 105–115.
More
