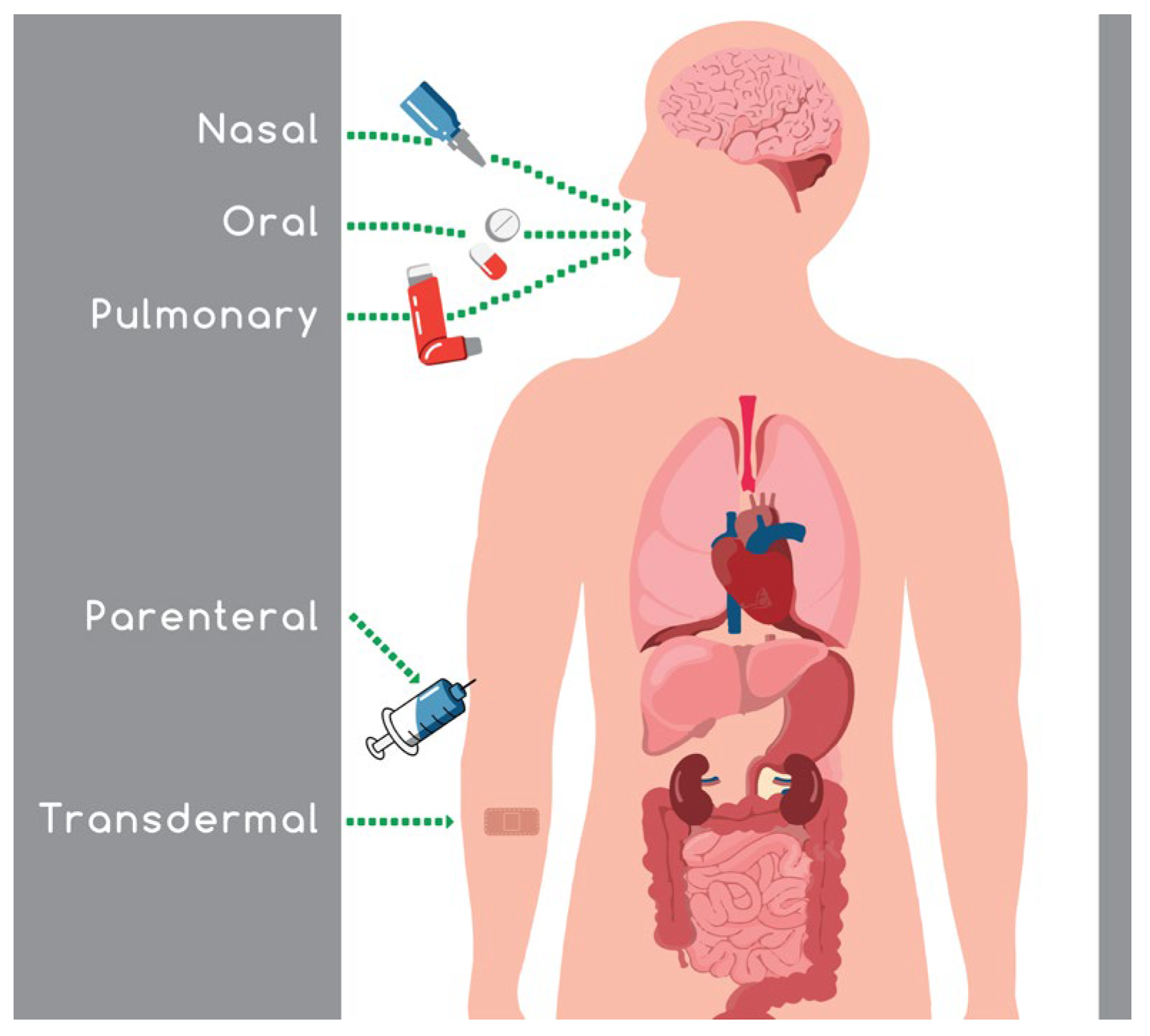
Intranasal absorption is a favored route because it avoids the gastrointestinal and hepatic metabolism, leading to an increase in drug bioavailability, and a reduction in the side effects and the required dose administered. The ongoing challenging task in the field of nasal drug delivery is the maintenance of an efficient concentration of the active substance in the target area for an adequate period of time.
- nasal drug delivery
- poloxamer
- chitosan
- gellan gum
- vaccines
- excipients
- nanoparticles
1. Introduction
Route | (Absorption Site) | Advantages | Disadvantages | Barrier Properties and Delivery Challenges | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Refs. | |||||||||||||||||||
Intravenous |
|
| None | ||||||||||||||||
NPs | extended | Encephalitis-chimeric virus | trimethyl CS, glycol CS, 6-maleimidohexanoic acid, 1-ethyl-3-(3-dimethylamino propyl)carbodiimide, N-hydroxysuccinimide, sodium tripolyphosphate, phenylmethylsulphonyl fluoride, fluorescein isothiocyanate-conjugated bovine serum albumin, bovine serum albumin, polystyrene microplates, IFN-γ, IL-4 cytokine |
[60] |
[51] |
||||||||||||||
Subcutaneous |
|
| |||||||||||||||||
NPs | slow | plasmid DNA encoding 5p36/LACK leishmanial antigen |
| ||||||||||||||||
CS microparticles, glyceraldehyde | [61] |
[52] |
Inhalation (lungs) |
| |||||||||||||||
NPs |
| controlled |
|
| |||||||||||||||
bovine serum albumin | aminated CS, aminated and thiolated CS, CS, N-(2-hydroxyethyl) ethylenediamine, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride, thioglycolic acid, (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), trypsin-EDTA |
[62] |
[53] |
Oral (intestines) |
|
|
| ||||||||||||
Transdermal (skin) |
|
|
| ||||||||||||||||
Nasal (nasal mucosa surface) |
|
|
| ||||||||||||||||
Buccal (oral mucosal surface) |
|
|
|
2. Anatomy and Physiology of the Nose
The primary roles of the nasal cavity are olfaction and breathing [12]. Nonetheless, the breathing air is filtered, humidified, and headed by the nasal cavity before reaching the lungs, whilst the inhaled particles and pathogens are trapped from the hair and the mucus layer present in the nasal cavity. Other functions of the nasal structure are the metabolism of endogenous substances and the immunological activities [13,14][13][14]. The nasal cavity is located between the roof of the mouth and the base of the skull supported from above by the ethmoid bones and from the side by the ethmoid, maxillary, and inferior conchae bones. The entire surface area is almost 150 cm2 and has a total volume of 15–20 mL [11,15][11][15]. The nasal cavity consist of three definite parts: the vestibule, the respiratory, and the olfactory regions (Figure 2) [16]. The anterior part of the nasal cavity is the nasal vestibule, which is part of the nostrils, covering an area of about 0.6 cm2, and includes the nasal hairs (vibrissae) [17]. Histologically this part is covered by a keratinized and stratified squamous epithelium with sebaceous glands [10]. These nasal regions prevent the insertion of toxic materials, but at the same time, drug absorption is also limited [18]. The respiratory region represents the largest part of the nasal cavity. It is split into three turbinates—the superior, middle, and inferior—which are responsible for the humidification and temperature adjustment of the inhaled air [19].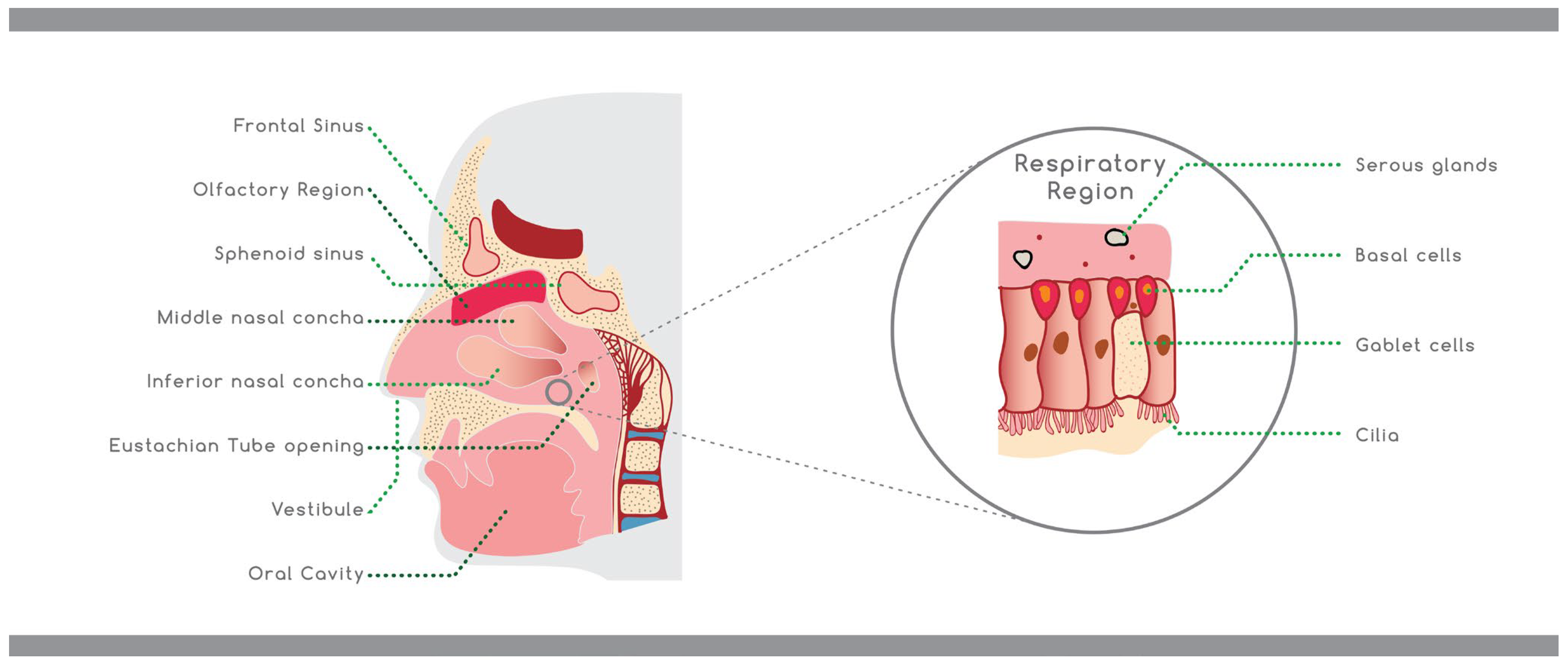
3. Nasal Drug Delivery
The nasal route of administration is used for treating local inflammation, allergic and common rhinitis, and nasal congestion. The active compounds that are commonly used against these diseases are antihistamines, glucocorticoids, or decongestants in the form of nasal spray, drops, solutions, gels, or powders and other types of formulations, including emulsions, suspensions, and microparticles [6,25][6][25]. The nasal route is used for either local or systemic action. The drug is administered locally for rapid alleviation of the symptoms of the disease, reducing the administered dose, as the drug is placed directly in the affected area, thus avoiding the systemic metabolism. On the other hand, the nasal administration of pharmacologically active substances for systemic action is used in the case of drugs with poor intestinal absorption and limited stability in the gastrointestinal fluids, with extensive hepatic first-pass metabolism, such as biologics and polar drugs [10,11][10][11]. The administration of the drug through the nasal passages can also bypass the blood–brain barrier (BBB), so it can be used for central nervous system (CNS) action. This route has been further studied for administrating vaccines [11,26][11][26]. The drug absorption through the nose is based on the physicochemical properties of the administered drug. The drug cannot penetrate the mucosa and manifest its action if it has a large size (greater than 1 kDa), a high degree of ionization, or is too lipophilic. Another factor that can affect absorption is the drug’s pH, which may affect the stability and the ionization of the drug, as well as cause nasal irritation. Formulations that have high viscosity can more easily enter the nasal mucosa, but simultaneous, they may be less absorbed. When the hyper- or hypotonicity is very high, the ciliary movement can be altered, resulting in lower absorption [6]. Finally, the drug’s concentration and quantity, the position of the head during administration, the nasal surface, and the physical condition of the dosage form all play a vital role in the absorption of the drug [27].4. Factors That Affect the Nasal Drug Absorption
Drug delivery through the nasal route of administration has some limitations, which are crucial because they influence drug concentration and bioavailability and therefore, the absorption and the pharmacological effect of the administered drug. The first main barrier is the range of pathological and physiological conditions linked to the nasal mucosa, which can affect the absorption and efficacy of the drug [28]. For example, a physiological change in nasal mucosa based on illness and allergy (irritation and the inflammation of the nasal cavity, which is intensified by itching and sneezing) may influence drug absorption [6]. In addition, there is a restriction regarding the absorption of the poorly water-soluble drugs due to the low volume of the nasal cavity, which reduces the administered amount to 100–150 μL [28]. The permeability also decreases for the polar and large molecules and for peptides and proteins [25]. However, by using the correct excipients, including bioadhesive polymers, enhancers, and enzymatic inhibitors, the drug permeability and residency in the nasal cavity can be improved [29]. Another crucial barrier is the mucociliary clearance (MCC) of the mucosa, which by replacing the mucus layer every ~15 min with 5–6 mm/min, the transmucosal absorption is decreased. The mucus can also decrease the drug absorption by binding the drug to mucin, which is the primary protein of the mucus. While the small moieties can pass through easily, the charged or larger units can be caught in the mucus gel [25]. Mucus also contains different enzymes which can influence the stability of protein- and peptide-based drugs; proteases degrade peptides and proteins by attacking them. These xenobiotic enzymes [e.g., P450 monooxygenase, Phase I enzymes (flavin monooxygenases, aldehyde dehydrogenases, epoxide hydrolases, carboxylesterases, etc.) and Phase II enzymes (glucuronyl and sulphate transferases, glutathione transferase) can also metabolize intranasally administered small-molecule drugs, such as opioids, histamines, corticosteroids, etc. [30]. Last but not least, there will always be concerns about the safety of nasal medicines, even though recent breakthroughs in both in vitro and in vivo models are a major benefit in speeding up clinical development and eventually, the time-to-market of new treatments [33][31].5. Excipients Used in Modified Drug Release Semi-Solid Pharmaceutical Dosage Forms for Nasal Administration
To improve nose-to-brain drug transfer and to extend drug residence time in the nasal cavity, several approaches could be employed, such as the use of semi-solid dosage forms, permeation enhancers, mucoadhesive and temperature responsive gels, or nano-sized drug carriers (Figure 3). In this section, the excipients and methods used in the recent achievements (2017–2022) in the modified release semi-solid formulations for nasal administration are reviewed and summarized, as shown in Table 2.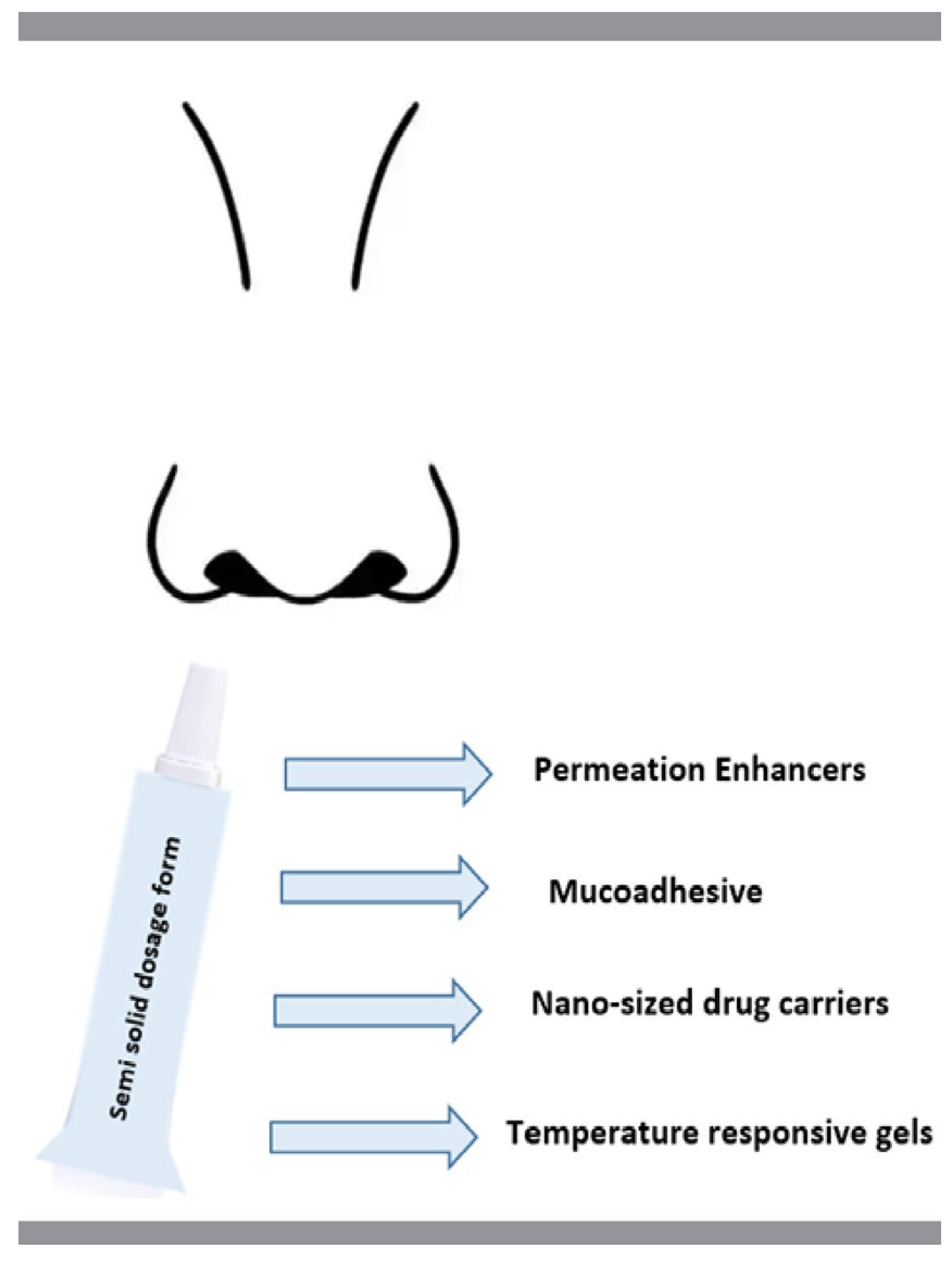
Nasal Dosage Form | Drug Release Rate * | API | Excipients | Refs. | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
in situ gel | biphasic | huperzine A | poloxamers (407, 188), CS, castor oil, polyoxyl 40 hydrogenated castor oil, 1,2- propanediol, Ringer’s solution |
[37] |
[32] |
|||||||||||||||||
in situ gel | biphasic | almotriptan | poloxamer (407, 188), Na-CMC, glyceryl behenate glyceryl palmitostearate, glyceryl monostearate, precirol |
[38] |
[33] |
|||||||||||||||||
in situ gel | biphasic | sumatriptan | poloxamers (407, 188), carrageenan, soybean phospholipids, cholesterol, tween 80, sodium caprate, sodium cholate, clostridium perfringens enterotoxin, sodium caprate |
[39] |
[34] |
|||||||||||||||||
in situ gel | controlled | |||||||||||||||||||||
hydrogel | ziprasidone | poloxamers (407, 188) β-cyclodextrin, HPMC E5, PEG 6000, PEG 4000, polyethylene, HPMCK4M | prolonged |
[40] |
antigen that generates nasal tissue resident memory CD8+ T cells | CS, poloxamers (188 and 407), ovalbumin protein, lipopolysaccharide |
[35] |
|||||||||||||||
[ | ] |
[54] |
in situ gel | controlled | ||||||||||||||||||
NPs | biphasic | geniposide | r4M2e.HSP70c | antigen | poloxamers (407, 188), HPMC, borneol, benzalkonium chloride, NaCl | N,N,N-trimethyl CS, trimethyl CS, glycerin |
[41] |
[36] |
||||||||||||||
[ | ] |
[55] |
in situ gel | sustained | rivastigmine | hydrogen tartrate | poloxamer 407, poly (lactic-co-glycolic acid), polymeric NPs | |||||||||||||||
NPs | [ |
biphasic | tetanus toxoid | 42] |
[37] |
|||||||||||||||||
CS, NPs, paraffin oil, nanospheres | [67] |
[56] |
in situ gel | sustained | mometasone furoate | |||||||||||||||||
NPs | biphasic | tetanus toxoid | poloxamer 407, Carbopol® 974P NF, PEG 400, NaCl, benzalkonium chloride, dexpanthenol, triethanolamine | N-trimethyl CS, CS, dextran microspheres, tripolyphosphate, lactose, Span 80, Tween 80 | [43] |
[38] |
||||||||||||||||
[ | ] |
[57] |
in situ gel | controlled | montelukast | sodium | poloxamer 407, HPMC K4M, PEG 400, methyl paraben |
[44] |
[39] |
|||||||||||||
NP | gradual | bovine serum albumin, ovalbumin, and myoglobin | low molecular weight CS, Compound 48/80, MTT (3-[4, 5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide), albumin-fluorescein isothiocyanate conjugate (FITC-BSA), trehalose, Dulbecco’s modified Eagle medium (DMEM) and Roswell Park Memorial Institute (RPMI), Bicinchoninic acid (BCA) assay and micro BCA kits, Fetal bovine serum (FBS), wheat germ agglutinin Alexa Fluor® 350 Conjugate and Lysotracker® Red DND 99 |
[69] |
[58] |
in situ gel | controlled | hydrocortisone | ||||||||||||||
NPs | extended | PPE17 antigen | (for tuberculosis) | poloxamer 188, Carbopol 934, PG, benzalkonium chloride, triethanolamine, isopropyl alcohol | CS, SA |
[45] |
[40] |
|||||||||||||||
[ | ] |
[59] |
NP | biphasic | pramipexole dihydrochloride | CS, sodium tripolyphosphate |
[46] | |||||||||||||||
NP | burst release prevented | PR8 influenza virus | SA, CS, N,N,N-trimethyl CS, concanavalin A |
[41] |
||||||||||||||||||
[ | ] |
[60] |
NP | biphasic | efavirenz | |||||||||||||||||
NPs | biphasic | inactivated influenza virus | CS chloral hydrate, glucosamine chloral hydrate, N-acetylglucosamine, HP-β-CD, Tween 80 | SA powder, class B CpG ODN 2007 with a phosphorothioated backbone, 2,3-bis-(2-methoxy-4-nitro-5- sulfophenyl)-2H -tetra- zolium-5-carboxanilide, Tween 80 and Span 80 |
[47] |
[42] |
||||||||||||||||
[ | ] |
[61] |
NP | |||||||||||||||||||
NPs | controlled | prolonged | sitagliptin | bovine serum | albumin | CS, glacial acetic acid, tripolyphosphate | Poly(D,L-lactide-co-glycolide), Bisphenol-A-ethoxylate di-acrylate, ethylenediamine, tetrahydrofuran, poly(vinyl alcohol) |
[48] |
[43] |
|||||||||||||
[ | ] |
[62] |
NP | delayed | ||||||||||||||||||
nanogel | gradually | human serum albumin | surface protein A fusion antigens | CS low molecular weight, acetic acid, mucin, sialic acid |
[ |
pullulan with 1.3% cholesterol and 23% amino residues | 49] |
[44] |
||||||||||||||
[ | ] |
[63] |
in situ misemgel | controlled | ||||||||||||||||||
nanogels | raloxifene |
complete release in 6 h | hydrochloride | Ovalbumin | peppermint oil, n-propanolol, n-butanol, Tween® 80, PEG 200, PG, GG, TPGS, linoleic acid, Kolliphor®, RH 40 | squalane oil, cyclohexane, surfactant sucrose laurate (L-195) | [50] |
[45] |
||||||||||||||
[ | ] |
[64] |
in situ gel loaded NPs | biphasic | voriconazole | GG, clove oil, nanotransferosomes, Tween 80, lecithin | ||||||||||||||||
nanodispersion | prolonged | Ovalbumin | Epsiliseen®-H (ϵ-polylysine), dextran sulfate sodium salt, hydrogen chloride, sodium hydroxide [51] |
[46] |
||||||||||||||||||
[ | ] |
[65] |
nanoemulsion | biphasic | quetiapine | Capmul MCM, Emalex LWIS 10, PEG 400, Transcutol P, Tween 80, water, Labrafil M 1944 CC, isopropyl myristate, sesame oil, Lauroglycol 90, miglyol 840 |
[52] |
[47] |
||||||||||||||
NPs | sustained | dolutegravir | sodium | HP-β-CD, DPC, Tween 80, DMSO |
[53] |
[48] |
||||||||||||||||
NPs | slow | acetylcholinesterase reactivator | L-α-phosphatidylcholine, 75% soybean phosphatidylcholine, dihexadecylmethylhydroxyethylammonium bromide, Tween 80, Phospholipon 80, Lipoid S75, 1-(o-tolylazo)-2-naphthol, pyrene, pyridine-2-aldoxime methochloride (Pralidoxime) |
[54] |
[49] |
* Drug release rate as stated by the author(s); CS: chitosan, DPC: diphenyl carbonate, GG: gellan gum, HPMC: hydroxypropylmethylcellulose, HP-β-CD: hydroxypropyl-β-cyclodextrin, Na-CMC: sodium carboxymethylcellulose, NPs: nanoparticles, PEG: polyethylene glycol, PG: propylene glycol, TPGS: d-α-tocopheryl polyethylene glycol 1000 succinate.
6. Excipients Used in Modified Drug Release Vaccines for Nasal Administration
Vaccine | Release Rate * | API | Excipients |
|---|
* Drug release rate as stated by the author(s); CS: chitosan, NPs: nanoparticles, SA: sodium alginate.
References
- Sadeghi, S.; Lee, W.K.; Kong, S.N.; Shetty, A.; Drum, C.L. Oral administration of protein nanoparticles: An emerging route to disease treatment. Pharmacol. Res. 2020, 158, 104685.
- Anselmo, A.C.; Gokarn, Y.; Mitragotri, S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov. 2018, 18, 19–40.
- Dubey, S.K.; Parab, S.; Dabholkar, N.; Agrawal, M.; Singhvi, G.; Alexander, A.; Bapat, R.A.; Kesharwani, P. Oral peptide delivery: Challenges and the way ahead. Drug Discov. Today 2021, 26, 931–950.
- Leonard, A.K.; Sileno, A.P.; Brandt, G.C.; Foerder, C.A.; Quay, S.C.; Costantino, H.R. In vitro formulation optimization of intranasal galantamine leading to enhanced bioavailability and reduced emetic response in vivo. Int. J. Pharm. 2007, 335, 138–146.
- Rapoport, A.; Winner, P. Nasal delivery of antimigraine drugs: Clinical rationale and evidence base. Headache 2006, 46, 192–201.
- Laffleur, F.; Bauer, B. Progress in nasal drug delivery systems. Int. J. Pharm. 2021, 607, 120994.
- Illum, L. Nasal drug delivery—Recent developments and future prospects. J. Control. Release 2012, 161, 254–263.
- Deli, M.A. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim. Biophys. Acta Biomembr. 2009, 1788, 892–910.
- Wolburg, H.; Wolburg-Buchholz, K.; Sam, H.; Horvát, S.; Deli, M.A.; Mack, A.F. Epithelial and endothelial barriers in the olfactory region of the nasal cavity of the rat. Histochem. Cell Biol. 2008, 130, 127–140.
- Illum, L. Nasal drug delivery—Possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198.
- Pires, A.; Fortuna, A.; Alves, G.; Falcão, A. Intranasal drug delivery: How, why and what for? J. Pharm. Pharm. Sci. 2009, 12, 288–311.
- Xi, J.; Si, X.A.; Kim, J.; Zhang, Y.; Jacob, R.E.; Kabilan, S.; Corley, R.A. Anatomical Details of the Rabbit Nasal Passages and Their Implications in Breathing, Air Conditioning, and Olfaction. Anat. Rec. 2016, 299, 853–868.
- Gizurarson, S. The effect of cilia and the mucociliary clearance on successful drug delivery. Biol. Pharm. Bull. 2015, 38, 497–506.
- Ugwoke, M.I.; Verbeke, N.; Kinget, R. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. J. Pharm. Pharmacol. 2010, 53, 3–21.
- Lazovic, G.D.; Daniel, R.K.; Janosevic, L.B.; Kosanovic, R.M.; Colic, M.M.; Kosins, A.M. Rhinoplasty: The nasal bones-anatomy and analysis. Aesthetic Surg. J. 2015, 35, 255–263.
- Kim, D.; Kim, Y.H.; Kwon, S. Enhanced nasal drug delivery efficiency by increasing mechanical loading using hypergravity. Sci. Rep. 2018, 8, 1–9.
- Bitter, C.; Suter-Zimmermann, K.; Surber, C. Nasal drug delivery in humans. Curr. Probl. Dermatol. 2011, 40, 20–35.
- Mujawar, N.; Ghatage, S.; Navale, S.; Sankpal, B.; Patil, S.; Patil, S. Nasal Drug Delivery: Problem Solution and Its Application. J. Curr. Pharma Res. 2014, 4, 1231–1245.
- Gosau, M.; Rink, D.; Driemel, O.; Draenert, F.G. Maxillary sinus anatomy: A cadaveric study with clinical implications. Anat. Rec. 2009, 292, 352–354.
- Inoue, D.; Tanaka, A.; Kimura, S.; Kiriyama, A.; Katsumi, H.; Yamamoto, A.; Ogawara, K.I.; Kimura, T.; Higaki, K.; Yutani, R.; et al. The relationship between in vivo nasal drug clearance and in vitro nasal mucociliary clearance: Application to the prediction of nasal drug absorption. Eur. J. Pharm. Sci. 2018, 117, 21–26.
- Tomazic, P.V.; Darnhofer, B.; Birner-Gruenberger, R. Nasal mucus proteome and its involvement in allergic rhinitis. Expert Rev. Proteom. 2020, 17, 191–199.
- Evans, C.M.; Koo, J.S. Airway mucus: The good, the bad, the sticky. Pharmacol. Ther. 2009, 121, 332–348.
- Roger, D.F. Physiology of airway mucus secretion and pathophysiology of hypersecretion. Respir. Care 2007, 52, 1134–1146.
- Charlton, S.; Jones, N.S.; Davis, S.S.; Illum, L. Distribution and clearance of bioadhesive formulations from the olfactory region in man: Effect of polymer type and nasal delivery device. Eur. J. Pharm. Sci. 2007, 30, 295–302.
- Ali, A.; Wahlgren, M.; Rembratt-Svensson, B.; Daftani, A.; Falkman, P.; Wollmer, P.; Engblom, J. Dehydration affects drug transport over nasal mucosa. Drug Deliv. 2019, 26, 831–840.
- Illum, L. Nasal drug delivery: New developments and strategies. Drug Discov. Today 2002, 7, 1184–1189.
- Costa, C.; Moreira, J.N.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J. Control. Release 2019, 295, 187–200.
- Costantino, H.R.; Illum, L.; Brandt, G.; Johnson, P.H.; Quay, S.C. Intranasal delivery: Physicochemical and therapeutic aspects. Int. J. Pharm. 2007, 337, 1–24.
- Tanaka, A.; Furubayashi, T.; Enomura, Y.; Hori, T.; Shimomura, R.; Maeda, C.; Kimura, S.; Inoue, D.; Kusamori, K.; Katsumi, H.; et al. Nasal drug absorption from powder formulations: Effect of fluid volume changes on the mucosal surface. Biol. Pharm. Bull. 2017, 40, 212–219.
- Keller, L.A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2022, 12, 735–757.
- Ugwoke, M.I.; Agu, R.U.; Verbeke, N.; Kinget, R. Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv. Drug Deliv. Rev. 2005, 57, 1640–1665.
- Chen, Y.; Cheng, G.; Hu, R.; Chen, S.; Lu, W.; Gao, S.; Xia, H.; Wang, B.; Sun, C.; Nie, X.; et al. A Nasal Temperature and pH Dual-Responsive In Situ Gel Delivery System Based on Microemulsion of Huperzine A: Formulation, Evaluation, and In Vivo Pharmacokinetic Study. AAPS PharmSciTech 2019, 20, 1–12.
- Youssef, N.A.H.A.; Kassem, A.A.; Farid, R.M.; Ismail, F.A.; EL-Massik, M.A.E.; Boraie, N.A. A novel nasal almotriptan loaded solid lipid nanoparticles in mucoadhesive in situ gel formulation for brain targeting: Preparation, characterization and in vivo evaluation. Int. J. Pharm. 2018, 548, 609–624.
- Omar, M.M.; Eleraky, N.E.; El Sisi, A.M.; Ali Hasan, O. Development and Evaluation of in-situ Nasal Gel Formulations of Nanosized Transferosomal Sumatriptan: Design, Optimization, in vitro and in vivo Evaluation. Drug Des. Dev. Ther. 2019, 13, 4413–4430.
- Londhe, V.; Krishnan, S. Formulation, Evaluation, and Pharmacodynamic Investigation of Ziprasidone-b-cyclodextrin In-Situ Nasal Gel. Proceedings 2020, 78, 42.
- Wang, Y.; Jiang, S.; Wang, H.; Bie, H. A mucoadhesive, thermoreversible in situ nasal gel of geniposide for neurodegenerative diseases. PLoS ONE 2017, 12, e0189478.
- Salatin, S.; Barar, J.; Barzegar-Jalali, M.; Adibkia, K.; Jelvehgari, M. Thermosensitive in situ nanocomposite of rivastigmine hydrogen tartrate as an intranasal delivery system: Development, characterization, ex vivo permeation and cellular studies. Colloids Surf. B Biointerfaces 2017, 159, 629–638.
- Altuntaş, E.; Yener, G. Formulation and Evaluation of Thermoreversible In Situ Nasal Gels Containing Mometasone Furoate for Allergic Rhinitis. AAPS PharmSciTech 2017, 18, 2673–2682.
- Durgapal, S.; Rana, M.; Mukhopadhyay, S.; Rana, A.J.; Goswami, L.; Joshi, S. Formulation and Evaluation of in-Situ Nasal Gel of Montelukast Sodium for the Effective Treatment of Asthma. Int. J. Pharm. Sci. Res. 2018, 9, 2792.
- Khandagale, P.M.; Rokade, M.M.; Phadtare, D.G. Formulation Development and Evaluation of Nasal In- Situ Gel of Hydrocortisone. Asian J. Pharm. Technol. 2018, 8, 92–102.
- Raj, R.; Wairkar, S.; Sridhar, V.; Gaud, R. Pramipexole dihydrochloride loaded chitosan nanoparticles for nose to brain delivery: Development, characterization and in vivo anti-Parkinson activity. Int. J. Biol. Macromol. 2018, 109, 27–35.
- Belgamwar, A.; Khan, S.; Yeole, P. Intranasal chitosan-g-HPβCD nanoparticles of efavirenz for the CNS targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 374–386.
- Wilson, B.; Mohamed Alobaid, B.N.; Geetha, K.M.; Jenita, J.L. Chitosan nanoparticles to enhance nasal absorption and brain targeting of sitagliptin to treat Alzheimer’s disease. J. Drug Deliv. Sci. Technol. 2021, 61, 102176.
- Piazzini, V.; Landucci, E.; D’Ambrosio, M.; Tiozzo Fasiolo, L.; Cinci, L.; Colombo, G.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Luceri, C.; Bergonzi, M.C. Chitosan coated human serum albumin nanoparticles: A promising strategy for nose-to-brain drug delivery. Int. J. Biol. Macromol. 2019, 129, 267–280.
- Ahmed, O.A.A.; Badr-Eldin, S.M. In situ misemgel as a multifunctional dual-absorption platform for nasal delivery of raloxifene hydrochloride: Formulation, characterization, and in vivo performance. Int. J. Nanomed. 2018, 13, 6325–6335.
- Kammoun, A.K.; Khedr, A.; Hegazy, M.A.; Almalki, A.J.; Hosny, K.M.; Abualsunun, W.A.; Murshid, S.S.A.; Bakhaidar, R.B. Formulation, optimization, and nephrotoxicity evaluation of an antifungal in situ nasal gel loaded with voriconazole–clove oil transferosomal nanoparticles. Drug Deliv. 2021, 28, 2229–2240.
- Boche, M.; Pokharkar, V. Quetiapine Nanoemulsion for Intranasal Drug Delivery: Evaluation of Brain-Targeting Efficiency. AAPS PharmSciTech 2017, 18, 686–696.
- Belgamwar, A.V.; Khan, S.A.; Yeole, P.G. Intranasal dolutegravir sodium loaded nanoparticles of hydroxypropyl-beta-cyclodextrin for brain delivery in Neuro-AIDS. J. Drug Deliv. Sci. Technol. 2019, 52, 1008–1020.
- Pashirova, T.N.; Zueva, I.V.; Petrov, K.A.; Lukashenko, S.S.; Nizameev, I.R.; Kulik, N.V.; Voloshina, A.D.; Almasy, L.; Kadirov, M.K.; Masson, P.; et al. Mixed cationic liposomes for brain delivery of drugs by the intranasal route: The acetylcholinesterase reactivator 2-PAM as encapsulated drug model. Colloids Surf. B Biointerfaces 2018, 171, 358–367.
- Tai, J.; Han, M.; Lee, D.; Park, I.-H.; Lee, S.H.; Kim, T.H. Different Methods and Formulations of Drugs and Vaccines for Nasal Administration. Pharmaceutics 2022, 14, 1073.
- Dumkliang, E.; Pamornpathomkul, B.; Patrojanasophon, P.; Ngawhirunpat, T.; Rojanarata, T.; Yoksan, S.; Opanasopit, P. Feasibility of chitosan-based nanoparticles approach for intranasal immunisation of live attenuated Japanese encephalitis vaccine. Int. J. Biol. Macromol. 2021, 183, 1096–1105.
- Oliveira Gomes, D.C.; Lilian da Silva Costa Souza, B.; Schwedersky, R.P.; Covre, L.P.; Leonel de Matos Guedes, H.; Lopes, U.G.; Inês Ré, M.; Rossi-Bergmann, B. Intranasal immunization with chitosan microparticles enhances lack-dna vaccine protection and induces specific long-lasting immunity against visceral leishmaniasis. Microbes Infect. 2021, 24, 104884.
- Sinani, G.; Sessevmez, M.; Gök, M.K.; Özgümüş, S.; Alpar, H.O.; Cevher, E. Modified chitosan-based nanoadjuvants enhance immunogenicity of protein antigens after mucosal vaccination. Int. J. Pharm. 2019, 569, 118592.
- Bedford, J.G.; Caminschi, I.; Wakim, L.M. Intranasal delivery of a chitosan-hydrogel vaccine generates nasal tissue resident memory CD8+ T cells that are protective against influenza virus infection. Vaccines 2020, 8, 572.
- Dabaghian, M.; Latifi, A.M.; Tebianian, M.; NajmiNejad, H.; Ebrahimi, S.M. Nasal vaccination with r4M2e.HSP70c antigen encapsulated into N-trimethyl chitosan (TMC) nanoparticulate systems: Preparation and immunogenicity in a mouse model. Vaccine 2018, 36, 2886–2895.
- Pirouzmand, H.; Khameneh, B.; Tafaghodi, M. Immunoadjuvant potential of cross-linked dextran microspheres mixed with chitosan nanospheres encapsulated with tetanus toxoid. Pharm. Biol. 2017, 55, 212–217.
- Kabiri, M.; Bolourian, H.; Dehghan, S.; Tafaghodi, M. The dry powder formulation of mixed cross-linked dextran microspheres and tetanus toxoid-loaded trimethyl chitosan nanospheres as a potent adjuvant for nasal delivery system. Iran. J. Basic Med. Sci. 2020, 24, 116–122.
- Bento, D.; Jesus, S.; Lebre, F.; Gonçalves, T.; Borges, O. Chitosan plus compound 48/80: Formulation and preliminary evaluation as a hepatitis B vaccine adjuvant. Pharmaceutics 2019, 11, 72.
- Najafi, A.; Ghazvini, K.; Sankian, M.; Gholami, L.; Amini, Y.; Zare, S.; Khademi, F.; Tafaghodi, M. T helper type 1 biased immune responses by PPE17 loaded core-shell alginate-chitosan nanoparticles after subcutaneous and intranasal administration. Life Sci. 2021, 282, 119806.
- Mosafer, J.; Sabbaghi, A.H.; Badiee, A.; Dehghan, S.; Tafaghodi, M. Preparation, characterization and in vivo evaluation of alginate-coated chitosan and trimethylchitosan nanoparticles loaded with PR8 influenza virus for nasal immunization. Asian J. Pharm. Sci. 2019, 14, 216–221.
- Dehghan, S.; Kheiri, M.T.; Abnous, K.; Eskandari, M.; Tafaghodi, M. Preparation, characterization and immunological evaluation of alginate nanoparticles loaded with whole inactivated influenza virus: Dry powder formulation for nasal immunization in rabbits. Microb. Pathog. 2018, 115, 74–85.
- Sinani, G.; Sessevmez, M.; Koray Gök, M.; Özgümüş, S.; Okyar, A.; Oya Alpar, H.; Cevher, E. Nasal vaccination with poly(β-amino ester)-poly(D,L-lactide-co-glycolide) hybrid nanoparticles. Int. J. Pharm. 2017, 529, 1–14.
- Yuki, Y.; Uchida, Y.; Sawada, S.I.; Nakahashi-Ouchida, R.; Sugiura, K.; Mori, H.; Yamanoue, T.; Machita, T.; Honma, A.; Kurokawa, S.; et al. Characterization and Specification of a Trivalent Protein-Based Pneumococcal Vaccine Formulation Using an Adjuvant-Free Nanogel Nasal Delivery System. Mol. Pharm. 2021, 18, 1582–1592.
- Kong, Q.; Kitaoka, M.; Tahara, Y.; Wakabayashi, R.; Kamiya, N.; Goto, M. Solid-in-oil nanodispersions for intranasal vaccination: Enhancement of mucosal and systemic immune responses. Int. J. Pharm. 2019, 572, 118777.
- Bonaccorso, A.; Carbone, C.; Tomasello, B.; Italiani, P.; Musumeci, T.; Puglisi, G.; Pignatello, R. Optimization of dextran sulfate/poly-L-lysine based nanogels polyelectrolyte complex for intranasal ovalbumin delivery. J. Drug Deliv. Sci. Technol. 2021, 65, 102678.
