Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Emily Davis.
The looming climate and energy crises, exacerbated by increased waste generation, are driving research and development of sustainable resource management systems. Research suggests that organic materials, such as food waste, grass, and manure, have potential for biotransformation into a range of products, including: high-value volatile fatty acids (VFAs); various carboxylic acids; bioenergy; and bioplastics. Valorizing these organic residues would additionally reduce the increasing burden on waste management systems.
- anaerobic digestion
- biorefinery
- fermentation
1. Introduction
As both climate change and the global energy crisis escalate, it becomes ever more critical to implement sustainable resource management strategies such as biorefineries and resource recovery systems. These systems typically utilize innovative resource recovery technologies and novel renewable materials. The valorization of biomass can play a foundational role within these systems, supporting the generation of energy (biofuel) as well as a wide range of bio-based products through the biorefinery concept [1,2,3,4][1][2][3][4]. Biomass can be broadly classified as either an energy crop or residue. Energy crops are specifically cultivated for energy generation. These crops are typically cultivated using intensive farming practices, and since they are often edible, using them for energy generation results in less food and less food-crop land. In contrast, biomass residues are non-edible and are generally composed of waste products or agro-industrial side streams.
Among biomass residues, food waste and agricultural waste have demonstrated their promising potential for biorefinery applications [2,3,5,6,7][2][3][5][6][7]. In Europe, these biomasses are valorized using biological, chemical, and thermochemical methods. However, variability in the quantity and composition of biomass limits the technological and economic viability of these valorization methods. Therefore, these highly variable biomass resources are better suited for processes such as anaerobic digestion (AD), which is able to convert a wide range of organics into products such as volatile fatty acids (VFAs), biohydrogen, polyhydroxyalkanoates (PHAs), and bioenergy. AD can serve as a sustainable and economically attractive biological pretreatment for lignocellulosic biomass, facilitating its conversion into bio-based products by exposing lignin and undigested fibers for further valorization.
The ultimate purpose of an AD-based biorefinery system is to optimize resource-use efficiency while minimizing waste; this is typically accomplished by maximizing energy/biogas production. The generation of alternative valuable by-products, in addition to biogas, represents a new opportunity to enhance resource recovery (Figure 1). An important value-added by-product, VFAs, are produced during the initial phases of AD in a process known as acidogenic fermentation. VFAs have a wide range of potential applications in the biorefinery industry where they can be used as feedstock for various bio-based products. For instance, VFAs are considered a potential platform for the production of biodegradable PHA polymers [8]. Currently, synthetically produced VFAs are used in the food and beverage industries, as well as in pharmaceutical and synthetic chemistry. The ratios of the specific volatile fatty acids that are produced via acidogenic fermentation are dependent on the feedstock biomass’ composition, the extent of hydrolysis, operational conditions, reactor design, and the structure of the microbial community. Research investigating these parameters is being carried out and promises to greatly improve the efficiency and stability of the acid-forming stage.
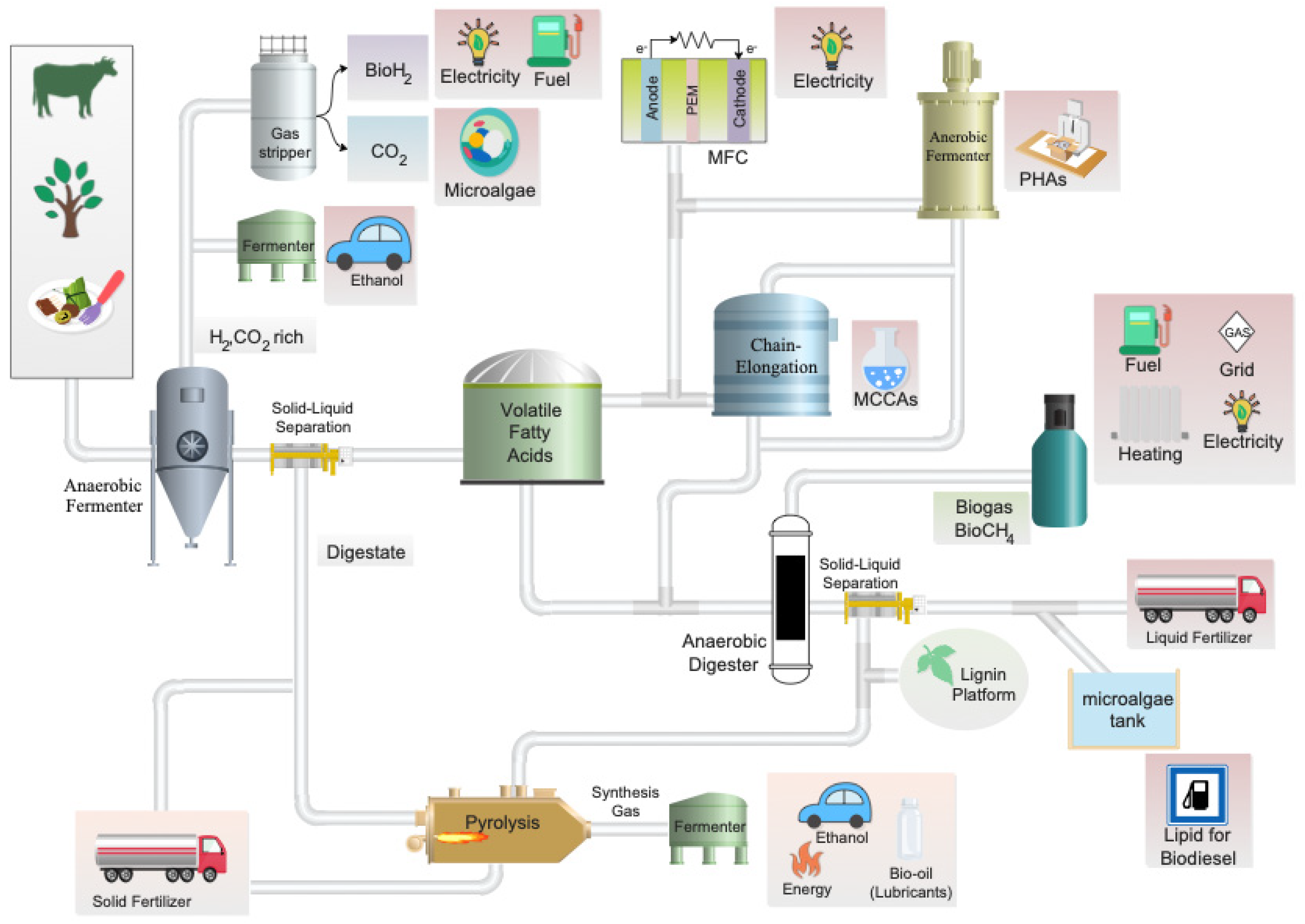
Figure 1. Potential biorefinery process focusing on maximizing VFA production. The process begins with the valorization of residual feedstocks and culminates in the potential production of various high-value end-products (highlighted in pink).
2. Methods of Valorizing Low-Value Feedstocks
2.1. Thermochemical Approach
Among the thermochemical conversion processes, gasification and pyrolysis are commonly used to produce heat, biochar, and syngas from lignocellulosic biomass. Conventional gasification technologies include fixed beds, fluidized beds, and entrained flow reactors [10][9]. However, these technologies still struggle with process inefficiencies related to biomass moisture content and tar production. Recently, efforts have been made to mitigate these factors—using technologies such as pyrolysis and supercritical water gasification [11,12,13,14,15,16][10][11][12][13][14][15]. Unlike gasification, pyrolysis is a technology that converts biomass into bio-oil, syngas, and biochar in the absence of oxygen [17][16]. Pyrolysis can be used to valorize different types of recalcitrant biomass, such as agricultural residues and wood wastes. The resulting syngas can then be converted by anaerobic bacteria into biochemicals and biofuels independent of the original biomass composition; a process known as hybrid thermochemical-biochemical [18][17]. However, the high cost and safety risk of the pyrolysis process make it unviable for large scale applications [17][16].
Recently, supercritical water gasification has been considered as a potential technique to valorize lignocellulosic biomass and wastes with high moisture content—up to 80% wet weight [16][15]. Supercritical water gasification is being studied especially for hydrogen production, as the composition of the resulting synthesis gas is higher in hydrogen and lower in carbon monoxide [19][18]; in addition, the low production of tar and char is an advantage compared to other technologies [20][19]. However, supercritical water gasification is a technology still unfeasible for large scale applications in biorefineries—its implementation requires improvements in terms of pump energy efficiency [16][15], and reactor designs which can withstand corrosion [16][15] and high pressure [21][20].
2.2. Biological Approach
Biological conversion processes encompass both AD and fermentation and are commonly used to valorize biomass such as food waste, agricultural residues, organic fraction of the municipal solid waste (OFMSW), and energy crops. Unlike the thermochemical conversion method where the primary product is biofuel, the biological conversion of biomass can produce biofuel and chemicals. Due to the high moisture content of most biomass, direct valorization using thermochemical technologies is challenging. Therefore, biological conversion technologies are reported to be more eco-friendly and appropriate for waste biomass with high moisture content [22][21].
AD is a well-established process for the sustainable management of solid organic feedstock [22][21]. AD can be used to convert various organic substrates into methane-rich gas destined for energy generation. In this context, organic residues are conveniently used to meet global energy demand while reducing the burden of fuel consumption and waste disposal. In Europe, the success of AD is witnessed by its dynamic ascent with a total of 18,202 biogas installations, producing 11,082 MW, and 63,511 GWh worth of biogas as recorded in 2018 [23,24][22][23]. Despite this continued growth, AD technology is still not cost-competitive with natural gas without fiscal incentives. This is due to high costs associated with biogas production, whereas natural gas is available at lower cost worldwide. Therefore, increasing the efficiency of AD processes is critical to improving its economic attractiveness. To this end, feedstock pre-treatment, reactor configuration, and feedstock co-digestion have been studied as potential means of improving resource recovery [25,26][24][25].
2.3. Valorization—Selecting a Method
The selection of a particular valorization method is highly dependent on the biomass characteristics and composition. For instance, biological approaches are suitable for readily degradable, high-moisture-content biomass such as food waste. Thermochemical methods are more commonly used for recalcitrant feedstocks such as lignocellulosic biomass. While both treatment options entail installation and operation costs, research and application suggest that the biological approach may be more flexible in terms of feedstock and products. Moreover, AD and fermentative processes result in fewer undesirable effects such as tar production.
3. Sustainable Feedstock Types
3.1. Food Waste
Food waste makes up a significant portion of anthropogenically derived organic waste and constitutes an environmental burden where landfill disposal is employed. One third of all food produced in the world for human consumption goes to waste [27][26], with 14% of food waste occurring during production processes alone [28][27]. While post-consumer waste can be minimized through prevention campaigns, production wastage (peelings, damaged or diseased matter, inedible plant parts) is likely to remain at similar or increasing values. Generally, food waste is composed of fruits, vegetables, and tubers [28][27]. These materials all have relatively high moisture and energy contents and, therefore, qualify as high value feedstock for AD [29][28]. Through AD, this waste stream can be converted into a renewable resource while simultaneously reducing waste-related challenges in the long term [30][29].
Food waste composition varies greatly but is fundamentally a mix of carbohydrates, proteins, and lipids. The ratio of these three biomolecules largely determines the material’s energy generation potential. Lipids have higher energy content than carbohydrates and proteins; however, they have been reported to be difficult to breakdown in AD bioreactors, even destabilizing digesters at high concentrations [31,32,33][30][31][32]. Most food waste is primarily composed of complex carbohydrates, including lignocellulosic and/or hemicellulosic compounds (25–30% of total solids (TS)) [34,35][33][34]. These carbohydrates originate from plant matter and are challenging to hydrolyze. Indeed, hydrolysis is frequently reported as the rate limiting step in AD [36][35]. Efforts to facilitate hydrolysis have been made, primarily the investigation of various pre-treatment methods including alkaline [25[24][36],37], thermal [38][37], acid [25][24] and enzymatic pre-treatments [39][38]. However, these treatments all increase operational costs. Whereas biological strategies, such as tailoring operational conditions to promote the growth and persistence of key microbial hydrolysers within AD bioreactors, represent a promising alternative [35][34].
3.2. Agricultural Residues
Agricultural residual biomasses comprise crop and plant residues, vegetable waste, forest residues, grass, and livestock manure [40][39]. These are largely composed of lignocellulose which can be converted via AD and fermentation to bioenergy and biochemicals. The efficiency of these conversions is determined by specific lignocellulosic characteristics such as lignin content, degree of polymerization, hemicellulose structure, cellulose crystallinity, porosity, and specific area [41,42][40][41].
Many studies in the literature review ese contents introduce the use of pre-treatments that would decrease the recalcitrance of this biomass by improving the accessibility of cellulose to cellulases. This is achieved either by decreasing the hemicellulose content (e.g., dilute acids and bases) or by applying physical treatment (e.g., high temperature and pressure) to disrupt the lignin matrix [40,42][39][41]. Of course, all pre-treatment processes entail a trade-off between the cost of pre-treatment and the desired end-product yield increase [43][42]. While ionic liquids and deep-eutectic solvents have been recently investigated, full-scale biorefineries generally employ steam explosion, organosolv, or dilute acids [43,44][42][43]. The use of these pretreatment technologies may negatively impact the indigenous microbiome of the feedstock, which can be critical to the fermentative process.
The use of lignocellulosic waste as feedstock for biogas production through AD is well established. However, the potential of lignocellulosic waste for VFA production has been garnering increased attention [45][44]. In a biorefinery context, carboxylic acids are a desirable product with high market value [46,47,48,49][45][46][47][48]. Among lignocellulosic wastes, grass is an abundant, renewable, and cheap feedstock that has been largely employed to produce biogas in AD [50][49]. Relatedly, silage is grass which has been fermented to facilitate preservation during storage. During fermentation, lactic acid bacteria use soluble carbohydrates present on the surface of grass in the production of lactic acid, causing a decrease in pH, which allows the feedstock to be preserved for animal feed without risk of spoilage [51][50].
While grass is considered a sustainable feedstock due to its carbon-sequestering capacity, co-digestion with other agricultural residues, such as cattle slurry, may further enhance the sustainability of the process [50,52][49][51]. Cattle slurry is an abundant agricultural waste and is cheaper and richer in nutrients than grassland feedstocks. Furthermore, by co-digesting this waste stream with grass, greenhouse gas (GHG) emissions from slurry are reduced [50][49]. The co-digestion of different grassland forages with grass could also improve AD yields due to an improvement in nutrient availability for the microbial community [53][52]. Moreover, the combined growth of multi-species grassland mixtures (herbs, legumes, and grass) in intensively managed grassland may enhance yield while mitigating disturbances, such as drought and environmental impact, when compared to monocultures [52][51].
3.3. Animal Residues
Animal manure is a primary contributor to environmental pollution in rural areas. This is usually due to emissions from land-spreading and manure storage facilities, which release harmful substances to the soil, water, and atmosphere. Animal manure/slurry has high concentrations of nutrients (such as nitrogen and phosphorus) and metals (such as copper, zinc, arsenic, and cadmium). Leaching of these metals into the surrounding environment increases phytotoxicity, reduces soil fertility and productivity, and increases toxicity of crops and food products grown on the contaminated soil [54][53]. Meanwhile, leaching of nutrients contributes to water quality degradation and eutrophication. Moreover, the storage and land-spreading of animal manure/slurry can release GHG, such as methane, nitrous oxide, and ammonia, into the air contributing to climate change [55,56][54][55].
To mitigate the environmental burden of the manure/slurry, researchers have engaged in developing techniques for its sustainable treatment. Although composting, incineration, pyrolysis, and gasification have been evaluated, AD is outstanding in its capacity to reduce pollution while generating valuable by-products such as fertilizers and renewable energy [54][53]. However, there are some factors at play which limit the use of slurry/manure fed AD: (i) slurry bio-methane potential is low due to high moisture and low organic content [55][54]; (ii) a large volume of feedstock, usually collected from multiple sites, is required for efficiencies of scale [54][53]; and (iii) slurry has a low C:N ratio, which tends to inhibit methane production.
These issues can be mitigated or avoided entirely by employing co-digestion, specifically, by making a mixed feedstock composed of slurry with other organic wastes/residues/energy crops that have a high C:N ratio. Several researchers have reported that co-digestions improved biogas production [57,58,59][56][57][58] or VFA production [60,61][59][60]. Although the co-digestion of manure/slurry with other feedstock provides a means to increase economic feasibility, the nutrient and metal-rich liquid digestate remains an issue in an AD-based facility. Therefore, complete valorization of the manure/slurry within an AD-based biorefinery concept could result in a more desirable digestate product.
4. State-of-the-Art System Designs
4.1. Single-Stage System Design and Application
Anaerobic bioreactors may be designed to optimize the processing of a selected biomass and for the production of a specific desired product. Many bioreactor types have the capacity to produce VFAs, hydrogen associated with VFA as a by-product, or biogas. Several reactors, including the continuous stirred tank reactor (CSTR), the packed bed biofilm column reactor, leach bed reactor (LBR), two-stage anaerobic bioreactor, and continuous flow fermentation reactors, have been used to produce VFAs (Table 1). Studies using solid feedstocks generally use CSTRs or LBRs and have generated promising results. The CSTR is perhaps the most widely used single-stage wet AD design [62][61]. CSTRs are suitable for materials with solids content up to 10% [63][62] and work by thoroughly mixing feedstock and microbes in the presence of suspended solids [64][63]. Previous studies have reported successful production of VFAs from food waste and OFMSW using the CSTR configuration (Table 1). However, this reactor design has significant inherent inefficiencies, including (i) a tendency for biomass washout, (ii) the need for size reduction of the substrates, (iii) energy input required for continuous stirring, and (iv) the low solids content (<10%) requirement [65,66][64][65]. In an attempt to overcome these limitations, a novel CSTR design consisting of a solid–liquid separator was proposed to retain undigested biomass with the active community in the system [67][66]. This approach addresses the issue of biomass washout, but not the limitations for feedstock processing (size reduction, low solids) or energy consumption. LBRs are a promising alternative to the CSTR for VFA production from high-solid waste such as food waste, OFMSW and vegetable waste, and grass (Table 1). Compared to CSTRs, these reactors have been reported to permit higher loads and high VFA production [35,68][34][67]. In LBRs, solid material is loaded into the reactor and irrigated with water, which is recycled through the system continuously. Hydrolysis occurs in the solid bed, while fermentation occurs in the liquid phase, thus, decoupling the hydrolysis and fermentation processes. The recirculation mechanism allows for the dilution of inhibitory compounds and increases the moisture in the solid bed which facilitates micro-organism growth and activity, all with a relatively low water requirement [69,70][68][69]. Compared to CSTRs, LBRs have several financial advantages—less instrumentation, maintenance, and investment are required, making it an attractively low-cost, high-solids AD reactor [71][70]. However, since LBRs process solid feedstock which is not stirred, VFA product accumulation can occur. Furthermore, high levels of VFA can inhibit micro-organisms involved in the hydrolysis and fermentation stages [72,73,74][71][72][73]. In-line VFA separation, which could remove VFA product from the LBR leachate, is currently being investigated [75,76][74][75]. However, there is currently no consensus or single outstanding technology being used to recover VFA from fermentation liquor. Therefore, researchers have focused on developing two-stage systems in which VFAs generated in LBRs are removed and valorized through processes such as chain elongation (CE), PHA production, or even biogas production.Table 1.
Chemical characteristics of feedstocks and inoculum sources used for VFA production.
| Raw Material | TS | VS | COD/TOC | Lignocellulosic | Remarks | Ref |
|---|---|---|---|---|---|---|
| (%ww) | (%ww) | (gO2.kg−1ww) | (%TS) | |||
| Feedstock: | ||||||
| Cattle manure | 5.0–9.5 | 7.0–7.3 | 44–54 | n/r | TKN: 1.9–3.6 gN.kg−1ww | [3] |
| Ryegrass silage | 35–40 | 31.3–36.0 | 312–360 | n/r | TKN: 4.7–5.9 gN.kg−1ww | [3] |
| Napier grass | 15.12 g.L−1 | 12.65 g.L−1 | 0.92 g.g−1 | Cel: 36.81%, Hem: 26.16%, Lig: 8.27% |
[77][76] | |
| Ryegrass silage | 25.5 | 24.1 | n/a | Cel: 34.3%, Hem: 29.6%, Lig: 8.6% |
Ddata based on fresh ryegrass before ensiling | [78][77] |
| Food waste | 42.46 ± 0.78 | 38.45 ± 1.87 | 53.02 ± 2.29% a | n/a | TKN: 2.10 ± 0.17% | [79][78] |
| Dried farmland grass | 83.6 ± 0.6 | 72.8 ± 1.1 | n/a | n/a | [80][79] | |
| OFMSW | 12 ± 1.4 | 10.7 ± 0.7 | 102.8 ± 13.0 | n/a | Mix of feed, inoculum, and tap water to a TS of 7–8 %ww. TKN: 3.12 ± 0.51 gN.kg−1ww |
[81][80] |
| OFMSW | 28.14 ± 4.01 | 25.98 ± 2.29 | 312.6 ± 120.8 | n/a | A mix of OFMSW and water was used as inoculum after it was acclimatized to 55 °C. TKN: 8.16 ± 1.83 gN.kg−1ww | [82][81] |
| Food waste | 28.19 ± 2.32 | 25.96 ± 2.08 | 376.4 ± 51.3 b, 28.6 ± 2.3 c |
Cel: 2.82 ± 0.95%, Hem: 32.58 ± 4.48% |
Lipids: 27.50 ± 1.45 %ww; Protein: 20.69 ± 1.17 %ww | [35][34] |
| Food waste | 17.8 | 17.1 | 320 gO2.L−1 b, 95 gO2.L−1 c |
n/a | Lipids: 0.59 %ww; Protein: 3.79 %ww | [83][82] |
| Food waste | 16.5 ± 0.2 | 15.5 ± 0.7 | 264 ± 27 gO2.L−1 b | n/a | Sludge inoculum acclimatized for 5 days at 37 °C. Inoculum was treated with BES to inhibit methanogenesis | [84][83] |
| Kitchen waste | 128.92 ± 2.33 g.L−1 | 115.91 ± 2.84 g.L−1 | n/a | n/a | [85][84] | |
| Inoculum: | ||||||
| Cow manure | 16.81 g.L−1 | 11.78 g.L−1 | 0.19 g.g−1 | Cel: 18.29%, Hem: 9.07%, Lig: 11.77% |
[77][76] | |
| Liquid digestate from the co-digestion of pig manure and grass silage | 2.30% | 1.60% | 0.58 gO2.L−1 c | n/a | Stored at 35 °C until CH4 production was minimal. | [78][77] |
| Cow manure | 18.22 ± 0.77 | 16.33 ± 0.76 | 50.76 ± 2.96% a | n/a | TKN: 1.40 ± 0.02% | [79][78] |
| Anaerobic digested food waste | 2.98 ± 0.00 | 2.67 ± 0.00 | 34.80 ± 0.98% a | n/a | TKN: 1.99 ± 0.03% | [79][78] |
| Anaerobic granular sludge | 9.01 ± 0.09 | 7.85 ± 0.04 | n/a | n/a | [35][34] | |
| Anaerobic digestion sludge | 0.4 ± 0.1 | 0.3 ± 0.1 | 10.5 ± 1.2 gO2.L−1 b, 5.1 ± 0.8 gO2.L−1,c |
n/a | [84][83] | |
| Anaerobic digestion sludge | 31.31 ± 0.49 g.L−1 | 19.67 ± 0.35 g.L−1 | n/a | n/a | Pre-treated with heat-shock at 70 °C for 30 min | [85][84] |
Acronyms: TS—total solids, vs.—volatile solids, COD—chemical oxygen demand, TOC—total organic carbon, TKN—total kjendahl nitrogen, Cel—cellulose, Hem—hemicellulose, Lig—lignin, BES—2-bromoethane sulfonic, and n/r—not reported. Notes: a—TOC, b—Total COD, and c—Soluble COD.
4.2. Multi-Stage System Design and Application
A multi-stage bioreactor is, broadly, any system with two or more bioreactors. This design facilitates the segregation of different microbial processes into separate reactors, allowing the environment of each reactor to be optimized for a specific functional microbiome. Such systems are capable of efficiently treating organic waste in terms of degradation yield and biogas production [86][85], and of producing valuable products such as VFA, lactic acid, alcohols, and medium-chain carboxylic acids (MCCAs) [87,88,89][86][87][88]. In a multi-stage system, hydrolysis and acidification stages occur in one reactor, while CE, PHA production, and methanogenesis occur in a separate reactor. In this way, the inhibition of the methanogens is avoided in the first reactor and different operating conditions can be used in each stage to maximize yields. This approach has been found to be more stable than single-stage systems in treating organic waste with high solid content [90,91][89][90]. The observed enhanced performance is reportedly due to the flexibility in process control offered by two-stage systems [8,92][8][91]. The number of multi-stage systems throughout Europe was expected to rise due to their ability to handle higher loading rates and improved process stability and flexibility. However, less than 10% of AD capacity in Europe are multi-stage systems [93,94][92][93]. This discrepancy is likely due to the complexity and cost of building and operating such systems. Nevertheless, the versatility and potential of multi-stage systems to improve process performance has encouraged ongoing research, especially within the biorefinery context. The viability of the multi-stage bioreactor systems was evaluated in a previous study in which one- and two-stage systems for the enzymatic hydrolysis of a municipal solid waste were compared using a techno-economic assessment (TEA) approach. The authors reported, on average, a 15–22% return on investment (ROI) and a 4–6 year payback period (PP) for two-stage systems, compared to 4–7% and 13–25 years for one-stage systems [95][94]. Regalado et al. (2022) pointed out that a multi-stage processing system, in which biogas is simultaneously recovered with other value-added products, offers a possible solution for achieving a more robust circular economy [96][95]. In addition, multi-stage systems allow for the treatment of large quantities of recalcitrant biomass which otherwise could not be treated with one-stage systems. This enhances the carbon-neutral energy output.References
- Badgujar, K.C.; Bhanage, B.M. Dedicated and Waste Feedstocks for Biorefinery: An Approach to Develop a Sustainable Society. In Waste Biorefinery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–38.
- Coma, M.; Martinez-Hernandez, E.; Abeln, F.; Raikova, S.; Donnelly, J.; Arnot, T.C.; Allen, M.J.; Hong, D.D.; Chuck, C.J. Organic Waste as a Sustainable Feedstock for Platform Chemicals. Faraday Discuss. 2017, 202, 175–195.
- Righetti, E.; Nortilli, S.; Fatone, F.; Frison, N.; Bolzonella, D. A Multiproduct Biorefinery Approach for the Production of Hydrogen, Methane and Volatile Fatty Acids from Agricultural Waste. Waste Biomass Valorization 2020, 11, 5239–5246.
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of Anaerobic Digestion for Volatile Fatty Acids, Hydrogen or Methane Production: A Critical Review. Bioengineered 2019, 10, 437–458.
- Lytras, G.; Lytras, C.; Mathioudakis, D.; Papadopoulou, K.; Lyberatos, G. Food Waste Valorization Based on Anaerobic Digestion. Waste Biomass Valorization 2021, 12, 1677–1697.
- Michalopoulos, I.; Lytras, G.M.; Mathioudakis, D.; Lytras, C.; Goumenos, A.; Zacharopoulos, I.; Papadopoulou, K.; Lyberatos, G. Hydrogen and Methane Production from Food Residue Biomass Product (FORBI). Waste Biomass Valorization 2020, 11, 1647–1655.
- Surendra, K.C.; Sawatdeenarunat, C.; Shrestha, S.; Sung, S.; Khanal, S.K. Anaerobic Digestion-Based Biorefinery for Bioenergy and Biobased Products. Ind. Biotechnol. 2015, 11, 103–112.
- Demirer, G.N.; Chen, S. Two-Phase Anaerobic Digestion of Unscreened Dairy Manure. Process Biochem. 2005, 40, 3542–3549.
- Farzad, S.; Mandegari, M.A.; Görgens, J.F. A Critical Review on Biomass Gasification, Co-Gasification, and Their Environmental Assessments. Biofuel Res. J. 2016, 3, 483–495.
- Bonilla, J.; Gordillo, G.; Cantor, C. Experimental Gasification of Coffee Husk Using Pure Oxygen-Steam Blends. Front. Energy Res. 2019, 7, 127.
- Guo, Y.; Wang, S.; Huelsman, C.M.; Savage, P.E. Products, Pathways, and Kinetics for Reactions of Indole under Supercritical Water Gasification Conditions. J. Supercrit. Fluids 2013, 73, 161–170.
- Heidenreich, S.; Foscolo, P.U. New Concepts in Biomass Gasification. Prog. Energy Combust. Sci. 2015, 46, 72–95.
- Kalinci, Y.; Hepbasli, A.; Dincer, I. Exergoeconomic Analysis and Performance Assessment of Hydrogen and Power Production Using Different Gasification Systems. Fuel 2012, 102, 187–198.
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An Overview of Advances in Biomass Gasification. Energy Environ. Sci. 2016, 9, 2939–2977.
- Lee, C.S.; Conradie, A.V.; Lester, E. Review of Supercritical Water Gasification with Lignocellulosic Real Biomass as the Feedstocks: Process Parameters, Biomass Composition, Catalyst Development, Reactor Design and Its Challenges. Chem. Eng. J. 2021, 415, 128837.
- Hu, X.; Gholizadeh, M. Biomass Pyrolysis: A Review of the Process Development and Challenges from Initial Researches up to the Commercialisation Stage. J. Energy Chem. 2019, 39, 109–143.
- Latif, H.; Zeidan, A.A.; Nielsen, A.T.; Zengler, K. Trash to Treasure: Production of Biofuels and Commodity Chemicals via Syngas Fermenting Microorganisms. Curr. Opin. Biotechnol. 2014, 27, 79–87.
- Gemechu, E.D.; Kumar, A. Chapter 12—The Environmental Performance of Hydrogen Production Pathways Based on Renewable Sources; Ren, J.B.T.-R.-E.-D.F., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 375–406. ISBN 978-0-12-820539-6.
- Casademont, P.; García-Jarana, M.B.; Sánchez-Oneto, J.; Portela, J.R.; Martínez de la Ossa, E.J. Supercritical Water Gasification: A Patents Review. Rev. Chem. Eng. 2017, 33, 237–261.
- De Blasio, C.; Järvinen, M. Supercritical Water Gasification of Biomass; Elsevier: Oxford, UK, 2017; pp. 171–195. ISBN 978-0-12-804792-7.
- Sevillano, C.A.; Pesantes, A.A.; Peña Carpio, E.; Martínez, E.J.; Gómez, X. Anaerobic Digestion for Producing Renewable Energy—The Evolution of This Technology in a New Uncertain Scenario. Entropy 2021, 23, 145.
- Cesaro, A. The Valorization of the Anaerobic Digestate from the Organic Fractions of Municipal Solid Waste: Challenges and Perspectives. J. Environ. Manag. 2021, 280, 111742.
- EBA European Biogas Association. EBA European Biogas Association Annual Report; EBA European Biogas Association: Brussels, Belgium, 2020; p. 38.
- Cheah, Y.-K.; Vidal-Antich, C.; Dosta, J.; Mata-Álvarez, J. Volatile Fatty Acid Production from Mesophilic Acidogenic Fermentation of Organic Fraction of Municipal Solid Waste and Food Waste under Acidic and Alkaline PH. Environ. Sci. Pollut. Res. 2019, 26, 35509–35522.
- Menzel, T.; Neubauer, P.; Junne, S. Role of Microbial Hydrolysis in Anaerobic Digestion. Energies 2020, 13, 5555.
- FAO. Food Loss and Food Waste: Causes and Solutions; Food and Agriculture Organization: Rome, Italy, 2011; ISBN 9781788975391.
- FAO. The State of Food and Agriculture 2019: Moving forward on Food Loss and Waste Reduction; Food and Agriculture Organization: Rome, Italy, 2019; ISBN 9781315764788.
- Matharu, A.S.; de Melo, E.M.; Houghton, J.A. Opportunity for High Value-Added Chemicals from Food Supply Chain Wastes. Bioresour. Technol. 2016, 215, 123–130.
- Morales-Polo, C.; Cledera-Castro, M.D.M.; Moratilla Soria, B.Y. Reviewing the Anaerobic Digestion of Food Waste: From Waste Generation and Anaerobic Process to Its Perspectives. Appl. Sci. 2018, 8, 1804.
- Fernández, A.; Sanchez, A.; Font, X. Anaerobic Co-Digestion of a Simulated Organic Fraction of Municipal Solid Wastes and Fats of Animal and Vegetable Origin. Biochem. Eng. J. 2005, 26, 22–28.
- Girault, R.; Bridoux, G.; Nauleau, F.; Poullain, C.; Buffet, J.; Peu, P.; Sadowski, A.G.; Béline, F. Anaerobic Co-Digestion of Waste Activated Sludge and Greasy Sludge from Flotation Process: Batch versus CSTR Experiments to Investigate Optimal Design. Bioresour. Technol. 2012, 105, 1–8.
- Neves, L.; Goncalo, E.; Oliveira, R.; Alves, M.M. Influence of Composition on the Biomethanation Potential of Restaurant Waste at Mesophilic Temperatures. Waste Manag. 2008, 28, 965–972.
- Fisgativa, H.; Tremier, A.; Dabert, P. Characterizing the Variability of Food Waste Quality: A Need for Efficient Valorisation through Anaerobic Digestion. Waste Manag. 2016, 50, 264–274.
- Nzeteu, C.O.; Trego, A.C.; Abram, F.; O’Flaherty, V. Reproducible, High-Yielding, Biological Caproate Production from Food Waste Using a Single-Phase Anaerobic Reactor System. Biotechnol. Biofuels 2018, 11, 108.
- Ma, J.; Frear, C.; Wang, Z.; Yu, L.; Zhao, Q.; Li, X.; Chen, S. A Simple Methodology for Rate-Limiting Step Determination for Anaerobic Digestion of Complex Substrates and Effect of Microbial Community Ratio. Bioresour. Technol. 2013, 134, 391–395.
- Fang, Q.; Ji, S.; Huang, D.; Huang, Z.; Huang, Z.; Zeng, Y.; Liu, Y. Impact of Alkaline Pretreatment to Enhance Volatile Fatty Acids (VFAs) Production from Rice Husk. Biochem. Res. Int. 2019, 2019, 8489747.
- Zhang, D.; Jiang, H.; Chang, J.; Sun, J.; Tu, W.; Wang, H. Effect of Thermal Hydrolysis Pretreatment on Volatile Fatty Acids Production in Sludge Acidification and Subsequent Polyhydroxyalkanoates Production. Bioresour. Technol. 2019, 279, 92–100.
- Kim, J.K.; Oh, B.R.; Chun, Y.N.; Kim, S.W. Effects of Temperature and Hydraulic Retention Time on Anaerobic Digestion of Food Waste. J. Biosci. Bioeng. 2006, 102, 328–332.
- Paudel, S.R.; Banjara, S.P.; Choi, O.K.; Park, K.Y.; Kim, Y.M.; Lee, J.W. Pretreatment of Agricultural Biomass for Anaerobic Digestion: Current State and Challenges. Bioresour. Technol. 2017, 245, 1194–1205.
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Rajendran, K.; Pugazhendhi, A.; Rao, C.V.; Atabani, A.E.; Kumar, G.; Yang, Y.-H. Renewable Biohydrogen Production from Lignocellulosic Biomass Using Fermentation and Integration of Systems with Other Energy Generation Technologies. Sci. Total Environ. 2020, 765, 144429.
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874.
- Galbe, M.; Wallberg, O. Pretreatment for Biorefineries: A Review of Common Methods for Efficient Utilisation of Lignocellulosic Materials. Biotechnol. Biofuels 2019, 12, 294.
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141.
- Sun, J.; Zhang, L.; Loh, K.-C. Review and Perspectives of Enhanced Volatile Fatty Acids Production from Acidogenic Fermentation of Lignocellulosic Biomass Wastes. Bioresour. Bioprocess. 2021, 8, 68.
- Cerrone, F.; Choudhari, S.K.; Davis, R.; Cysneiros, D.; O’Flaherty, V.; Duane, G.; Casey, E.; Guzik, M.W.; Kenny, S.T.; Babu, R.P.; et al. Medium Chain Length Polyhydroxyalkanoate (Mcl-PHA) Production from Volatile Fatty Acids Derived from the Anaerobic Digestion of Grass. Appl. Microbiol. Biotechnol. 2014, 98, 611–620.
- Cysneiros, D.; Thuillier, A.; Villemont, R.; Littlestone, A.; Mahony, T.; O’Flaherty, V. Temperature Effects on the Trophic Stages of Perennial Rye Grass Anaerobic Digestion. Water Sci. Technol. 2011, 64, 70–76.
- Joyce, A.; Ijaz, U.Z.; Nzeteu, C.; Vaughan, A.; Shirran, S.L.; Botting, C.H.; Quince, C.; O’Flaherty, V.; Abram, F. Linking Microbial Community Structure and Function during the Acidified Anaerobic Digestion of Grass. Front. Microbiol. 2018, 9, 540.
- Tilman, D.; Hill, J.; Lehman, C. Carbon-Negative Biofuels from Low-Input High-Diversity Grassland Biomass. Science 2006, 314, 1598–1600.
- Murphy, J.; Korres, N.; Singh, A.; Smyth, B.; Nizami, A.S.; Thamsiriroj, T. The Potential for Grass Biomethane as a Biofuel Compressed Biomethane Generated from Grass, Utilised as A Transport Biofuel CCRP Report; Environmental Protection Agency: Wexford, Ireland, 2013; ISBN 9781840954272.
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage Review: Interpretation of Chemical, Microbial, and Organoleptic Components of Silages; Elsevier: Amsterdam, The Netherlands, 2018; Volume 101.
- Grange, G.; Finn, J.A.; Brophy, C. Plant Diversity Enhanced Yield and Mitigated Drought Impacts in Intensively Managed Grassland Communities. J. Appl. Ecol. 2021, 58, 1864–1875.
- Cong, W.-F.; Moset, V.; Feng, L.; Møller, H.B.; Eriksen, J. Anaerobic Co-Digestion of Grass and Forbs—Influence of Cattle Manure or Grass Based Inoculum. Biomass Bioenergy 2018, 119, 90–96.
- Díaz-Vázquez, D.; Alvarado-Cummings, S.C.; Meza-Rodríguez, D.; Senés-Guerrero, C.; de Anda, J.; Gradilla-Hernández, M.S. Evaluation of Biogas Potential from Livestock Manures and Multicriteria Site Selection for Centralized Anaerobic Digester Systems: The Case of Jalisco, México. Sustainability 2020, 12, 3527.
- Nolan, S.; Thorn, C.E.; Ashekuzzaman, S.M.; Kavanagh, I.; Nag, R.; Bolton, D.; Cummins, E.; O’Flaherty, V.; Abram, F.; Richards, K.; et al. Landspreading with Co-Digested Cattle Slurry, with or without Pasteurisation, as a Mitigation Strategy against Pathogen, Nutrient and Metal Contamination Associated with Untreated Slurry. Sci. Total Environ. 2020, 744, 140841.
- Wolter, M.; Prayitno, S.; Schuchardt, F. Greenhouse Gas Emission during Storage of Pig Manure on a Pilot Scale. Bioresour. Technol. 2004, 95, 235–244.
- Clemens, J.; Trimborn, M.; Weiland, P.; Amon, B. Mitigation of Greenhouse Gas Emissions by Anaerobic Digestion of Cattle Slurry. Agric. Ecosyst. Environ. 2006, 112, 171–177.
- Kougias, P.G.; Kotsopoulos, T.A.; Martzopoulos, G.G. Effect of Feedstock Composition and Organic Loading Rate during the Mesophilic Co-Digestion of Olive Mill Wastewater and Swine Manure. Renew. Energy 2014, 69, 202–207.
- Orive, M.; Cebrián, M.; Zufía, J. Techno-Economic Anaerobic Co-Digestion Feasibility Study for Two-Phase Olive Oil Mill Pomace and Pig Slurry. Renew. Energy 2016, 97, 532–540.
- Tampio, E.A.; Blasco, L.; Vainio, M.M.; Kahala, M.M.; Rasi, S.E. Volatile Fatty Acids (VFAs) and Methane from Food Waste and Cow Slurry: Comparison of Biogas and VFA Fermentation Processes. GCB Bioenergy 2019, 11, 72–84.
- Yin, D.; Mahboubi, A.; Wainaina, S.; Qiao, W.; Taherzadeh, M.J. The Effect of Mono-and Multiple Fermentation Parameters on Volatile Fatty Acids (VFAs) Production from Chicken Manure via Anaerobic Digestion. Bioresour. Technol. 2021, 330, 124992.
- Moran, S. An Applied Guide to Process and Plant Design; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 0128148616.
- Van, D.P.; Fujiwara, T.; Leu Tho, B.; Song Toan, P.P.; Hoang Minh, G. A Review of Anaerobic Digestion Systems for Biodegradable Waste: Configurations, Operating Parameters, and Current Trends. Environ. Eng. Res. 2020, 25, 1–17.
- Pastor-Poquet, V.; Papirio, S.; Steyer, J.-P.; Trably, E.; Escudié, R.; Esposito, G. High-Solids Anaerobic Digestion Model for Homogenized Reactors. Water Res. 2018, 142, 501–511.
- Kariyama, I.D.; Zhai, X.; Wu, B. Influence of Mixing on Anaerobic Digestion Efficiency in Stirred Tank Digesters: A Review. Water Res. 2018, 143, 503–517.
- Lindmark, J.; Thorin, E.; Fdhila, R.B.; Dahlquist, E. Effects of Mixing on the Result of Anaerobic Digestion. Renew. Sustain. Energy Rev. 2014, 40, 1030–1047.
- Karthikeyan, O.P.; Selvam, A.; Wong, J.W.C. Hydrolysis–Acidogenesis of Food Waste in Solid–Liquid-Separating Continuous Stirred Tank Reactor (SLS-CSTR) for Volatile Organic Acid Production. Bioresour. Technol. 2016, 200, 366–373.
- Doğan, E.; Demirer, G.N. Volatile Fatty Acid Production from Organic Fraction of Municipal Solid Waste through Anaerobic Acidogenic Digestion. Environ. Eng. Sci. 2009, 26, 1443–1450.
- Pommier, S.; Chenu, D.; Quintard, M.; Lefebvre, X. A Logistic Model for the Prediction of the Influence of Water on the Solid Waste Methanization in Landfills. Biotechnol. Bioeng. 2007, 97, 473–482.
- Sanphoti, N.; Towprayoon, S.; Chaiprasert, P.; Nopharatana, A. The Effects of Leachate Recirculation with Supplemental Water Addition on Methane Production and Waste Decomposition in a Simulated Tropical Landfill. J. Environ. Manag. 2006, 81, 27–35.
- Degueurce, A.; Trémier, A.; Peu, P. Dynamic Effect of Leachate Recirculation on Batch Mode Solid State Anaerobic Digestion: Influence of Recirculated Volume, Leachate to Substrate Ratio and Recirculation Periodicity. Bioresour. Technol. 2016, 216, 553–561.
- Cadavid-Rodríguez, L.S.; Horan, N.J. Production of Volatile Fatty Acids from Wastewater Screenings Using a Leach-Bed Reactor. Water Res. 2014, 60, 242–249.
- Dahiya, S.; Sarkar, O.; Swamy, Y.V.; Mohan, S.V. Acidogenic Fermentation of Food Waste for Volatile Fatty Acid Production with Co-Generation of Biohydrogen. Bioresour. Technol. 2015, 182, 103–113.
- Hussain, A.; Filiatrault, M.; Guiot, S.R. Acidogenic Digestion of Food Waste in a Thermophilic Leach Bed Reactor: Effect of PH and Leachate Recirculation Rate on Hydrolysis and Volatile Fatty Acid Production. Bioresour. Technol. 2017, 245, 1–9.
- Arslan, D.; Zhang, Y.; Steinbusch, K.J.J.; Diels, L.; Hamelers, H.V.M.; Buisman, C.J.N.; De Wever, H. In-Situ Carboxylate Recovery and Simultaneous PH Control with Tailor-Configured Bipolar Membrane Electrodialysis during Continuous Mixed Culture Fermentation. Sep. Purif. Technol. 2017, 175, 27–35.
- Roume, H.; Arends, J.; Ameril, C.P.; Patil, S.A.; Rabaey, K. Enhanced Product Recovery from Glycerol Fermentation into 3-Carbon Compounds in a Bioelectrochemical System Combined with in Situ Extraction. Front. Bioeng. Biotechnol. 2016, 4, 73.
- Kullavanijaya, P.; Chavalparit, O. The Production of Volatile Fatty Acids from Napier Grass via an Anaerobic Leach Bed Process: The Influence of Leachate Dilution, Inoculum, Recirculation, and Buffering Agent Addition. J. Environ. Chem. Eng. 2019, 7, 103458.
- Xie, S.; Lawlor, P.G.; Frost, J.P.; Wu, G.; Zhan, X. Hydrolysis and Acidification of Grass Silage in Leaching Bed Reactors. Bioresour. Technol. 2012, 114, 406–413.
- Yan, B.H.; Selvam, A.; Wong, J.W.C. Application of Rumen Microbes to Enhance Food Waste Hydrolysis in Acidogenic Leach-Bed Reactors. Bioresour. Technol. 2014, 168, 64–71.
- Khor, W.C.; Andersen, S.; Vervaeren, H.; Rabaey, K. Electricity-Assisted Production of Caproic Acid from Grass. Biotechnol. Biofuels 2017, 10, 180.
- Valentino, F.; Munarin, G.; Biasiolo, M.; Cavinato, C.; Bolzonella, D.; Pavan, P. Enhancing Volatile Fatty Acids (VFA) Production from Food Waste in a Two-Phases Pilot-Scale Anaerobic Digestion Process. J. Environ. Chem. Eng. 2021, 9, 106062.
- Gottardo, M.; Micolucci, F.; Bolzonella, D.; Uellendahl, H.; Pavan, P. Pilot Scale Fermentation Coupled with Anaerobic Digestion of Food Waste—Effect of Dynamic Digestate Recirculation. Renew. Energy 2017, 114, 455–463.
- Lukitawesa; Patinvoh, R.J.; Millati, R.; Sárvári-Horváth, I.; Taherzadeh, M.J. Factors Influencing Volatile Fatty Acids Production from Food Wastes via Anaerobic Digestion. Bioengineered 2020, 11, 39–52.
- Xiong, Z.; Hussain, A.; Lee, J.; Lee, H.-S. Food Waste Fermentation in a Leach Bed Reactor: Reactor Performance, and Microbial Ecology and Dynamics. Bioresour. Technol. 2019, 274, 153–161.
- Swiatkiewicz, J.; Slezak, R.; Krzystek, L.; Ledakowicz, S. Production of Volatile Fatty Acids in a Semi-Continuous Dark Fermentation of Kitchen Waste: Impact of Organic Loading Rate and Hydraulic Retention Time. Energies 2021, 14, 2993.
- Pohland, F.G.; Ghosh, S. Developments in Anaerobic Stabilization of Organic Wastes-the Two-Phase Concept. Environ. Lett. 1971, 1, 255–266.
- Grootscholten, T.I.M.; Strik, D.; Steinbusch, K.J.J.; Buisman, C.J.N.; Hamelers, H.V.M. Two-Stage Medium Chain Fatty Acid (MCFA) Production from Municipal Solid Waste and Ethanol. Appl. Energy 2014, 116, 223–229.
- Kannengiesser, J.; Sakaguchi-Söder, K.; Mrukwia, T.; Jager, J.; Schebek, L. Extraction of Medium Chain Fatty Acids from Organic Municipal Waste and Subsequent Production of Bio-Based Fuels. Waste Manag. 2016, 47, 78–83.
- Xu, J.; Hao, J.; Guzman, J.J.L.; Spirito, C.M.; Harroff, L.A.; Angenent, L.T. Temperature-Phased Conversion of Acid Whey Waste into Medium-Chain Carboxylic Acids Via Lactic Acid: No External e-Donor. Joule 2019, 3, 885–888.
- Fezzani, B.; Cheikh, R. Ben Two-Phase Anaerobic Co-Digestion of Olive Mill Wastes in Semi-Continuous Digesters at Mesophilic Temperature. Bioresour. Technol. 2010, 101, 1628–1634.
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The Anaerobic Digestion of Solid Organic Waste. Waste Manag. 2011, 31, 1737–1744.
- Cho, J.K.; Park, S.C.; Chang, H.N. Biochemical Methane Potential and Solid State Anaerobic Digestion of Korean Food Wastes. Bioresour. Technol. 1995, 52, 245–253.
- De Baere, L.; Mattheeuws, B. Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste in Europe-Status, Experience and Prospects. In Proceedings of the ISTANBUL3WCONGRESS 2013, Istanbul, Turkey, 22–24 May 2013; Volume 38.
- Rapport, J.; Zhang, R.; Jenkins, B.M.; Williams, R.B. Current Anaerobic Digestion Technologies Used for Treatment of Municipal Organic Solid Waste; California Integrated Waste Management Board: Sacramento, CA, USA, 2008; Volume 236.
- Climent Barba, F.; Grasham, O.; Puri, D.J.; Blacker, A.J. A Simple Techno-Economic Assessment for Scaling-Up the Enzymatic Hydrolysis of MSW Pulp. Front. Energy Res. 2022, 10.
- Hernández Regalado, R.E.; Häner, J.; Brügging, E.; Tränckner, J. Techno-Economic Assessment of Solid–Liquid Biogas Treatment Plants for the Agro-Industrial Sector. Energies 2022, 15, 4413.
More
