Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Vicky Zhou and Version 2 by Vicky Zhou.
Metal-ion batteries are systems for electrochemical energy conversion and storage with only one kind of ion shuttling between the negative and the positive electrode during discharge and charge. This concept also known as rocking-chair battery has been made highly popular with the lithium-ion battery as its most popular example. The principle can also be applied with other cations both mono- and multivalent. This might have implications and advantages in terms of increased safety, lower expenses, and utilizing materials, in particular metals, not being subject to resource limitations.
- metal-ion battery
- rocking-chair battery
- secondary battery
- accumulator
In a secondary battery commonly employed for electrochemical energy conversion and storage commonly two electrodes and an electrolyte (solution) are employed with electrode reactions proceeding at both electrodes with more or less significant changes in the composition of the electrolyte (solution). Frequently constituents of the electrolyte solution are consumed or new ones are generated. The associated changes of properties of the electrolyte (solution) are mostly unwelcome because they might e.g., result in reduced ionic conductivity and increased internal resistance of the device. In addition, these changes require a certain minimum amount of electrolyte (solution) for the electrode reactions to proceed. These disadvantages are absent with the metal-ion battery. Because all known examples work with a common cation An+ the electrode reactions can be written for the negative electrode during discharge as
A → An+ + en−- (1)
and for the positive electrode during discharge assuming a host material B capable of accommodating A or An+ with associated valency changes in
B + An+ + en−- → BA (2)
Assuming a metal electrode as the negative one the principle is illustrated in Figure 1. For various reasons addressed for specific materials below a metal may not be suitable, instead a host material H is also employed as a negative electrode. This results in a modified electrode reaction of the negative electrode
H(A) → H + A+ + en− - (3)
illustrated in Figure 2.
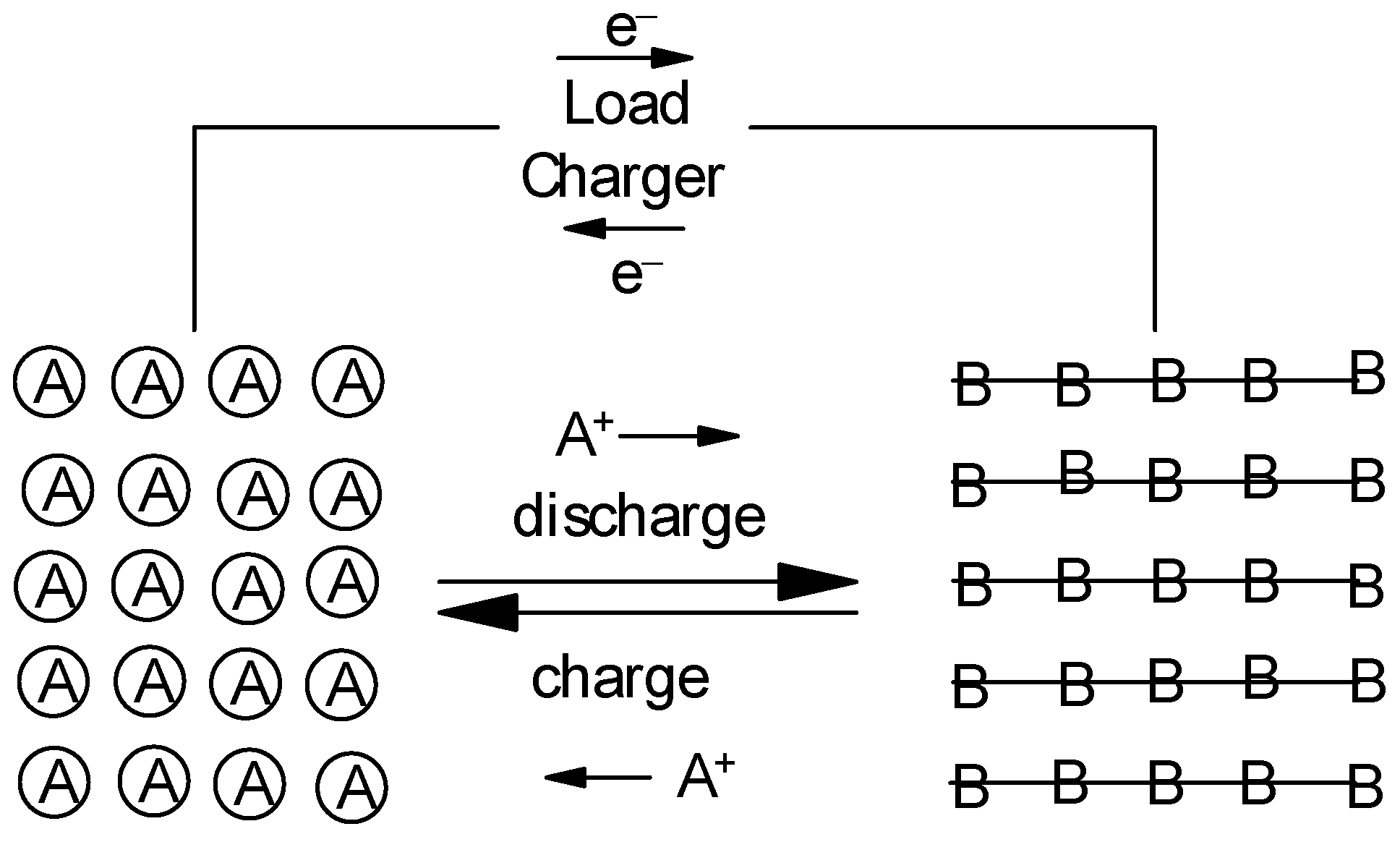
Figure 1. Principle of a rocking-chair battery, a positive intercalation-type electrode is assumed.
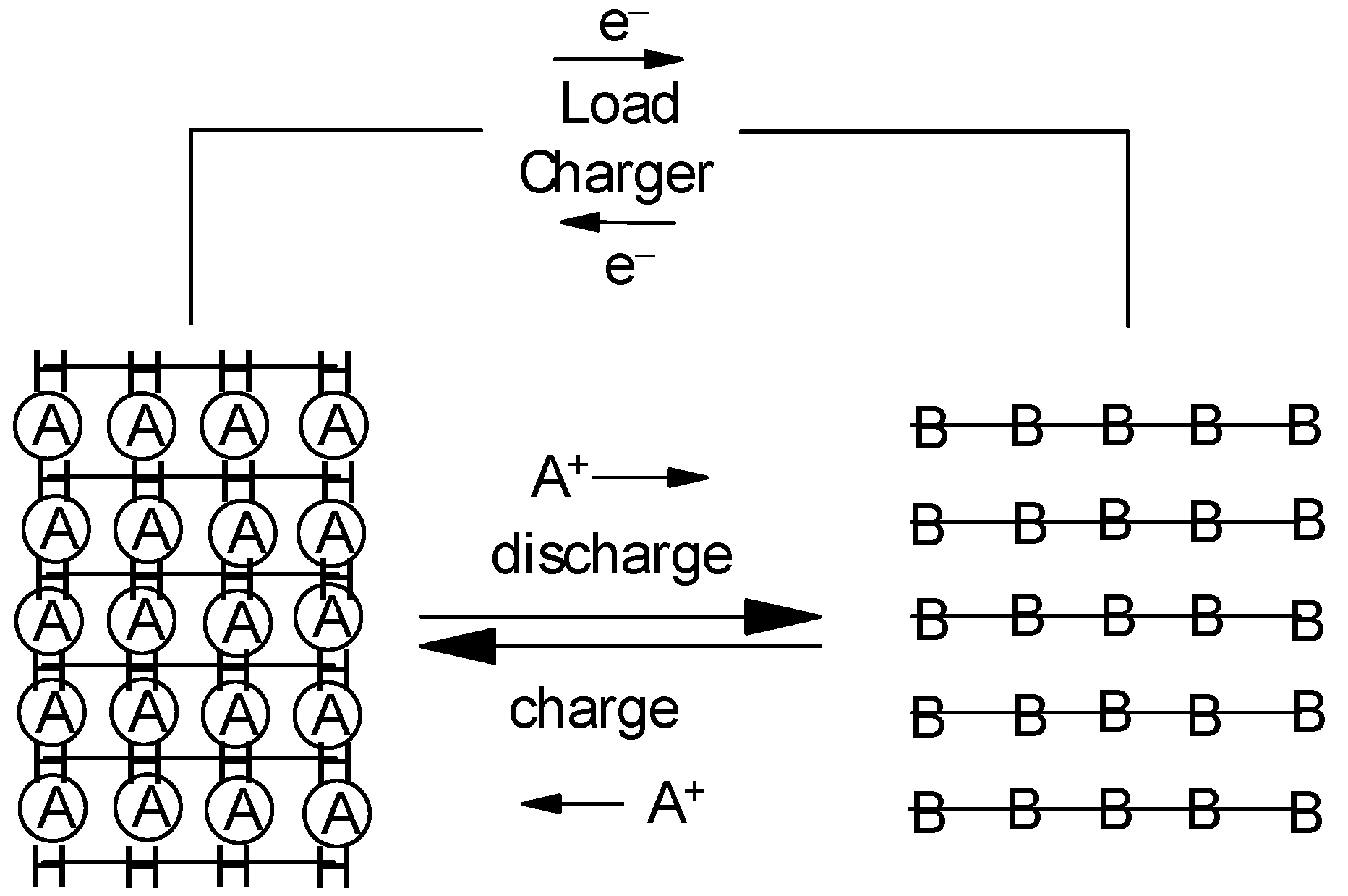
Figure 2. Principle of a rocking-chair battery, both negative (H) and positive (B) intercalation-type electrodes are assumed.
