Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Rita Xu and Version 4 by Alberto Ortiz.
Chronic kidney disease (CKD) is projected to become the fifth global cause of death by 2040 as a result of key shortcomings in the current methods available to diagnose and treat kidney diseases. In this regard, the novel holobiont concept, used to describe an individual host and its microbial community, may pave the way towards a better understanding of kidney disease pathogenesis and progression. Microbiota-modulating or -derived interventions include probiotics, prebiotics, synbiotics and postbiotics.
- chronic kidney disease
- postbiotics
- prebiotics
- probiotics
- kidney
- hypertension
- acute kidney injury
- microbiota
- hyperoxaluria
1. The Global Burden of Kidney Disease
Chronic kidney disease (CKD) is to date defined by the measurement of the estimated glomerular filtration rate (eGFR, i.e., a measure of kidney function) and albuminuria (i.e., a measure of kidney injury), which are associated with a combined risk of premature all-cause and cardiovascular death, CKD progression and acute kidney injury (AKI) [1]. Despite recent advances in the field, CKD is estimated to become the fifth global cause of death by 2040 and the second cause of death by the end of this century in countries with a long-life expectancy [2]. These projections illustrate key shortcomings in the current methods used to diagnose and treat kidney diseases. As such, an improved understanding of the underlying pathogenesis of CKD may help researchers identifying novel diagnostic clues and therapeutic targets. In this regard, the holobiont concept, a term used to describe an individual host and its microbial community, including viruses and cellular microorganisms [3], may pave the way to a better understanding of the pathogenesis of CKD. There is indeed increasing evidence suggesting that health and disease may be the result of a bilateral interaction between a host and its microbiota, particularly the gut microbiota. Such interaction may be affected by several factors: disease condition in the host, diet, drugs and antibiotics [4]. Attempts to modulate the host-microbiota interactions have resulted in the design and prescription of prebiotics, probiotics, synbiotics and postbiotics.
2. The Gut Microbiota: A Key Modifier of Kidney Disease and Health
In recent years, a key role of the gut microbiota as a homeostasis modulator, influencing both health and disease, has emerged in the context of CKD [5][6]. Indeed, there is a close connection between intestinal dysbiosis, hypervolemia, systemic inflammation, myocardial stunning and the malnutrition-inflammation syndrome in CKD populations [7]. Kidney disease may influence the gut microbiota through several mechanisms, ranging from the influence of the uremic milieu to the impact of prescribed diets (e.g., low potassium diets are often low in dietary fiber), as well as the frequent use of antibiotics and polypharmacy which may adversely modulate the gut microbiota [8][9][10]. Conversely, an altered gut microbiota may contribute to CKD development and progression by mediating increased inflammation and/or generating uremic toxins and their precursors [11]. The increase in intestinal permeability resulting from the degradation of the barrier integrity, as a consequence of an altered microbiota, allows for the transition of endotoxins and bacterial products to the blood. This potentially leads to an increase in oxidative stress and inflammation, contributing to CKD complications, such as cardiovascular disease and mineral metabolism disorders. The gut microbiota may also generate indoles, amines and phenols from dietary components, mainly proteins. These gut-derived metabolites can be metabolized further into the uremic toxins p-cresyl sulphate (pCS), indoxyl sulphate (IS) and trimethylamine N-Oxide (TMAO) [8][10][12]. The presence of uremic toxins in the circulation may lead to proinflammatory events, promoting the expression of transforming factor-β (TGF-β1) and the production of reactive oxygen species (ROS) in kidney tubular epithelial cells [13]. IS and pCS also inhibit Klotho expression via gene hypermethylation [14]. Klotho has antiaging and nephroprotective effect. All these factors can lead to an acceleration of CKD progression and of its associated cardiovascular disorders. The recognition of the interplay between the kidneys and the microbiota has led to increased efforts in the development of novel therapeutic approaches focused on targeting the microbiota.3. Prebiotics, Probiotics, Synbiotics and Postbiotics
Diet has a large impact on the composition of the microbiota as both humans and their gut microbiota use dietary components as nutrients. As a result, microbiota-modulating or -derived interventions have emerged, including probiotics, prebiotics, synbiotics and postbiotics, that could be available as drugs, diet or food supplements [15][16] (Figure 1). The concepts of probiotics, prebiotics and synbiotics have all been well described in the scientific literature.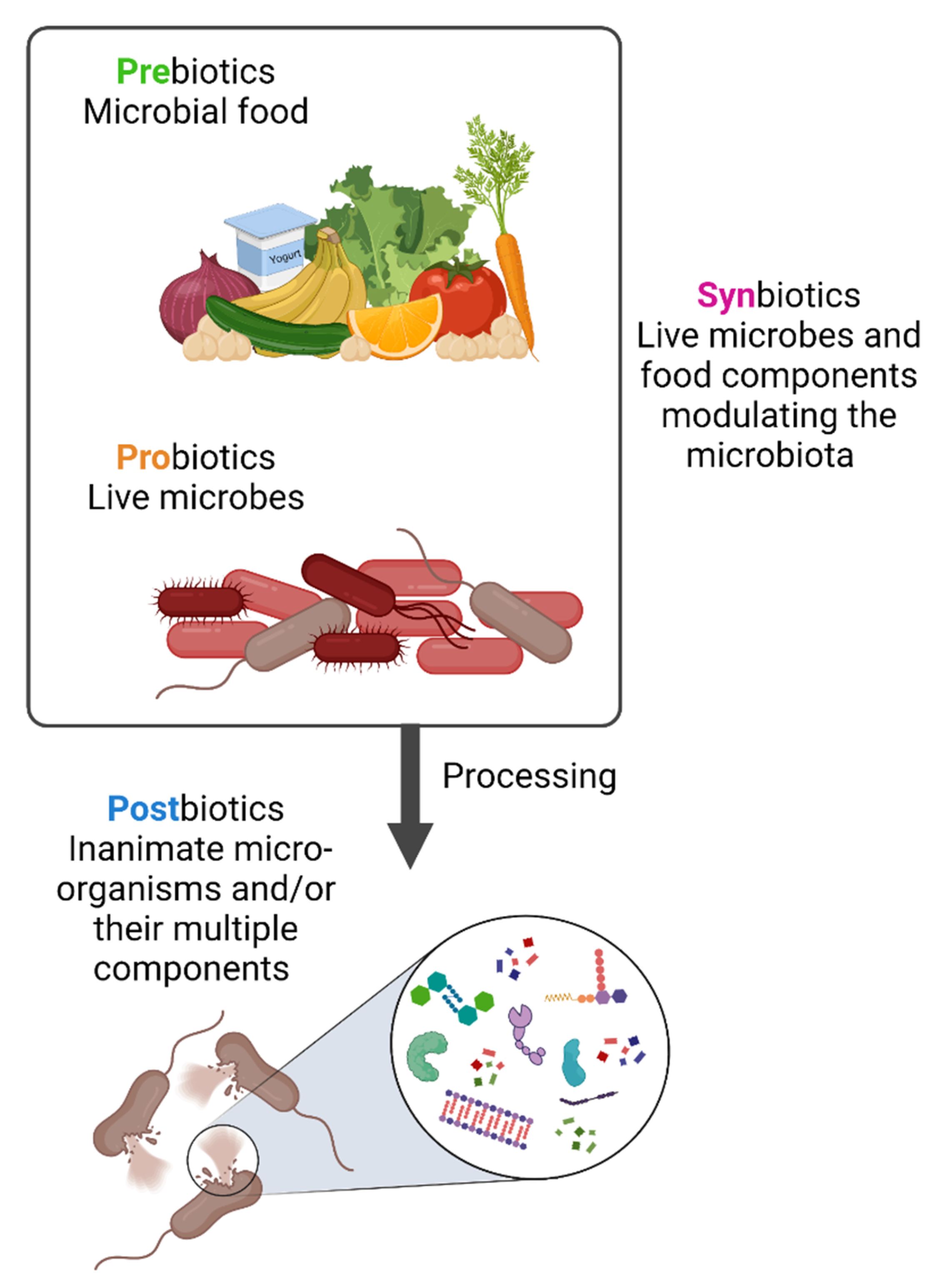
Figure 1. Prebiotics, probiotics, synbiotics and postbiotics. Prebiotics are molecules that can be metabolized by the microbiota while probiotics are live strains microorganisms, i.e., individual live components of the microbiota. When prebiotics and probiotics are used in combination these are named synbiotics. In contrast, postbiotics are the result of specific bacterial inactivation procedures. Figure created with Biorender.com (license number 9E1540F2).
4. The 2019 Concept of Postbiotic: What Is and What Is Not a Postbiotic
Although probiotics are live microorganisms, by the end of their shelf life they might get injured and die. Little attention has been paid to the contribution of these dead microorganisms and of the products of fermentation with regards to any biological impact of probiotics [20]. In 2019, the International Scientific Association of Probiotics and Prebiotics (ISAPP) defined the concept of postbiotic from a scientific, commercial, and regulatory point of view as a “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host” [20]. “Preparation” indicates that it should have a specific formulation achieved through certain inactivation methods to confer any beneficial effects. “Inanimate” refers to the microorganisms that were alive and once they have been killed, they retain their beneficial effect. The word “components” focuses on the beneficial effect that might be played by microbial components, such as cell wall components and pili. While microbial metabolites might be present in postbiotic preparations and might play an essential role, the definition does not include the purified metabolites lacking cellular biomass. However, until 2019, the literature has frequently used the term incorrectly by referring to vaccines and purified metabolites, such as SCFAs, proteins and peptides as postbiotics [21][22][23]. This potentially renders obsolete any older literature using the term postbiotic and calls for an urgent reset. The preparation of a postbiotic should follow defined criteria: firstly, the composition of the microorganism must be characterized prior to inactivation, for example through genome sequencing. Secondly, the process of inactivation should be described and verified. Thirdly, the postbiotic preparation must be reported [20]. The main advantages of postbiotics compared to probiotics lies in their stability. Their preparation and composition render them extremely stable for several years at room temperature, thus making them particularly suitable for those areas where the maintenance of the cold chain for transport and storage are still a limitation. In addition, they may have a better safety profile compared to probiotics as they cannot replicate and cause bacteremia or fungaemia. Nevertheless, safety should still be assessed for any kind of postbiotic [20]. The possible mechanisms of actions of postbiotics include the modulation of resident microbiota, enhancement of epithelial barrier function, modulation of systemic or local immune responses, modulation of systemic metabolic responses and system signaling via the nervous system [20] (Figure 2).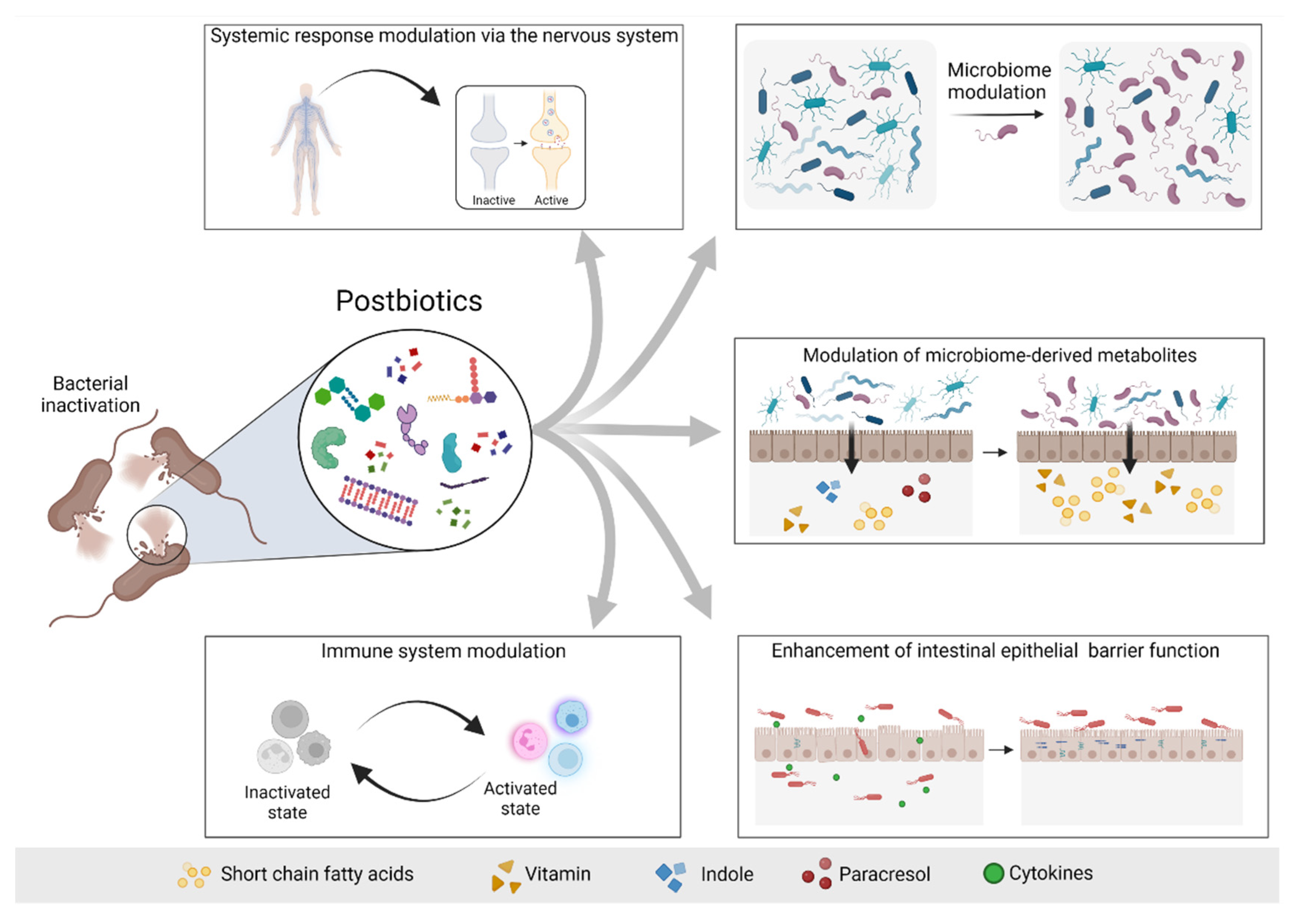
Figure 2. Possible mechanisms of actions of postbiotics. Postbiotics mediate their beneficial effects to the host via different mechanisms, including modulation of the systemic response via the nervous system (e.g., GABA), modulation of the microbiome and consequently modulation of the microbiome-derived metabolites, enhancement of the intestinal epithelial barrier and modulation of the immune system response. Based on [20]. Figure created with Biorender.com (license number 9E1540F2).
5. Postbiotics in Kidney Disease
There are no human studies conducted so far that investigated the use of postbiotics in kidney disease. However, a PubMed search performed in May 2022 identified several preclinical studies that examined the role and function of postbiotics in kidney-related diseases in animal models. Researchers also found manuscripts published between 2020–2022 that used the term “postbiotic” to refer to compounds that would not be considered postbiotics according to the 2019 consensus definition [20]. In this regard, the short-chain fatty acid (SCFA) butyric acid and its derivative N-[2-(2-Butyrylamino-ethoxy)-ethyl]-butyramide (BA-NH-NH-BA) are produced by Cutibacterium acnes and reported to solubilize calcium phosphate [24]. A study that applied BA-NH-NH-BA topically in a murine model of uremic itching, considered this compound as a postbiotic [24]. However, this does not comply with the novel definition proposed by the ISAAP panel since a purified microbial metabolite itself cannot be considered a postbiotic [24].
Several studies on postbiotics and kidney disease were not very informative as they studied healthy animals or were too preliminary and did not address in vivo and functional consequences following administration. In aged or adult mice, treatment with probiotics or probiotics and postbiotics mix (Lactobacillus and Bifidobacterium strains and their postbiotics compounds selected for potential antioxidative activity) decreased oxidative stress as assessed by MDA (malondialdehyde) in the kidneys [25]. However, an impact on kidney function was not assessed and whether or not the combination of postbiotics with probiotics added up to the impact of probiotics alone was not formally assessed, although a trend towards a greater impact was observed in the higher dose groups.
Fifteen weeks of a diet supplemented by a postbiotic based on lactic acid bacteria in healthy male rabbits was not associated with differences in kidney function parameters, including serum urea and creatinine [26].
The postbiotic OM-85 is a standardized lysate of 21 bacterial strains, often found in human airways, that is undergoing clinical trials for diverse respiratory conditions and it has already been authorized in several European countries [27]. The EMA limits its use to the prevention of recurrent respiratory infections [28]. A clinical trial investigating children following the first episode of idiopathic nephrotic syndrome is not yet recruiting (NCT05044169), but plans to enroll 83 patients to whom OM-85 will be administered for 6 months after remission with a primary endpoint of one year relapse-free survival rate. Since nephrotic syndrome relapse is frequently preceded by infections, OM-85 is hypothesized to reduce the incidence of bacterial respiratory infections and, thus, reduce infection-related relapses. Unfortunately, a comparison to placebo was not considered, making the results of the trial difficult to interpret. In cultured epithelial cells, including kidney-derived Vero E6 monkey cells, OM-85 downregulated ACE2 and TMPRSS2 and, as a result, inhibited SARS-CoV-2 cell infection [29]. Whilst these results are promising, the absence of in vivo and clinical studies hampers the translatability and applicability of these observations.
Oxalobacter formigenes postbiotic could contribute to the maintenance of the balance between renal and enteric oxalate as it reduced urinary oxalate excretion and supported colonic oxalate secretion in hyperoxaluric rats with renal insufficiency [30]. GABA-salt also has features of a postbiotic and was protective in preclinical diet-induced kidney injury [31] ad potentially in nephrotoxic acute kidney injury [32].
References
- Perez-Gomez, M.V.; Bartsch, L.-A.; Castillo-Rodriguez, E.; Fernandez-Prado, R.; Fernandez-Fernandez, B.; Martin-Cleary, C.; Gracia-Iguacel, C.; Ortiz, A. Clarifying the Concept of Chronic Kidney Disease for Non-Nephrologists. Clin. Kidney J. 2019, 12, 258–261.
- Ortiz, A.; Roger, M.; Jiménez, V.M.; Perez, J.C.R.; Furlano, M.; Atxer, L.S.; Zurro, D.G.; Casabona, C.M.R.; Gómez, C.G.; Bermúdez, P.P.; et al. RICORS2040: The need for collaborative research in chronic kidney disease. Clin. Kidney J. 2021, 15, 372–387.
- Douglas, A.E.; Werren, J.H. Holes in the Hologenome: Why Host-Microbe Symbioses Are Not Holobionts. mBio 2016, 7, e02099.
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive Impact of Non-Antibiotic Drugs on Human Gut Bacteria. Nature 2018, 555, 623–628.
- Zhang, P.; Zou, J.-Z.; Chen, J.; Tan, X.; Xiang, F.-F.; Shen, B.; Hu, J.-C.; Wang, J.-L.; Wang, Y.-Q.; Yu, J.-B.; et al. Association of Trimethylamine N-Oxide with Cardiovascular and All-Cause Mortality in Hemodialysis Patients. Ren. Fail. 2020, 42, 1004–1014.
- Zhao, J.; Ning, X.; Liu, B.; Dong, R.; Bai, M.; Sun, S. Specific Alterations in Gut Microbiota in Patients with Chronic Kidney Disease: An Updated Systematic Review. Ren. Fail. 2021, 43, 102–112.
- Zsom, L.; Zsom, M.; Salim, S.A.; Fülöp, T. Estimated Glomerular Filtration Rate in Chronic Kidney Disease: A Critical Review of Estimate-Based Predictions of Individual Outcomes in Kidney Disease. Toxins 2022, 14, 127.
- Cigarran Guldris, S.; González Parra, E.; Cases Amenós, A. Gut Microbiota in Chronic Kidney Disease. Nefrologia 2017, 37, 9–19.
- Favero, C.; Carriazo, S.; Cuarental, L.; Fernandez-Prado, R.; Gomá-Garcés, E.; Perez-Gomez, M.V.; Ortiz, A.; Fernandez-Fernandez, B.; Sanchez-Niño, M.D. Phosphate, Microbiota and CKD. Nutrients 2021, 13, 1273.
- Chi, M.; Ma, K.; Wang, J.; Ding, Z.; Li, Y.; Zhu, S.; Liang, X.; Zhang, Q.; Song, L.; Liu, C. The Immunomodulatory Effect of the Gut Microbiota in Kidney Disease. J. Immunol. Res. 2021, 2021, 5516035.
- Castillo-Rodriguez, E.; Fernandez-Prado, R.; Esteras, R.; Perez-Gomez, M.V.; Gracia-Iguacel, C.; Fernandez-Fernandez, B.; Kanbay, M.; Tejedor, A.; Lazaro, A.; Ruiz-Ortega, M.; et al. Impact of Altered Intestinal Microbiota on Chronic Kidney Disease Progression. Toxins 2018, 10, 300.
- Fernandez-Prado, R.; Esteras, R.; Perez-Gomez, M.V.; Gracia-Iguacel, C.; Gonzalez-Parra, E.; Sanz, A.B.; Ortiz, A.; Sanchez-Niño, M.D. Nutrients Turned into Toxins: Microbiota Modulation of Nutrient Properties in Chronic Kidney Disease. Nutrients 2017, 9, 489.
- Poveda, J.; Sanchez-Niño, M.D.; Glorieux, G.; Sanz, A.B.; Egido, J.; Vanholder, R.; Ortiz, A. P-Cresyl Sulphate Has pro-Inflammatory and Cytotoxic Actions on Human Proximal Tubular Epithelial Cells. Nephrol. Dial. Transplant. 2014, 29, 56–64.
- Sun, C.-Y.; Chang, S.-C.; Wu, M.-S. Suppression of Klotho Expression by Protein-Bound Uremic Toxins Is Associated with Increased DNA Methyltransferase Expression and DNA Hypermethylation. Kidney Int. 2012, 81, 640–650.
- Gut Microbiota in Chronic Kidney Disease—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27553986/ (accessed on 11 July 2022).
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients 2019, 11, 923.
- Kim, S.-K.; Guevarra, R.B.; Kim, Y.-T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.-H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340.
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021.
- Holscher, H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes 2017, 8, 172–184.
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667.
- Muralitharan, R.R.; Jama, H.A.; Xie, L.; Peh, A.; Snelson, M.; Marques, F.Z. Microbial Peer Pressure: The Role of the Gut Microbiota in Hypertension and Its Complications. Hypertension 2020, 76, 1674–1687.
- Vrzáčková, N.; Ruml, T.; Zelenka, J. Postbiotics, Metabolic Signaling, and Cancer. Molecules 2021, 26, 1528.
- Rhys-Jones, D.; Climie, R.E.; Gill, P.A.; Jama, H.A.; Head, G.A.; Gibson, P.R.; Kaye, D.M.; Muir, J.G.; Marques, F.Z. Microbial Interventions to Control and Reduce Blood Pressure in Australia (MICRoBIA): Rationale and Design of a Double-Blinded Randomised Cross-over Placebo Controlled Trial. Trials 2021, 22, 496.
- Keshari, S.; Wang, Y.; Herr, D.R.; Wang, S.-M.; Yang, W.-C.; Chuang, T.-H.; Chen, C.-L.; Huang, C.-M. Skin Cutibacterium Acnes Mediates Fermentation to Suppress the Calcium Phosphate-Induced Itching: A Butyric Acid Derivative with Potential for Uremic Pruritus. J. Clin. Med. 2020, 9, 312.
- Lin, W.-Y.; Lin, J.-H.; Kuo, Y.-W.; Chiang, P.-F.R.; Ho, H.-H. Probiotics and Their Metabolites Reduce Oxidative Stress in Middle-Aged Mice. Curr. Microbiol. 2022, 79, 104.
- Díaz Cano, J.V.; Argente, M.-J.; García, M.-L. Effect of Postbiotic Based on Lactic Acid Bacteria on Semen Quality and Health of Male Rabbits. Animals 2021, 11, 1007.
- Notification to the CHMP/EMA Secretariat of a Referral under Article 31 of Directive 2001/83/EC. Available online: https://www.ema.europa.eu/en/documents/referral/bacterial-lysate-medicines-article-31-referral-notification_en.pdf (accessed on 5 June 2022).
- EMA/351772/2019 Bacterial Lysate Medicines for Respiratory Conditions to Be Used Only for Prevention of Recurrent Infections. Available online: https://www.ema.europa.eu/en/documents/press-release/bacterial-lysate-medicines-respiratory-conditions-be-used-only-prevention-recurrent-infections_en.pdf (accessed on 5 June 2022).
- Pivniouk, V.; Pivniouk, O.; DeVries, A.; Uhrlaub, J.L.; Michael, A.; Pivniouk, D.; VanLinden, S.R.; Conway, M.Y.; Hahn, S.; Malone, S.P.; et al. The OM-85 Bacterial Lysate Inhibits SARS-CoV-2 Infection of Epithelial Cells by Downregulating SARS-CoV-2 Receptor Expression. J. Allergy Clin. Immunol. 2022, 149, 923–933.e6.
- Hatch, M.; Cornelius, J.; Allison, M.; Sidhu, H.; Peck, A.; Freel, R.W. Oxalobacter Sp. Reduces Urinary Oxalate Excretion by Promoting Enteric Oxalate Secretion. Kidney Int 2006, 69, 691–698, doi:10.1038/sj.ki.5000162.
- Son, M.; Oh, S.; Lee, H.S.; Choi, J.; Lee, B.-J.; Park, J.-H.; Park, C.H.; Son, K.H.; Byun, K. Gamma-Aminobutyric Acid-Salt Attenuated High Cholesterol/High Salt Diet Induced Hypertension in Mice. Korean J Physiol Pharmacol 2021, 25, 27–38, doi:10.4196/kjpp.2021.25.1.27
- Lee, H.; Ji, S.Y.; Hwangbo, H.; Kim, M.Y.; Kim, D.H.; Park, B.S.; Park, J.-H.; Lee, B.-J.; Kim, G.-Y.; Jeon, Y.-J.; et al. Protective Effect of Gamma Aminobutyric Acid against Aggravation of Renal Injury Caused by High Salt Intake in Cisplatin-Induced Nephrotoxicity. Int J Mol Sci 2022, 23, 502, doi:10.3390/ijms23010502.
More
