Photocatalysis, a unique process that occurs in the presence of light radiation, can potentially be utilized to control environmental pollution, and improve the health of society. Photocatalytic removal, or disinfection, of chemical and biological species has been known for decades; however, its extension to indoor environments in public places has always been challenging. Many efforts have been made in this direction sin the last two–three years since the COVID-19 pandemic started. TFurthermore, the development of efficient photocatalytic nanomaterials through modifications to improve their photoactivity under ambient conditions for fighting with such a pandemic situation is a high research priority. SIn recent years, several metal oxides-based nano-photocatalysts have been designed to work efficiently in outdoor and indoor environments for the photocatalytic disinfection of biological species.
1. Introduction
Even with the fast growing technology and industrial developments, the modern world is still lacking in the control of environmental and health issues. The best example is the current COVID-19 pandemic, which has made
peopleus realize that the modern world should also take care of the development of novel technologies, materials and medical innovations to control such health- and environment-related issues
[1]. Various unwanted components present in the environment affect human health directly or indirectly. In this context in particular, different microbial pathogens such as viruses, bacteria, protozoa, etc. present in the environment may sometimes threaten human health and cause dangerous infectious illnesses
[1][2][1,2]. Recent developments suggest that nanotechnology-based methods and materials could be alternate options with the huge potential for controlling such bacterial/viral outbreaks
[3][4][5][6][3,4,5,6] which have been a serious issue and increased at a disquieting rate over the past decades
[2].
Photocatalysis, which uses nano-photocatalysts, is one of the unique processes occurs in the presence of solar radiation
[7][8][7,8]. This process is promising for the control of environmental issues and for improving the health of the society due to the presence of unspent solar energy on the Earth
[9][10][9,10]. Photocatalysis has multifunctional applications in the field of environmental studies, including the photocatalytic degradation of toxic/harmful organic compounds and gases
[11][12][13][11,12,13], and the photocatalytic viral and bacterial disinfection of water, air, or on surfaces, which ultimately protects the environment and improves human health
[14][15][16][17][14,15,16,17]. Photocatalytic removal or disinfection of such species is a promising and environmentally friendly process using suitable photocatalysts under the influence of solar radiation. Furthermore, it is also very cost-effective and promising in the open environment
[1][9][1,9]. In recent years, several metal oxide semiconductor photocatalysts such as TiO
2, ZnO, CuO, WO
3, etc. have been designed as visible active photocatalysts. Their properties have been improved through some modifications which enable them to work efficiently in solar light towards photocatalytic degradation and disinfection of chemical and biological species
[18][19][18,19], respectively. These are found to be very useful for disinfecting surfaces, air, and water by killing several microorganisms i.e., bacteria and fungi, and inactivating several viruses including influenza virus, hepatitis C virus, coronavirus, etc.,
[20]. These photocatalysts exhibit oxidative capabilities via the photocatalytic production of cytotoxic reactive oxygen species (ROS) for photo-degradation/inactivation of such species in outdoor as well as indoor environment. It has been found to be very beneficial for the treatment of various bacterial/viral diseases such as measles, influenza, herpes, Ebola, current COVID-19, etc.,
[1][2][1,2].
2. Metal Oxide Based Nano-Photocatalysts
The photocatalysis method of disinfection using metal oxide semiconductors shows great potential in outdoor and indoor environments as compared to the conventional methods for the removal of bacteria or viruses. These nano-photocatalysts can effectively inactivate the bacteria and viruses in the presence of light radiation under ambient conditions without producing any other by-products as compared to that of using chemicals
[1][24][1,24].
Metal oxide semiconductor-based nano-photocatalysts such as TiO
2, and ZnO have been extensively investigated for inactivation of several bacteria and viruses. The basic mechanism behind their photoinduced inactivation involves the photocatalytic production of short-lived but effective biocidal ROS, i.e., hydroxyl radicals (•OH), superoxide (•O
2−), and hydrogen peroxide (H
2O
2), through photochemical redox reactions under light irradiation.
[25][26][25,26]. The formation mechanism of various ROS in various cases is shown in
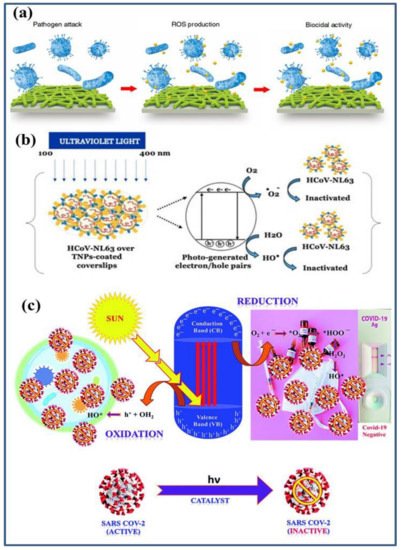
Figure 3. Such an effectively biocidal ROS further inactivates the bacteria and viruses by damaging deoxyribonucleic acid (DNA), Ribonucleic acid (RNA), proteins, and lipids
[2][17][26][27][2,17,26,27]. The generation of ROS and the disinfection of bacteria and virus, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus inactivation, are shown schematically in
Figure 1a–c.
Figure 1. Schematic representation of photocatalytic disinfection of: (
a) bacteria
[26]; (
b) HCoV-NL63 virus
[28]; and (
c) SARS-CoV-2 virus
[27]. Under the influence of light irradiation, the photocatalysts produce electrons and holes that undergo oxidation and reduction processes with O
2 and H
2O generating strong free radical on their surfaces. These radicals interact with the adsorbed bacteria or viruses and inactivate them.
Under the influence of ultra-violet (UV) light radiation, these nano-photocatalysts absorb the radiation resulting in excitation and promotion of valance band (VB) electrons into the conduction band (CB). The holes in VB interact with the adsorbed water molecules and produce active OH and H
2O
2 free radicals. These free radicals are powerful oxidants which generally oxidize the components/chemical in the shell and capsid of the bacteria and viruses
[27]. Subsequently, whereas electrons in CB generally reduce the atmospheric O
2 (or available from the medium) and produce •O
2− radicals
[27][28][27,28]. Similarly, •O
2− radicals produced in photocatalysis are effective in rupturing the capsid shell that results in the leakage and rapid destruction of capsid proteins and RNA
[29] (
Figure 1b,c).
The ROS as produced generally attack or interact with the cytoplasmic membrane and cell wall of the bacteria or viruses during the inactivation mechanism
[30]. However, the rate of photo inactivation/disinfection depends on the photocatalyst used and the amount of ROS produced under the influence of the available wavelength of light irradiation, and also depends on the internal as well as external cell structures of the type of pathogens, because all the bacteria or viruses do not have similar cell wall and membrane structures. These components may have complicated layered structures and contain various types of RNA/DNA, proteins, or enzymes
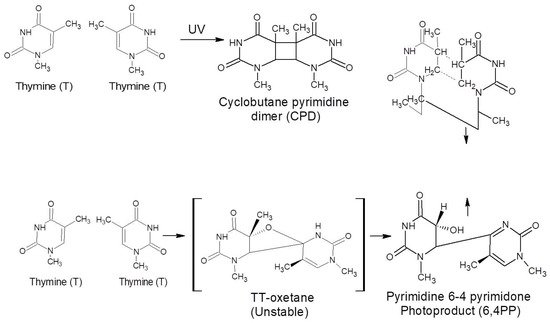
[1][27][30][1,27,30]. For instance, cyclobutene pyrimidine dimers (CPDs) and pyrimidine-6,4-pyrimidone (6.4 PP) photoproducts, together with the Dewar-valence isomers, are the most studied and best described UV-induced photoreactions between and within nucleic acids
[30][31][30,31]. Following UV light exposure, pyrimidine dimers (see
Scheme 1) are formed. CPD and 6,4PP dimers are mainly responsible for bending the double helix 7–9 and 44 degrees, respectively, once they are formed. DNA replication is stopped because of these alterations.
Scheme 1. Schematic diagram of how pyrimidine dimers form after DNA is exposed to UV light. between two adjacent thymine (T) nitrogenous bases, the production of cyclobutane pyrimidine dimers (CPDs); and pyrimidine-6.4-pyrimidone photoproducts (6.4 PP). Similar reactions for uracil in the case of RNA could take place (U)
[30].
Because of their wide band gap, the TiO
2 (3.2 eV) and ZnO (3.37 eV) nano-photocatalysts absorb the high energy UV radiation. This limits their potential photocatalytic applicability more efficiently in outdoor environments under the sunlight because it has only 3–5% UV radiation. Furthermore, photocatalytic disinfection processes in indoor environments are challenging, and modifications of these metal oxide nano-photocatalysts to make them visible light active photocatalysts need to be explored
[9][25][9,25]. There are several ways to modify these nano-photocatalysts, such as doping with metals/non-metals
[32], surface modification via sensitizing or heterojunction formation
[10][33][10,33] with other functional nanomaterials such as noble metals, carbon based nanomaterials (i.e., graphene, carbon nanotubes, graphene oxide, etc.)
[8][34][8,34] other metal oxides, etc.
[35][36][37][35,36,37] Emphasis has been given to enhance the surface area, prevent the recombination of photogenerated charge carriers, and bandgap modification to extend into visible light absorption for effective use in ambient conditions
[11][38][11,38]. For example, Yu et al.
[39][40][39,40] demonstrated the enhanced photocatalytic activity of mesoporous TiO
2 via F doping attributed to the stronger absorption in UV-visible region with a red shift in the band gap transition. Fe doped TiO
2 were found to be very effective in visible region with excellent antifungal activities under natural environment
[41]. Similarly, Ag doped ZnO
[23] and TiO
2 NPs
[11] showed better antibacterial activities in normal room conditions due to Ag ion-induced visible light activity in these nano-photocatalysts. These nano-photocatalysts, modified with plasmonic noble metals
[42][43][44][42,43,44], are effective antibacterial and antiviral agents in dark conditions
[1][45][1,45]. Interestingly, such nano-photocatalysts have also been used as memory catalysis because of their unique talent to retain the catalytic performance in dark conditions
[33][45][46][33,45,46]. For example, Tatsuma et al.
[47] demonstrated that TiO
2–WO
3 heterojuction nanocomposite photocatalyst films could be charged by UV light irradiation which showed good antibacterial effect on Escherichia coli in dark environment. Similarly, Ag-modified TiO
2 films were also shown to exhibit disinfection memory activity
[45].
As discussed above, a great deal of research has been performed in real practical applications of such nano-photocatalysts. Recent developments show that modified metal oxide nano-photocatalysts are promising disinfection agents in indoor environments in ambient room conditions when applied in the form of surface coating/thin films on commonly used surfaces in hospitals, offices, home, etc. Additionally, potential practical applications have been carried out which show excellent results while using these nanomaterials for the disinfection of polluted water and air (in indoor as well as outdoor environments) which show their potential to combat pandemics such as COVID-19. The disinfection applications of such nano-photocatalysts in air, water and on surfaces have been discussed in the next sections, with an emphasis on their mechanism of actions.