1. Types of Anionic Dyes
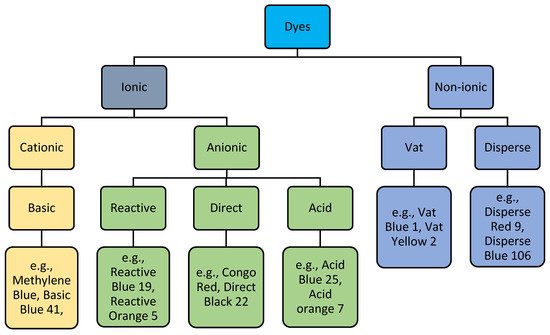
Based on their ionic nature, dyes can be classified into two categories: ionic and nonionic (
Figure 12). Anionic dyes are a type of ionic dye in which anions are the key active groups. Some nonionic dyes, such as vat and disperse dyes, need to be converted into soluble anionic forms before being applied on a substrate
[1][7]. Anionic dyes can be classified into reactive, direct, and acid dyes based on their application process.
Figure 12.
Ion-based-dye classification with examples.
Historically, the chemistry of dyes and pigments has mostly been studied in the context of textile materials, as the coloration of textiles demands that extensive quality parameters be met, which are not relevant to other industries. Understanding their mechanism of interaction with fibers (such as cellulose) can also provide an indication of their interaction ability when adsorbents from agricultural wastes are used for dye removal, as the chemical nature of these wastes is also mostly cellulosic or lignocellulosic.
1.1. Reactive Dye
Reactive dyes can form a covalent bond with certain fiber active sites, such as the hydroxyl group (-OH) or amino group (-NH
2)
[2][19]. Lignocellulosic biomasses contain plenty of hydroxyl groups around their structures, thus making them accessible to interact with reactive dyes. Commercial reactive dyes commonly contain the dichlorotriazine, trichloropyrimidine, aminochloro-s-triazine, sulphatoethylsulphone, dichloroquinoxaline, aminofluoro-s-triazine, and difluorochloropyrimide groups
[3][20]. Because reactive dyes and the surface of cellulosic fibers become negatively charged in water, electrolytes (such as sodium chloride or Glauber salt) are often used to neutralize the fiber surface. No matter how reactive the chemical groups of these dyes are, it is hard to achieve over 80% dye fixation in actual dyeing, which causes the obvious discharge of unfixed dyes as hazardous effluent
[4][21].
1.2. Direct Dye
Direct dyes are named from their high affinity towards cellulosic fiber, and they are thus said to be directly appliable onto cellulose-based fiber systems during dyeing. The major chromophores in the structure of direct dyes are the azo, anthraquinoid, phthalocyanine, and metal complexes
[2][19]. These dyes are highly substantive, although not reactive. The use of electrolytes is also required for the successful attachment of direct dyes to cellulosic fibers.
1.3. Acid Dye
Acid dyes have acidic groups in their structures and are mainly applied on noncellulosic fibers, such as protein and polyamides in acidic conditions. The chromophoric systems in acid dyes are sulphonated azo, anthraquinone, nitrodiphenylamine, triphenylmethane, and xanthenes
[5][22]. Cationized fibers can produce a bond with an anion of acid dyes through an electrostatic force
[6][23].
2. Chemical Nature of Plant-Derived Agricultural Wastes
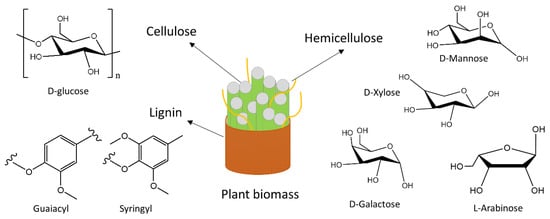
Common plant-based agricultural wastes are a complex combination of cellulose, hemicellulose, and lignin, and are thus also widely known as lignocellulosic biomass. As shown in
Figure 23, these constituents are complexly attached in the lignocellulosic structure. Cellulose is the major component that is linearly formed from the D-glucose subunits, and it is commonly known for its higher crystallinity
[7][24]. However, the hemicellulose part (such as pentose and hexose) is randomly oriented within cellulose chains and is known to impact negatively on the crystallinity. Their chain length is much shorter than the typical chain length of cellulose. The other part, lignin, is the outer layer that holds together both cellulose and hemicellulose
[8][25]. It is a complex structure of cross-linked phenolic subunits, although its actual structure is still to be identified
[9][26]. Throughout the lignocellulosic structure, there are abundant hydroxyl groups, which make this material highly hydrophilic and ideal for dye adsorption from water. For the same reason, their surface is electronegative, and they attract cationic dyes more as the cations of dye become easily attached to the anionic hydroxyl groups. Therefore, their properties need to be altered for efficient anionic dye removal, which has frequently been attempted by researchers over the years.
Figure 23. Common chemical groups in plant-derived agricultural waste.
3. Adsorbents for Anionic Dyes
As mentioned above, due to the electronegative nature of the surface of agricultural wastes (i.e., lignocellulosic structure), anionic dyes show a repulsive effect during the attachment through ionic interactions, if the adsorbent surface is not modified
[1][7]. The modification of these materials can be divided into three main broad concepts: (1) mechanical, (2) chemical, and (3) thermal modifications. The concept of mechanical modification is mainly to reduce the particle size of the initial material by cutting or mechanical milling
[10][27]. This may involve some chemical treatment (e.g., for cleaning purposes), but it will not necessarily alter the chemical groups or ionic behavior. However, the principle of the second concept (i.e., chemical modification) is exactly the opposite, where the modification of the chemical groups is performed to make them chemically more interactive with anionic dyes
[11][16]. This method may involve mechanical milling as a preliminary step, although chemical treatments are the main driving factor for the dye adsorption. The other concept is thermal modification, where both mechanical milling and chemical treatment may be present, although the main principle is to convert the biomass into a carbonaceous material (such as activated carbon) by high-temperature carbonization processes
[12][28], which often result in a high surface area, which leads to the key improvement in the adsorption property. All three methods are promising from different aspects and have their benefits and drawbacks.
3.1. Mechanical Modifications
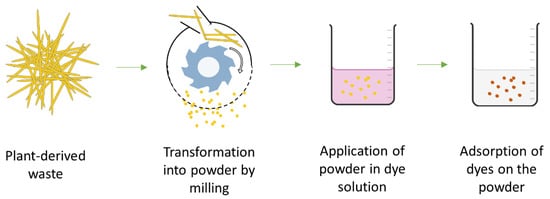
The mechanical modification of biomass mainly involves the mechanical-milling operation, where the materials are crushed into fine or coarse powder (
Figure 34 and
Table 1). Although the chemical properties are not altered, the adsorption capacity largely depends on bath conditions and is often found as a complex synergistic influence from many parameters. Although anionic dyes have a general repulsive effect on the lignocellulosic surface, at the initial stage, the dye adsorption largely depends on the force generated by the dye-concentration gradient from the solution
[13][29]. At the beginning of adsorption, this force helps the dye molecules to reach the adsorbent surface, overcoming the repulsive effect. However, after a certain level, when the concentration gradient is reduced by some of the dyes adsorbed onto the adsorbent, the repulsive effect starts to take the active role and limits the adsorption rate. Hence, even with a similar surface area, mechanically modified biomass can often adsorb more cationic dyes than anionic dyes
[14][30]. This indicates that there is the possibility of remaining vacant sites inside the adsorbent (not completely saturated), even when the equilibrium is reached.
Figure 34.
Schematic of the key concept of the mechanical modification of plant-derived agricultural waste for anionic dye removal.
Table 1.
Maximum adsorption capacities (q
max
) of anionic dye adsorbents derived from mechanically modified agricultural wastes.
| Resources |
Particle Size (µm) |
Dye |
qmax (mg/g) |
Reference |
Waste
tea residue |
0.3475 |
Acid Blue 25 |
127.14 |
[15][31] |
| Date stones and jujube shells |
50–100 |
Congo Red |
45.08–59.55 |
[16][32] |
| Waste banana pith |
>53 |
Direct Red |
5.92 |
[17][33] |
| Waste banana pith |
>53 |
Acid
Brilliant Blue |
4.42 |
[17][33] |
| Jujuba seeds |
53–150 |
Congo Red |
55.56 |
[18][34] |
| Cotton plant wastes |
75–500 |
Remazol Black
B |
35.7–50.9 |
[19][35] |
| Lotus |
<100 |
Congo Red |
0.783–1.179 |
[20][36] |
| Almond shell |
100–500 |
Eriochrome Black T |
123.92 |
[21][37] |
| Waste of corn silk |
250–500 |
Reactive Blue 19 |
71.6 |
[10][27] |
| Waste of corn silk |
250–500 |
Reactive Red 218 |
63.3 |
[10][27] |
| Banana peel, cucumber peel, and potato peel |
250–500 |
Orange G |
20.9–40.5 |
[14][30] |
| Eucalyptus bark |
250–700 |
Solar Red BA |
43.5 |
[22][17] |
| Eucalyptus bark |
250–700 |
Solar Brittle Blue A |
49 |
[22][17] |
| Saccharomyces cerevisiae (yeast) |
315–400 |
Acid Red 14 |
18–23 |
[23][38] |
| Mushroom waste |
<400 |
Direct Red 5B |
18 |
[24][39] |
| Mushroom waste |
<400 |
Direct Black 22 |
15.46 |
[24][39] |
| Mushroom waste |
<400 |
Direct Black 71 |
20.19 |
[24][39] |
| Mushroom waste |
<400 |
Reactive Black 5 |
14.62 |
[24][39] |
| Ash seed |
≤1000 |
Cibacron Blue |
67.114 |
[25][40] |
| Bean peel |
≤1000 |
Cibacron Blue |
28.490 |
[26][41] |
| Jute processing waste |
10,000 |
Congo Red |
13.18 |
[24][27][39,42] |
| Corn stigmata |
Ground (size not mentioned) |
Indigo Carmine |
63.7 |
[24][28][39,43] |
| Cotton gin trash |
Film form |
Acid Blue 25 |
35.46 |
[1][7] |
It is almost a rule of thumb that a smaller-size particle possesses more surface area, and hence, it can hold a greater amount of dye. This has been found to be true in countless studies, and it has been proven for anionic dye adsorption as well. For example, the adsorption capacity of eucalyptus bark towards Solar Red BA (a direct dye) was below 10 mg/g when a particle size of 0.51–0.71 mm was used, although it reached 18.15 mg/g when a <0.255 mm size was used
[22][17]. A similar observation was also found in another report in which the adsorption of Eriochrome Black T was significantly improved (~22.8%) when the particle size of an almond-shell adsorbent was reduced from >500 µm to <100 µm
[21][37].
3.2. Chemical Modifications
Due to the poor interaction of lignocellulosic biomass with anionic dyes
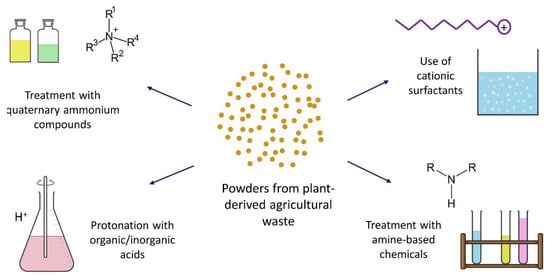
[29][1], the chemical modification of their surface has received keen attention. In most cases, the main idea was to bring cationic groups to the surface, which facilitated the dye adsorption. Several methods of achieving the cationized adsorbent are reported in varied dimensions. As shown in
Figure 45, these can be divided into four main categories: treatment with amine-based chemicals, cationic surfactants, quaternary ammonium compounds, and acid solutions.
Figure 45. Different chemical-treatment methods are proposed for modifying plant-derived agricultural waste.
Different amine-rich compounds have mostly been chosen by researchers for the chemical modification of lignocellulosic biomass because they contain a high number of amines that easily become attached to lignocellulose and attract anionic dyes. However, these methods often require multiple steps of chemical treatment to effectively modify the structure. For instance, Wong et al. used polyethylenimine to treat coffee waste for the adsorption of reactive and acid dye
[30][18]. The method included the impregnation of coffee-waste powder in a polyethylenimine solution at a moderate temperature (65 °C) for 6 h, and then cross-linking using glutaraldehyde. This delivered 77.5 mg/g and 34.4 mg/g maximum adsorptions of Reactive Black 5 and Congo Red dyes, respectively. Nevertheless, a comparison with the unmodified coffee waste was not reported to validate the true influence of the cationization. In another study, nanocrystalline cellulose extracted from hardwood bleached pulp was amino-functionalized by ethylenediamine
[31][44]. The process involved the treatment of ethylenediamine at 30 °C for 6 h, and an in situ reduction by NaBH
4 at room temperature for 3 h, which resulted in the 555.6 mg/g maximum adsorption of Acid Red GR, although no comparison was shown with unmodified lignocellulose. In a different study, wheat straw was treated with epichlorohydrin and N,N-dimethylformamide at 85 °C for 1 h. Furthermore, an ethylenediamine treatment (45 min at 8 °C) and trimethylamine treatments (120 min at 85 °C) were applied to achieve the final amino-modified wheat-straw powder, which showed maximum adsorption capacities of 714.3 mg/g for anionic dye (Acid Red 73) and 285.7 mg/g for reactive dye (Reactive Red 24)
[32][12].
Coating with amine-based polymer was also performed, where sawdust coated with polyaniline nanofibers removed up to 212.97 mg/g of Acid Red G
[33][45]. These nanofibers were prepared by the polymerization of an aniline monomer and oxidant ((FeCl
3). The inclusion of polyaniline nanofibers enhanced the surface area of the biomass (from 1.22 to 16.26 m
2/g), which was useful for the adsorption. In one study, an amine-rich biopolymer, (chitosan) was used to modify lignocellulose for anionic dye adsorption
[1][7]. The chitosan was dissolved in an acetic acid solution, and the cotton-gin-trash film was impregnated for 1 h at room temperature. The resultant modified adsorbent showed a significant increase in the adsorption capacity of Acid Blue 25 (151.5 mg/g) compared with that of the unmodified biomass (35.5 mg/g). The amine-based adsorbent was also prepared as a form of a nanocomposite. For instance, banana-peel cellulose was combined with chitosan and silver nanoparticles to investigate its suitability to adsorb Reactive Orange 5, reaching a maximum adsorption capacity of 125 mg/g
[34][14].
In the second category, treatment with cationic surfactants commonly increases the aliphatic carbon content in biomass
[35][15], but most importantly, they induce the cationic groups on the surface, which help anionic dye removal. Several studies have been performed to modify lignocellulosic biomass using different cationic surfactants. These include cetylpyridinium bromide
[35][15], hexadecylpyridinium bromide
[36][37][46,47], hexadecylpyridinium chloride
[38][48], hexadecylpyridinium chloride monohydrate
[39][49], cetyltrimethylammonium bromide
[40][50], and hexadecyltrimethylammonium bromide
[41][51]. This technique commonly involves the continuous shaking of the crushed lignocellulose particles in the surfactant solutions, and mostly at room temperature. However, the maximum adsorption achieved through this technique is somewhat lower than the amine-based methods. For example, a few of the adsorption capacities reported for this method are 70 mg/g dye by modified wheat straw
[37][47], 30.8–31.1 mg/g dye by modified corn stalks
[35][15], 55–89 mg/g dye by modified coir pith
[41][51], and 146.2 ± 2.4 mg/g dye by modified orange-peel powder
[11][16], which are comparatively lower than most of those reported for amine-based modifications. This is probably related to the abundance of amide groups
[30][18] in the amine-based compounds, which are more prone to attach to the lignocellulosic structure, as well as with the anionic dyes, by electrostatic attraction
[1][30][7,18].
There are few studies on the modification of lignocellulose by quaternary ammonium compounds for anionic dye adsorption, which mostly showed high efficiency. For example, Jiang and Hu reported hydroxypropyloctadecyldimethylammonium-modified rice husk cellulose for reactive (Diamine Green B) and acid-dye (Congo Red and Acid Black 24) adsorption. This modification required multiple steps, which included alkaline and ultrasonic treatments, and treatments with epichlorohydrin and N,N-dimethyl-1-octadecylamine/isopropanol. The resultant adsorbent was able to adsorb up to ~580 mg/g of the anionic dye (Congo Red)
[42][52]. In another study, biomass from palm kernel shell was quaternized using N-(3-chloro-2-hydroxypropyl)trimethylammonium chloride in an alkaline aqueous mixture, which resulted in 207.5 mg/g of maximum adsorption
[43][53].
The fourth category of the chemical modification of the lignocellulose surface is through acidic treatment. For instance, it was claimed that a phosphoric acid treatment worked by increasing the surface area and led to the grafting of phosphate onto the lignocellulose structure. However, the adsorption capacity reported in the proposed method was quite low (15.96 mg/g)
[44][54]. A similar result (adsorption capacity of 13.39 mg/g of Reactive Red 196) was reported when concentrated HCl was used for sawdust treatment
[45][13]. Slightly higher adsorption has also been reported, such as 40.98 mg/g of Drimarine Black CL-B removal by HCl-modified peanut husk
[46][55]. The only exception (a higher removal efficiency) was the treatment of fermentation wastes with nitric acid, where the raw biomass structure was reported to be protonated, replacing the naturally available ionic species, and thereby resulting in a 185.2 mg/g adsorption of Reactive Black 5
[47][56]. However, there was no following study on the nitric acid treatment.
A combination of amine-based chemicals and acid treatment, such as cationization by dichloroethane and methyl amine, followed by protonation using acetic acid, was also reported in one study to modify the biomass surface
[11][16]. The resultant orange-peel adsorbent showed a maximum adsorption capacity of 163 mg/g of Congo Red. Furthermore, the silylation of biomass was also found to be effective for anionic dye adsorption, where a maximum adsorption of 208.33 mg/g was reported
[48][57]. Moreover, Lin et al. proposed an amphoteric-modification method of delignified wheat straw by a monochloroacetic acid treatment, and then grafting with 2-dimethylamino ethyl methacrylate. The resultant adsorbent could be used to adsorb both anionic and cationic dyes by switching between acidic or alkaline conditions ranging from pH 2 to 10
[49][58].
Aside from the four major kinds of treatments, some other methods, such as modifications of biomass using oxides and metal salts, have also been reported. For example, the modification of wood biowaste using Al
2O
3 by the solvo-reaction method showed a high adsorption capacity of Reactive Blue 19 (441.9 mg/g)
[50][59]. Furthermore, the modification of sawdust using FeCl
3 (maximum 212.97 mg/g Acid Red G removal)
[33][45], the use of ZnCl
2 on pulp waste (maximum 285.7 mg/g Methyl Orange removal)
[51][60], hazelnut bagasse (maximum 450 mg/g Acid Blue 350 removal)
[52][61], and pine cone (maximum 118.06 mg/g Alizarin Red S removal)
[53][62], and the use of CaCl
2 with peanut husk (maximum 40.98 mg/g Drimarine Black CL-B removal)
[46][55], have also been reported, showing considerable efficiency.
Overall, it is showed that effective dye removal by chemically modified adsorbents is more related to the modification methods, rather than to the type of dye. For example, the acidic modification of lignocellulose often produced a lower efficiency either for acid or reactive dyes, although the adsorption capacity was reported to be higher for both when amine-based chemicals were used. When the same adsorbent was used to adsorb acid and reactive dyes, mostly the acid dyes showed a higher removal efficiency
[32][41][42][12,51,52], although an exception was also seen
[38][48]. Modification with different amine-based chemicals was found to be more effective for anionic dye adsorption, which was probably related to the abundance of cationic amine groups in these compounds.
3.3. Thermal Modifications
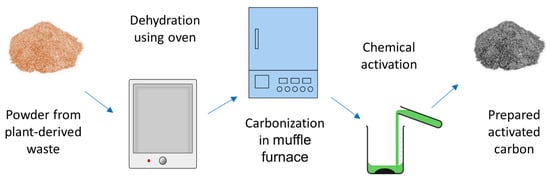
The thermal modification of plant-derived wastes is mainly performed to prepare activated carbon (AC). AC is an advanced form of charcoal that is basically a porous carbonaceous structure with an extended surface area
[54][65]. It has been accepted as an effective adsorbent and has been applied widely in water and wastewater treatment, air purification, and solvent recovery, as well as in the pharmaceutical and medical industries and industrial processes. According to a market research report, the market demand for AC was estimated to be USD 5.7 billion worldwide in 2021, and it is predicted to reach USD 8.9 billion by 2026
[55][66]. A variety of plant-derived wastes have been used as raw materials for the preparation of AC for anionic dye adsorption, as these resources are rich in carbon content, cost effective, renewable, and abundant
[56][67].
Carbonization and activation are basically the two steps that are involved in preparing AC
[57][68]. Activation can be performed by different methods, such as mechanical or thermal, chemical, or a combination of both (i.e., physiochemical)
[56][67], and it can also be biological
[58][69]. These include several methods and their combinations, such as chemical treatment, air oxidation, electrochemical oxidation, and plasma, microwave, and ozone treatment for enhancing the adsorption performance of AC
[59][60][61][62][63][70,71,72,73,74]. However, for AC prepared for anionic dye adsorption, the chemical-activation technique (using activating agents, such as zinc chloride or phosphoric acid)
[64][75] is mostly preferred (
Figure 56) because of its faster activation time, higher carbon yield, simplicity, lower operating temperature, and well-developed pore structure. The proper selection of materials can enhance the adsorption potentiality of AC through the development of its pore structure (total pore volume, pore size distribution) and surface functionalities
[65][76].
Figure 56.
Schematic of thermal-modification steps for plant-derived agricultural waste towards the preparation of activated carbon for anionic dye removal.
The main functional groups on the surface of AC that are responsible for removing dyes are carboxyl, carbonyl, phenols, lactones, and quinones. A positive surface charge on AC can be attained by applying an alkaline treatment, which can accelerate the adsorption of anionic dyes. The porous carbon of alkali-treated AC can enhance the reaction with acid-dye molecules by dipole–dipole, H-bonding, and covalent bonding
[58][69]. ACs prepared from many agricultural byproducts, such as peanut hull, coir pith, rice husk, coffee husk, and water hyacinth, have successfully been used for eliminating anionic dyes from wastewater.
The dye-adsorption capacity of different ACs is substantially controlled by the pH of the dye solution, possibly because the electrostatic interaction between ionized dye molecules and carbon surface functionalities that change the solution pH and rate of adsorption also varies. In the case of anionic dye, high dye uptake is favored at a lower pH or acidic medium, as the complete protonation of the carbon surface functionalities enhances the electrostatic attraction with anionic dyes, thereby increasing the dye uptake. From previous studies, it was observed that the OH group in the solution increased with an increasing pH, producing electrostatic repulsion with anionic dye, and thus decreasing the dye uptake. Therefore, the maximum adsorption of anionic dyes was observed in a pH range of 2–6
[66][67][79,84]. Although the surface area of the AC is known as a key factor for dye adsorption because it provides more spaces for the dye molecules to be attached, it can be perceived that this was not always true for anionic dye adsorption due to ionic interactions, which could have a positive or negative impact, depending on the specific adsorption system. For example, the maximum anionic dye absorption (960 mg/g) was found in AC obtained from pink shower seed pods, although it contained a moderate surface area (283.4 m
2/g)
[64][75], where the strong electrostatic attraction between the functional groups of the AC surface and anionic dye was facilitated with π–π interactions between the free electrons of the aromatic rings of the Congo Red structure, and the delocalized π-electrons on the basal planes of carbon.
3.4. The Effect of Process Conditions
Other than the modification performed on the biomass, if the amount of adsorbent is kept constant, then different process parameters, which include the pH, temperature, dye concentration, and chemical composition of the biomass, can impact the overall adsorption.
The pH of the solution plays a vital role during the adsorption. In numerous studies, for anionic dye adsorption, the favorable pH was reported as 2
[10][14][24][28][27,30,39,43], and in some cases, even 1
[15][19][31,35]. This is directly related to the change in the surface charge in lignocellulose that is altered at a lower pH. A lower pH reduces the electronegativity of the surface, and can even alter it to a positive surface charge
[21][37], thus possibly attracting anionic dyes more conveniently. This was found to be true in cases of different kinds of anionic dyes, such as acid dyes
[14][28][30,43], reactive dyes
[10][27], and direct dyes
[24][39]. Although this was seen as a common trend, some exceptions were also reported in which the pH showed a minor impact
[23][38], or in which a moderate pH (7.5) was found to be more effective for better adsorption
[68][88].
The bath temperature is also important because a higher temperature can often improve the mobility of dye molecules, and thus, the hindering force from the electronegative adsorbent surface diminishes
[69][89]. As a result, a greater amount of dye can be transported into the adsorbent surface. Added to that, swelling can result in the adsorbent, which can promote intraparticle diffusion inside the structure
[69][70][89,90], allowing more dyes be adsorbed onto the surface. However, the opposite phenomenon was also frequently reported (exothermic adsorption)
[21][22][26][17,37,41], where a higher temperature was unfavorable for dye uptake. This was explained as a possible weakening of the related forces of attraction by a higher temperature
[22][25][17,40].
The concentration of dye present in a solution largely affects the adsorption rate, and particularly at the initial stage. If the dye concentration is high, then the rate of adsorption will be higher, and vice versa. The content of cellulose in the biomass is also reported as an influencing factor in the adsorption. In a study, three different biomasses (i.e., coconut shells, cauliflower cores, and broccoli stalks) were used for the adsorption of the same anionic dyes, although the chemical compositions of the biomasses were different. The total cellulose, hemicellulose, starch, and pectinic sugar in the cauliflower cores were the highest among the three and resulted in a higher uptake of reactive and acid dyes
[69][89]. Furthermore, the adsorption capacity was particularly significantly affected by the impact of cellulose as the external layer, rather than hemicellulose and lignin
[69][89]. The chemical groups in lignocellulosic biomass (such as –OH, C=O, and C–O) are also good candidates to form hydrogen bonding with anionic dyes
[14][28][30,43].