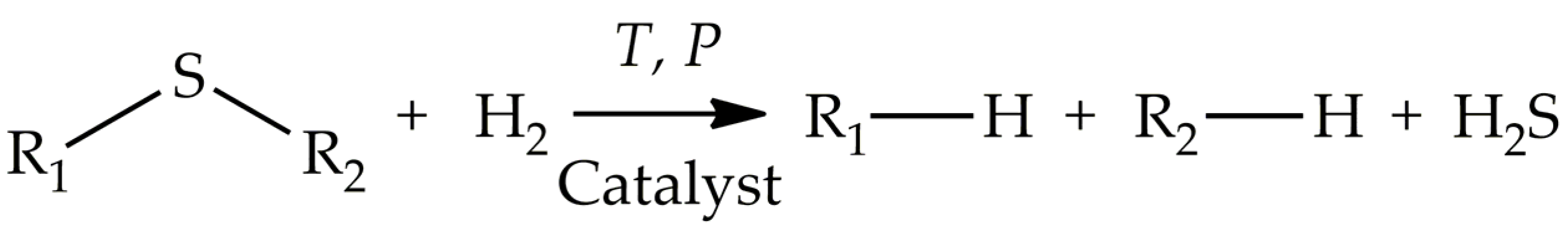
Desulfurization of fuels such as diesel, gasoline, kerosene, and jet fuel has been a challenging operation and remains critical to the petrochemical industry. The main naturally occurring sulfur-containing organic compounds (SCCs) are sulfides, disulfides, mercaptans, thiophene (Th) and its derivatives (benzothiophene (BT), dibenzothiophenes (DBTs), 4-methylbenzothiophene (4-MBT), 4,6-dimethyldibenzothiophene (4,6-DMDBT), 3,7-dimethyldibenzothiophene (3,7-DMDBT), and 2,8-dimethyldibenzothiophene (2,8-DMDBT)). The presence of these SCCs in fuels is undesirable since they create problems during refining, namely deactivation of some catalysts and corrosion of equipment. Moreover, sulfur compounds release toxic SOx and cause severe environmental problems: water and air pollution, global warming, ecological instability, as well as the harmful impact on living organisms. Many countries (USA, European Union, Japan, China, and so etc.on) have introduced strict standards to limit the content of sulfur in fuels to 10 ppm.
- desulfurization
- sulfur-containing organic compounds
- photocatalysis
- photocatalysts
1. Introduction
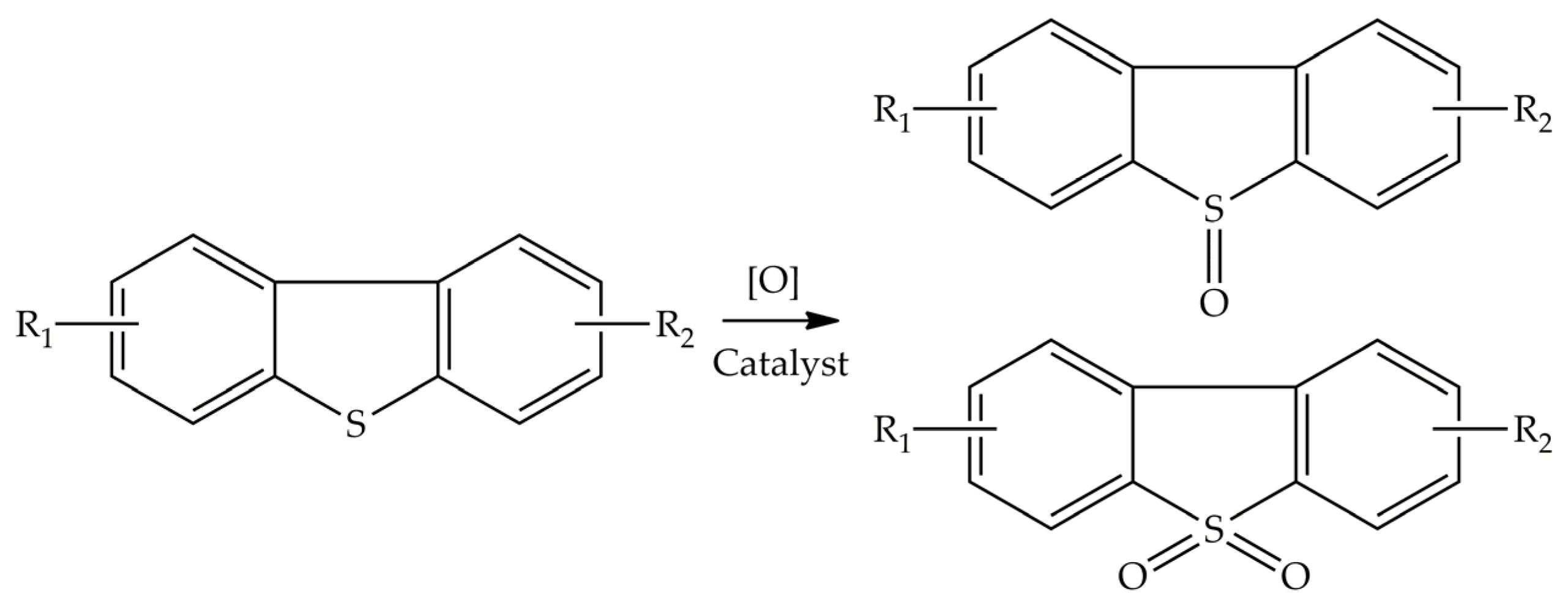
2. Photocatalysis and Desulfurization
2.1. General Principles
2.2. The Most Common Photocatalysts for Desulfurization
3. Advances in Photocatalytic Desulfurization under Visible Light
3.1. Band Gap Engineering (Non-Metal Doping)
3.2. Metal Doping
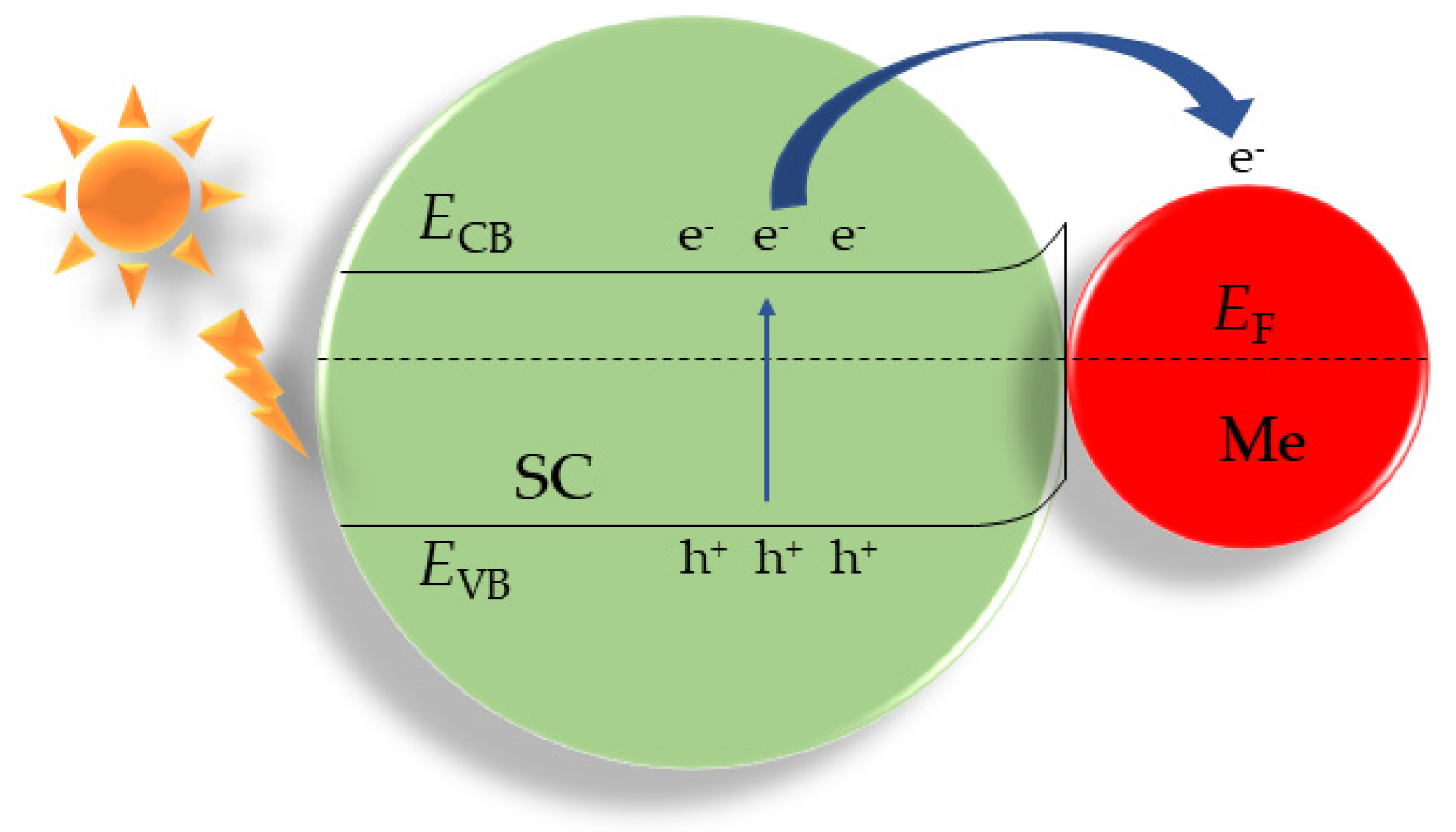
3.3. Creation of Heterojunctions
3.4. Supported Photocatalysts
Many materials used for PODS have a tendency toward severe particle agglomeration, which reduces the photocatalytic activity as well as the number of surface active sites. To overcome these problems, support materials can be used to disperse and immobilize nanoparticles. The performance of supported photocatalysts has been recently reviewed in detail by Hitam et al. [5][1] and Zhou et al. [9][6]. On the other hand, since 2020, there have been several reports related to the supported photocatalytic materials, which should be mentioned. It was shown that the most popular photocatalyst, TiO2, can be immobilized onto SBA-15 [147][91] and porous glass [148][92]. For the photocatalytic oxidation of DBT, Guo and colleagues [147][91] prepared TiO2@SBA-15 composites and sensitized these with organic dye (2,9-dichloroquinacridone, DCQ) to extend the spectral response range of the photocatalyst from UV light to visible light. The authors found that the DCQ-TiO2@SBA-15 photocatalyst has a better performance than the unsensitized TiO2@SBA-15, and the desulfurization rate can reach up to 96% within 90 min. Although photocatalyst sensitization has been a research hotspot in PODS, there is still a need to reduce the cost of this technology and improve the stability of the process. From the point of view of the practical application of the PODS technology, the most attractive is the use of materials that are sensitive to visible light without sensitization with organic dyes. For instance, the core-shell MoS2–g-C3N4–BiOBr@MCM-41 photocatalyst adsorbents with different percentages of MoS2 (1, 3, and 5 wt%) were investigated in a one-step photooxidative-adsorptive desulfurization under simulated solar light [44][41]. Among the different samples, the sample with 5 wt% of MoS2 had the highest catalytic activity in 75 min due to strong interaction between components, highest coverage of MCM-41 with active phases, high capability of light absorption, and low recombination of charge carriers. The authors revealed the direct Z-scheme transfer of the photogenerated charges in this dual heterojunction. It could be assumed that a suitable selection of support materials with a high surface area might play crucial roles in enhancing the PODS performance. The support materials can not only disperse and immobilize photocatalyst nanoparticles, preventing their agglomeration, but are also capable of improving the adsorption capacity towards SCCs.References
- Hitam, C.N.C.; Jalil, A.A.; Abdulrasheed, A.A. A review on recent progression of photocatalytic desulphurization study over decorated photocatalysts. J. Ind. Eng. Chem. 2019, 74, 172–186.
- Gao, X.-M.; Fu, F.; Zhang, L.-P.; Li, W.-H. The preparation of Ag–BiVO4 metal composite oxides and its application in efficient photocatalytic oxidative thiophene. Phys. B Condens. Matter 2013, 419, 80–85.
- Saha, B.; Vedachalam, S.; Dalai, A.K. Review on recent advances in adsorptive desulfurization. Fuel Process. Technol. 2021, 214, 106685.
- Lee, K.X.; Valla, J.A. Adsorptive desulfurization of liquid hydrocarbons using zeolite-based sorbents: A comprehensive review. React. Chem. Eng. 2019, 4, 1357–1386.
- Kulkarni, P.S.; Afonso, C.A.M. Deep desulfurization of diesel fuel using ionic liquids: Current status and future challenges. Green Chem. 2010, 12, 1139–1149.
- Zhou, X.; Wang, T.; Liu, H.; Gao, X.; Wang, C.; Wang, G. Desulfurization through photocatalytic oxidation: A critical review. ChemSusChem 2020, 14, 492–511.
- Majid, M.F.; Mohd Zaid, H.F.; Kait, C.F.; Jumbri, K.; Yuan, L.C.; Rajasuriyan, S. Futuristic advance and perspective of deep eutectic solvent for extractive desulfurization of fuel oil: A review. J. Mol. Liq. 2020, 306, 112870.
- Ibrahim, M.H.; Hayyan, M.; Hashim, M.A.; Hayyan, A. The role of ionic liquids in desulfurization of fuels: A review. Renew. Sustain. Energy Rev. 2017, 76, 1534–1549.
- Ye, J.; Zhang, P.; Zhang, G.; Wang, S.; Nabi, M.; Zhang, Q.; Zhang, H. Biodesulfurization of high sulfur fat coal with indigenous and exotic microorganisms. J. Clean. Prod. 2018, 197, 562–570.
- Chen, L.; Yuan, Z.-Y. Design strategies of supported metal-based catalysts for efficient oxidative desulfurization of fuel. J. Ind. Eng. Chem. 2022, 108, 1–14.
- Tanimu, A.; Tanimu, G.; Ganiyu, S.A.; Gambo, Y.; Alasiri, H.; Alhooshani, K. Metal-free catalytic oxidative desulfurization of fuels – a review. Energy Fuels 2022, 36, 3394–3419.
- Hossain, M.; Park, H.; Choi, H. A comprehensive review on catalytic oxidative desulfurization of liquid fuel oil. Catalysts 2019, 9, 229.
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336.
- Parrino, F.; Bellardita, M.; García-López, E.I.; Marcì, G.; Loddo, V.; Palmisano, L. Heterogeneous photocatalysis for selective formation of high-value-added molecules: Some chemical and engineering aspects. ACS Catal. 2018, 8, 11191–11225.
- Michelin, C.; Hoffmann, N. Photosensitization and photocatalysis—perspectives in organic synthesis. ACS Catal. 2018, 8, 12046–12055.
- Braslavsky, S.E.; Braun, A.M.; Cassano, A.E.; Emeline, A.V.; Litter, M.I.; Palmisano, L.; Parmon, V.N.; Serpone, N. Glossary of terms used in photocatalysis and radiation catalysis (IUPAC recommendations 2011). Pure Appl. Chem. 2011, 83, 931–1014.
- Zhu, S.; Wang, D. Photocatalysis: Basic principles, diverse forms of implementations and emerging scientific opportunities. Adv. Energy Mater. 2017, 7, 1700841.
- Yang, X.; Wang, D. Photocatalysis: From fundamental principles to materials and applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693.
- Cui, J.; Wang, G.; Liu, W.; Ke, P.; Tian, Q.; Li, X.; Tian, Y. Synthesis BiVO4 modified by CuO supported onto bentonite for molecular oxygen photocatalytic oxidative desulfurization of fuel under visible light. Fuel 2021, 290, 120066.
- Zhou, X.; Wang, T.; Zhang, L.; Che, S.; Liu, H.; Liu, S.; Wang, C.; Su, D.; Teng, Z. Highly efficient Ag2O/Na-g-C3N4 heterojunction for photocatalytic desulfurization of thiophene in fuel under ambient air conditions. Appl. Catal. B Environ. 2022, 316, 121614.
- Pham, X.N.; Nguyen, M.B.; Ngo, H.S.; Doan, H.V. Highly efficient photocatalytic oxidative desulfurization of dibenzothiophene with sunlight irradiation using green catalyst of /Al-SBA-15 derived from natural halloysite. J. Ind. Eng. Chem. 2020, 90, 358–370.
- Belousov, A.S.; Suleimanov, E.V.; Parkhacheva, A.A.; Fukina, D.G.; Koryagin, A.V.; Titaev, D.N.; Lazarev, M.A. Synthesis and characterization of Bi2WxMo1–xO6 solid solutions and their application in photocatalytic desulfurization under visible light. Processes 2022, 10, 789.
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38.
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725.
- Boyjoo, Y.; Sun, H.; Liu, J.; Pareek, V.K.; Wang, S. A review on photocatalysis for air treatment: From catalyst development to reactor design. Chem. Eng. J. 2017, 310, 537–559.
- Wang, Q.; Domen, K. Particulate photocatalysts for light-driven water splitting: Mechanisms, challenges, and design strategies. Chem. Rev. 2019, 120, 919–985.
- Belousov, A.S.; Suleimanov, E.V. Application of metal–organic frameworks as an alternative to metal oxide-based photocatalysts for the production of industrially important organic chemicals. Green Chem. 2021, 23, 6172–6204.
- Kovačič, Ž.; Likozar, B.; Huš, M. Photocatalytic CO2 reduction: A review of ab initio mechanism, kinetics, and multiscale modeling simulations. ACS Catal. 2020, 10, 14984–15007.
- Matsuzawa, S.; Tanaka, J.; Sato, S.; Ibusuki, T. Photocatalytic oxidation of dibenzothiophenes in acetonitrile using TiO2: Effect of hydrogen peroxide and ultrasound irradiation. J. Photochem. Photobiol. A Chem. 2002, 149, 183–189.
- Li, F.-T.; Liu, Y.; Sun, Z.-M.; Zhao, Y.; Liu, R.-H.; Chen, L.-J.; Zhao, D.-S. Photocatalytic oxidative desulfurization of dibenzothiophene under simulated sunlight irradiation with mixed-phase Fe2O3 prepared by solution combustion. Catal. Sci. Technol. 2012, 2, 1455–1462.
- Kang, M.; Wang, X.; Zhang, J.; Lu, Y.; Chen, X.; Yang, L.; Wang, F. Boosting the photocatalytic oxidative desulfurization of dibenzothiophene by decoration of MWO4 (M = Cu, Zn, Ni) on WO3. J. Environ. Chem. Eng. 2019, 7, 102809.
- Li, X.; Li, F.; Lu, X.; Zuo, S.; Yao, C.; Ni, C. Development of Bi2W1−xMoxO6/montmorillonite nanocomposite as efficient catalyst for photocatalytic desulfurization. J. Alloy. Compd. 2017, 709, 285–292.
- Li, B.; Song, H.; Han, F.; Wei, L. Photocatalytic oxidative desulfurization and denitrogenation for fuels in ambient air over Ti3C2/g-C3N4 composites under visible light irradiation. Appl. Catal. B Environ. 2020, 269, 118845.
- Huang, Z.; Song, H.; Li, A.; An, Z.; Zhang, K.; Xiang, X.; Shu, X.; He, J. Z-scheme ZnM-LDHs/g-C3N4 (M = Al, Cr) photocatalysts: Their desulfurization performance and mechanism for model oil with air. Energy Fuels 2020, 34, 14676–14687.
- Bagheri, M.; Masoomi, M.Y.; Morsali, A. A MoO3–metal–organic framework composite as a simultaneous photocatalyst and catalyst in the pods process of light oil. ACS Catal. 2017, 7, 6949–6956.
- Flihh, S.M.; Ammar, S.H. Zeolitic imidazolate framework grafted by cobalt tungstate as an efficient photocatalyst for photocatalytic oxidative desulfurization of dibenzothiophene. Mater. Sci. Semicond. Process. 2022, 149, 106894.
- Kalantari, K.; Kalbasi, M.; Sohrabi, M.; Royaee, S.J. Synthesis and characterization of N-doped TiO2 nanoparticles and their application in photocatalytic oxidation of dibenzothiophene under visible light. Ceram. Int. 2016, 42, 14834–14842.
- Zhao, N.; Li, S.; Zhang, X.; Huang, X.; Wang, J.; Gao, R.; Zhao, J.; Wang, J. Photocatalytic performances of Ag/ALa4Ti4O15 (A = Ca, Sr and Ba) on H2O2 oxidative desulfurization. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 125–132.
- Chen, Y.; Shen, C.; Wang, J.; Xiao, G.; Luo, G. Green synthesis of Ag–TiO2 supported on porous glass with enhanced photocatalytic performance for oxidative desulfurization and removal of dyes under visible light. ACS Sustain. Chem. Eng. 2018, 6, 13276–13286.
- Amiri, O.; Beshkar, F.; Ahmed, S.S.; Mahmood, P.H.; Dezaye, A.A. Hierarchical p-BiOI/n-BiPO4 heterojunction nanocomposite with enhanced visible-light photocatalytic desulfurization of thiophene under mild conditions. Int. J. Hydrog. Energy 2021, 46, 6547–6560.
- Abedini, F.; Allahyari, S.; Rahemi, N. One-step oxidative-adsorptive desulfurization of DBT on simulated solar light-driven nano photocatalyst of MoS2-C3N4 Adv. Powder Technol. 2022, 33, 103611.
- Dedual, G.; MacDonald, M.J.; Alshareef, A.; Wu, Z.; Tsang, D.C.W.; Yip, A.C.K. Requirements for effective photocatalytic oxidative desulfurization of a thiophene-containing solution using TiO2. J. Environ. Chem. Eng. 2014, 2, 1947–1955.
- Hosseini, S.A.; Majidi, V.; Abbasian, A.R. Photocatalytic desulfurization of dibenzothiophene by NiCo2O4 nanospinel obtained by an oxidative precipitation process modeling and optimization. J. Sulfur Chem. 2018, 39, 119–129.
- Shafiq, I.; Hussain, M.; Rashid, R.; Shafique, S.; Akhter, P.; Yang, W.; Ahmed, A.; Nawaz, Z.; Park, Y.-K. Development of hierarchically porous LaVO4 for efficient visible-light-driven photocatalytic desulfurization of diesel. Chem. Eng. J. 2021, 420, 130529.
- Shafiq, I.; Hussain, M.; Shafique, S.; Rashid, R.; Akhter, P.; Ahmed, A.; Jeon, J.-K.; Park, Y.-K. Oxidative desulfurization of refinery diesel pool fractions using LaVO4 photocatalyst. J. Ind. Eng. Chem. 2021, 98, 283–288.
- Mahboob, I.; Shafiq, I.; Shafique, S.; Akhter, P.; Amjad, U.-E.-S.; Hussain, M.; Park, Y.-K. Effect of active species scavengers in photocatalytic desulfurization of hydrocracker diesel using mesoporous Ag3VO4. Chem. Eng. J. 2022, 441, 136063.
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852.
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009.
- Belousov, A.S.; Suleimanov, E.V.; Fukina, D.G. Pyrochlore oxides as visible light-responsive photocatalysts. New J. Chem. 2021, 45, 22531–22558.
- Lu, X.; Li, X.; Chen, F.; Chen, Z.; Qian, J.; Zhang, Q. Biotemplating synthesis of N-doped two-dimensional CeO2–TiO2 nanosheets with enhanced visible light photocatalytic desulfurization performance. J. Alloy. Compd. 2020, 815, 152326.
- Zeng, X.; Xiao, X.; Li, Y.; Chen, J.; Wang, H. Deep desulfurization of liquid fuels with molecular oxygen through graphene photocatalytic oxidation. App. Catal. B Environ. 2017, 209, 98–109.
- Ma, Y.; Zhang, M.; Sun, M.; Xie, D.; Mominou, N.; Jing, C.; Wang, L. Ultra-deep photocatalytic desulfurization of dibenzothiophene over hollow core-shell N-doped graphene nanospheres anchored bimetallic single atoms under visible light. Fuel 2022, 324, 124577.
- Li, X.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Xie, J. Graphene in photocatalysis: A review. Small 2016, 12, 6640–6696.
- Ramalingam, G.; Perumal, N.; Priya, A.K.; Rajendran, S. A review of graphene-based semiconductors for photocatalytic degradation of pollutants in wastewater. Chemosphere 2022, 300, 134391.
- Arora, I.; Chawla, H.; Chandra, A.; Sagadevan, S.; Garg, S. Advances in the strategies for enhancing the photocatalytic activity of TiO2: Conversion from UV-light active to visible-light active photocatalyst. Inorg. Chem. Commun. 2022, 143, 109700.
- Panayotov, D.A.; Frenkel, A.I.; Morris, J.R. Catalysis and photocatalysis by nanoscale Au/TiO2: Perspectives for renewable energy. ACS Energy Lett. 2017, 2, 1223–1231.
- Salomatina, E.V.; Fukina, D.G.; Koryagin, A.V.; Titaev, D.N.; Suleimanov, E.V.; Smirnova, L.A. Preparation and photocatalytic properties of titanium dioxide modified with gold or silver nanoparticles. J. Environ. Chem. Eng. 2021, 9, 106078.
- Kavitha, R.; Kumar, S.G. A review on plasmonic Au-ZnO heterojunction photocatalysts: Preparation, modifications and related charge carrier dynamics. Mater. Sci. Semicond. Process. 2019, 93, 59–91.
- Hak, C.H.; Sim, L.C.; Leong, K.H.; Lim, P.F.; Chin, Y.H.; Saravanan, P. M/g-C3N4 (M = Ag, Au, and Pd) composite: Synthesis via sunlight photodeposition and application towards the degradation of bisphenol A. Environ. Sci. Pollut. Res. 2018, 25, 25401–25412.
- Nasir, J.A.; Rehman, Z.U.; Shah, S.N.A.; Khan, A.; Butler, I.S.; Catlow, C.R.A. Recent developments and perspectives in CdS-based photocatalysts for water splitting. J. Mater. Chem. A 2020, 8, 20752–20780.
- Cushing, S.K.; Li, J.; Meng, F.; Senty, T.R.; Suri, S.; Zhi, M.; Li, M.; Bristow, A.D.; Wu, N. Photocatalytic activity enhanced by plasmonic resonant energy transfer from metal to semiconductor. J. Am. Chem. Soc. 2012, 134, 15033–15041.
- Gellé, A.; Jin, T.; de la Garza, L.; Price, G.D.; Besteiro, L.V.; Moores, A. Applications of plasmon-enhanced nanocatalysis to organic transformations. Chem. Rev. 2019, 120, 986–1041.
- Wang, D.; Xue, G.; Zhen, Y.; Fu, F.; Li, D. Monodispersed Ag nanoparticles loaded on the surface of spherical Bi2WO6 nanoarchitectures with enhanced photocatalytic activities. J. Mater. Chem. 2012, 22, 4751–4758.
- Lin, F.; Wang, D.; Jiang, Z.; Ma, Y.; Li, J.; Li, R.; Li, C. Photocatalytic oxidation of thiophene on BiVO4 with dual co-catalysts Pt and RuO2 under visible light irradiation using molecular oxygen as oxidant. Energy Environ. Sci. 2012, 5, 6400–6406.
- Amiri, O.; Beshkar, F.; Ahmed, S.S.; Hamad, B.W.; Mahmood, P.H.; Dezaye, A.A. Magnetically-driven Ag/Fe3O4/graphene ternary nanocomposite as efficient photocatalyst for desulfurization of thiophene under visible-light irradiation. Int. J. Hydrog. Energy 2021, 46, 19913–19925.
- Ibadurrohman, M.; Hellgardt, K. Photoelectrochemical performance of graphene-modified TiO2 photoanodes in the presence of glycerol as a hole scavenger. Int. J. Hydrog. Energy 2014, 39, 18204–18215.
- Chiam, S.-L.; Pung, S.-Y.; Yeoh, F.-Y. Recent developments in MnO2-based photocatalysts for organic dye removal: A review. Environ. Sci. Pollut. Res. 2020, 27, 5759–5778.
- Belousov, A.S.; Fukina, D.G.; Koryagin, A.V. Metal–organic framework-based heterojunction photocatalysts for organic pollutant degradation: Design, construction, and performances. J. Chem. Technol. Biotechnol. 2022, 97, 2675–2693.
- Wang, L.; Zuo, N.; Sun, M.; Ma, Y.; Mominou, N.; Jiang, W.; Li, S.; Jing, C. Deep desulfurization and denitrogenation of diesel fuel over Ir/Pr-N-CQDs-TiO2 under ultraviolet radiation. Sep. Purif. Technol. 2021, 272, 118861.
- Wang, L.; Ma, Y.; Xie, D.; Zhang, M.; Zuo, N.; Mominou, N.; Jing, C. Ultra-deep desulfurization of model diesel fuel over Pr/Ce–N–TiO2 assisted by visible light. Microporous Mesoporous Mater. 2021, 323, 111258.
- Wang, L.; Xie, D.; Ma, Y.; Sun, M.; Mominou, N.; Jiang, W.; Shufeng, C.; Jing, C. Simultaneous desulfurization and denitrogenation of diesel over Er/W-N-TiO2 photocatalyst. Fuel Process. Technol. 2021, 216, 106802.
- Sun, Y.; Jian, W.; Tong, S.; Ma, D.; Zhuang, B.; Jia, F. Study on photocatalytic desulfurization and denitrification performance of Cu- and Cr-modified MWCNT. Processes 2021, 9, 1823.
- Xiao, H.; Luo, C.; Huangfu, G.; Guo, Y. Boosting the photocatalytic ability of bandgap engineered (Na0.5Bi0.5)TiO3–BaTiO3 by N–Ni codoping. J. Phys. Chem. C 2020, 124, 11810–11818.
- Zhang, X.; Song, H.; Sun, C.; Chen, C.; Han, F.; Li, X. Photocatalytic oxidative desulfurization and denitrogenation of fuels over sodium doped graphitic carbon nitride nanosheets under visible light irradiation. Mater. Chem. Phys. 2019, 226, 34–43.
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694.
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-Scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559.
- Zhang, G.; Wang, Z.; Wu, J. Construction of a Z-scheme heterojunction for high-efficiency visible-light-driven photocatalytic CO2 reduction. Nanoscale 2021, 13, 4359–4389.
- Que, M.; Cai, W.; Chen, J.; Zhu, L.; Yang, Y. Recent advances in g-C3N4 composites within four types of heterojunctions for photocatalytic CO2 reduction. Nanoscale 2021, 13, 6692–6712.
- Mishra, B.P.; Parida, K. Orienting z scheme charge transfer in graphitic carbon nitride-based systems for photocatalytic energy and environmental applications. J. Mater. Chem. A 2021, 9, 10039–10080.
- Xu, F.; Chai, B.; Liu, Y.; Liu, Y.; Fan, G.; Song, G. Superior photo-fenton activity toward tetracycline degradation by 2D α-Fe2O3 anchored on 2D g-C3N4: S-scheme heterojunction mechanism and accelerated Fe3+/Fe2+ cycle. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129854.
- Wen, C.; Li, D.; Zhong, J.; Wang, Z.; Huang, S.; Liu, H.; Wu, J.; Chen, P.; Lv, W.; Liu, G. In situ synthesis of S-scheme AgBr/BiOBr for efficient degradation of sulfonamide antibiotics: Synergistic effects of oxygen vacancies and heterojunctions promote exciton dissociation. Chem. Eng. J. 2022, 450, 138075.
- Ren, W.; Yang, J.; Chen, W.; Zhang, J.; Sun, Y.; Zheng, Y.; Zhao, H.; Liang, B. In situ synthesis of novel 0D/2D SnO2 nanoparticles/SnS2 nanosheets s-scheme heterojunction for enhancing the photocatalytic pollutant degradation. Mater. Res. Bull. 2022, 153, 111884.
- Ning, R.; Pang, H.; Yan, Z.; Lu, Z.; Wang, Q.; Wu, Z.; Dai, W.; Liu, L.; Li, Z.; Fan, G.; et al. An innovative s-scheme AgCl/MIL-100(Fe) heterojunction for visible-light-driven degradation of sulfamethazine and mechanism insight. J. Hazard. Mater. 2022, 435, 129061.
- Jiang, Y.; Sun, Z.; Chen, Q.; Cao, C.; Zhao, Y.; Yang, W.; Zeng, L.; Huang, L. Fabrication of 0D/2D TiO2 nanodots/g-C3N4 S-scheme heterojunction photocatalyst for efficient photocatalytic overall water splitting. Appl. Surf. Sci. 2022, 571, 151287.
- Guo, W.; Luo, H.; Jiang, Z.; Shangguan, W. In-situ pressure-induced BiVO4/Bi0.6Y0.4VO4 S-scheme heterojunction for enhanced photocatalytic overall water splitting activity. Chin. J. Catal. 2022, 43, 316–328.
- Fang, W.; Yao, S.; Wang, L.; Li, C. Enhanced photocatalytic overall water splitting via hollow structure Pt/g-C3N4/BiOBr photocatalyst with S-scheme heterojunction. J. Alloy. Compd. 2022, 891, 162081.
- Dai, Y.-M.; Li, C.-Y.; Ting, W.-H.; Jehng, J.-M. Plasmon Ag/AgVO3/TiO2-nanowires S-scheme heterojunction photocatalyst for CO2 reduction. J. Environ. Chem. Eng. 2022, 10, 108045.
- Qaraah, F.A.; Mahyoub, S.A.; Hezam, A.; Qaraah, A.; Xin, F.; Xiu, G. Synergistic effect of hierarchical structure and s-scheme heterojunction over O-doped g-C3N4/N-doped Nb2O5 for highly efficient photocatalytic CO2 reduction. App. Catal., B 2022, 315, 121585.
- Zhao, X.; Li, J.; Song, X.; Liu, X.; Zhou, W.; Wang, H.; Huo, P. Au nanoparticles-modified S-scheme In2O3/H1.5CN heterojunction with enhanced photocatalytic CO2 reduction activity. Appl. Surf. Sci. 2022, 601, 154246.
- Afroz, K.; Moniruddin, M.; Bakranov, N.; Kudaibergenov, S.; Nuraje, N. A heterojunction strategy to improve the visible light sensitive water splitting performance of photocatalytic materials. J. Mater. Chem. A 2018, 6, 21696–21718.
- Guo, G.; Guo, H.; Wang, F.; France, L.J.; Yang, W.; Mei, Z.; Yu, Y. Dye-sensitized TiO2@SBA-15 composites: Preparation and their application in photocatalytic desulfurization. Green Energy Environ. 2020, 5, 114–120.
- Liu, Y.; Tian, J.-Z.; Hao, X.; Zheng, Y.-J.; Jing, T.; Zhao, Y.-P.; Yang, W.-L. Preparation of TiO2/porous glass-H with the coupling of photocatalysis oxidation–adsorption system in the initial position and its desulfurization performance on model fuel. RSC Adv. 2021, 11, 28508–28520.
