Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Rojita Mishra.
The implementation of cutting-edge agricultural practices provides tools and techniques to drive climate-smart agriculture, reduce carbon emissions, and lower the carbon footprint. The alteration of climate conditions due to human activities poses a serious threat to the global agricultural systems. Greenhouse gas emissions (GHG) from organic waste management need urgent attention to optimize conventional composting strategies for organic wastes.
- vermicomposting
- greenhouse gas emissions
- carbon sequestration
1. Introduction
The changing temperature conditions, precipitation variability, and the incidence of extreme weather events are increasing day by day throughout the world, including the Indian subcontinent [1]. The Paris Climate Conference (COP21) created the goal of limiting the amount of heat-trapping gases, i.e., carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and fluorinated gases (F-gases) emitted globally. The largest climatic impacts will be greatest in those regions where agricultural production is vital for securing livelihoods and promoting economic growth [2]. Soil is a major source and sink of greenhouse gases, producing approximately one-fifth of global CO2 emissions [3], roughly one-third of global CH4 emissions, and two-thirds of N2O emissions [4]. The green revolution has increased global food production which indirectly increased the dependence of farmers on synthetic chemical fertilizers, pesticides, and insecticides. The repetitive use of chemical fertilizers causes severe environmental and land degradation. Vermicomposting is an integrated biological process of converting organic waste into vermicast by employing earthworms and naturally occurring microbes under a mesophilic environment. Vermicomposting has been reported as a sustainable technique for the treatment and management of different organic wastes [5]. Earthworms increase the bacterial abundance in the soil as their gut conditions are favorable for the multiplication of bacteria and the suppression of fungi [6].
2. Greenhouse Gas Emissions by Organic Waste Management
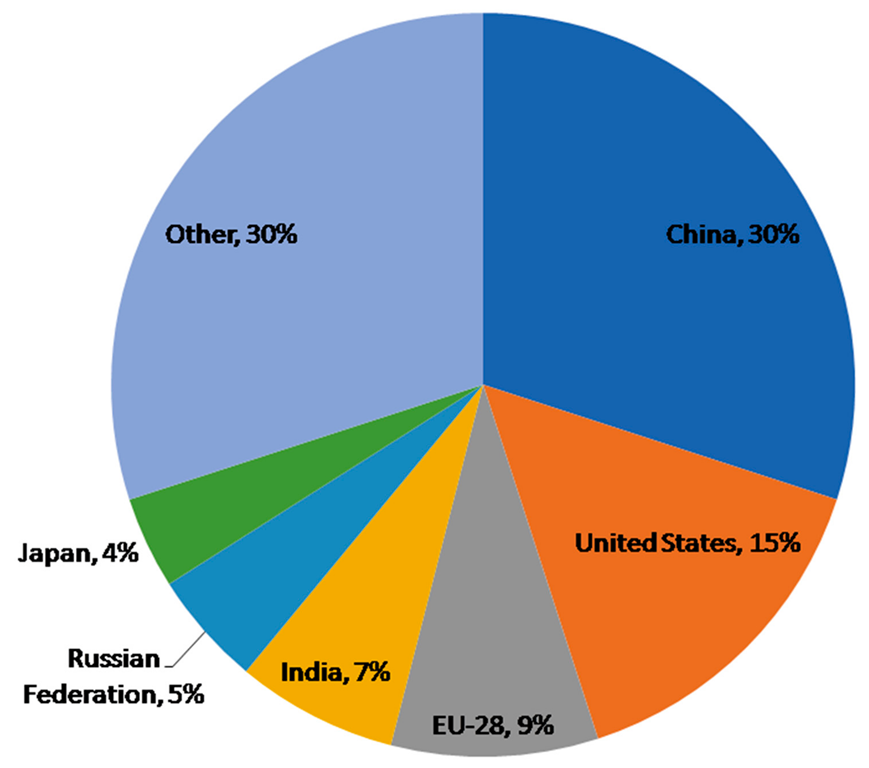
Greenhouse gas emission is one of the foremost problems connected with waste management; CO2 is generated under strictly aerobic conditions, whereas CH4 and N2O are produced from the anaerobic mineralization of organic waste [23][7]. The COP21 Paris climate change conference established the desire to reduce GHG in all sectors [24][8]. China had the highest CO2 emissions from agriculture, forestry, and other land use in 2014, followed by the United States, the European Union, India, the Russian Federation, and Japan (Figure 1). Greenhouse gas emissions have many detrimental ecosystem impacts such as the melting of glaciers, rise in sea level, global warming, acidification of the ocean, etc.2.1. Mitigating GHGs Emission during the Vermicomposting of Waste
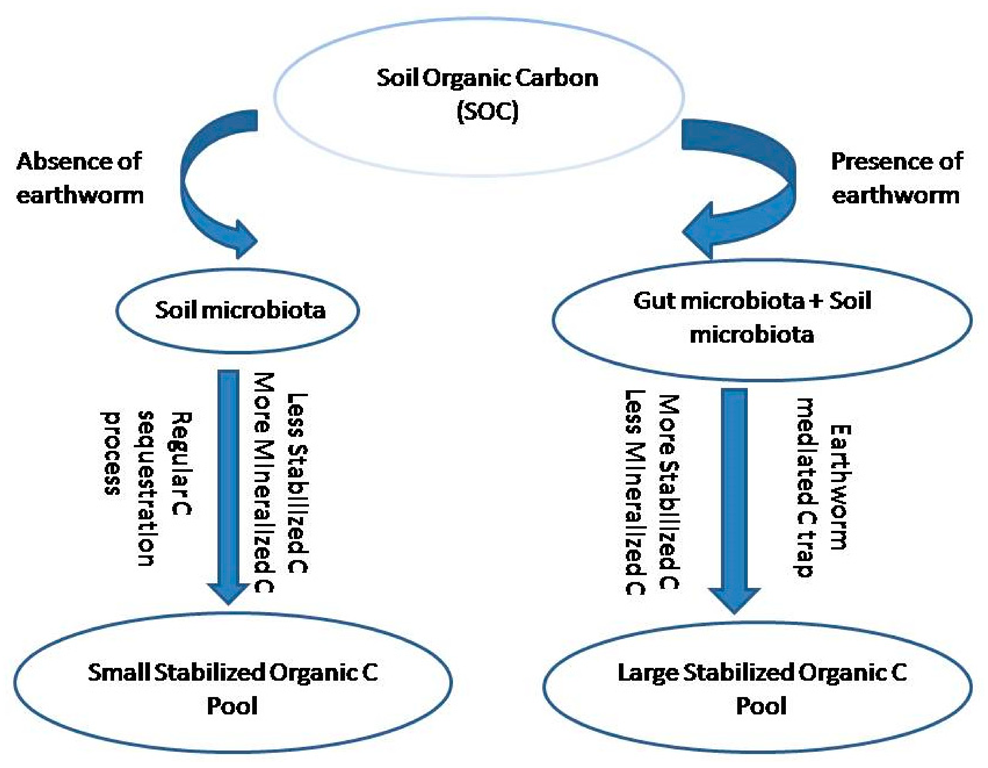
The mitigation policies to lessen the GHG emission during vermicomposting comprise the following: use of C-rich amendment material such as sawdust [28][10], red mud and fly ash [29][11], inorganic material [30][12], and an aeration system. The selection of a bulking agent is based on locally available agricultural residues such as wood chips [31][13], corn stalks [8][14], and spent mushrooms and cotton gins [32][15]. The role of biochar in mitigating greenhouse gas (GHG) emissions has been documented in many pieces of literature [29,33][11][16]. Greenhouse gas emission during composting and vermicomposting is controlled by various factors such as aeration, the addition of a bulking agent, pH, temperature, and C/N ratio [34][17]. Many studies suggest that the incorporation of sewage sludge and cow dung significantly reduces greenhouse gas emissions [35][18]. Wang et al. found that a reed straw addition to duck dung reduces N2O generation. However, manure cannot be used in vermicomposting as it enhances GHG production [34][17]. Greenhouse gas emission is linked with the carbon content of the waste used. Vermicomposting can be used for reducing the methane emission from the sewage sludge with an additive of pelletized wheat straw [36][19]. There are reports that worm composting reduces CH4 emissions in comparison to controlled conditions due to the aerobic environment maintained in the pile by the earthworms. The increased aeration period and suitable maintenance reduce CH4 emissions during the vermicomposting process. Moisture is also an important factor that regulates greenhouse gas emissions during vermicomposting. Excess moisture in worm composting bins causes the death of worms, increases N2O emission by enhancing the process of nitrification and denitrification, and induces CH4 emissions by supporting the growth of methanogenic bacteria in vermibeds. The center of the anoxic earthworm gut contains the highest concentration of N2O [37,38][20][21]. An increase in the radius of the earthworm might increase the probability that the N2O is further reduced to N2 before it leaves the alimentary canal [39][22]. There are reports that gut passage time and competing redox processes may be important factors for the in vivo emission of N2O and N2 by earthworms [40][23]. The passage through the earthworm gut accelerates the decomposition of organic matter due to mineralization, fragmentation, and the consequent increase in microbial activity (Figure 2) thus, higher C storage occurs with the physical protection of soil organic matter inside cast aggregates [41][24]. The feeding ratio is also an important parameter for the determination of GHG emissions during vermicomposting.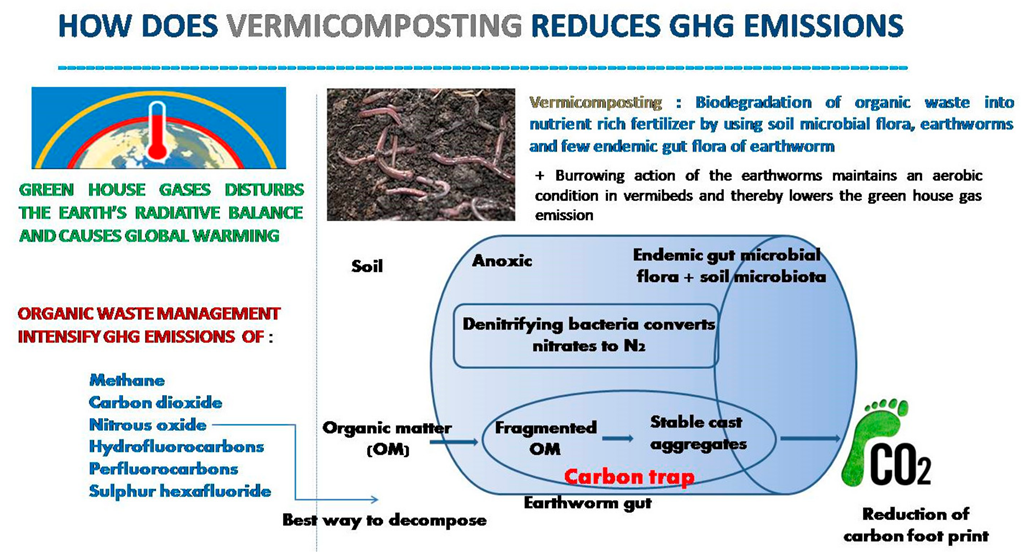
Figure 2. Vermicomposting in climate-smart agriculture.
2.2. Effect of Feeding Ratio on Greenhouse Gas Emission (GHG)
The feeding ratio is the ratio of substrate added over earthworm biomass [43][25]. The optimum feeding ratio helps in decreasing GHGE by 23–48% as compared to non-earthworm-treated composting. A low amount of GHGE during vermicomposting is because of many factors such as (i) earthworms help to increase aeration due to the continuous turning of the substrates [11,16][26][27] and (ii) stabilization of the substrate after passing through the gut of the earthworm [44][28]. In case of a high feeding ratio, the conditions are reversed and GHGE increases. The findings for GHGE during vermicomposting are contradictory; for example, [15,45][29][30] had a different view than that of [8,11,16][14][26][27]. This difference in result can be well explained in terms of the feeding and burrowing behavior of earthworms [45][30], carbon quality and nitrogen content [16[27][28],44], temperature, and scale of the experiment [11,44][26][28]. The denitrification process that takes place inside the earthworm gut is the main physiological process for N2O emission in anecic earthworms [45][30]. A high feeding ratio decreases the conversion rate of fresh materials into vermicompost. Previous studies revealed that more food supply decreases the biomass and reproduction of earthworms [44][28]. However, a low feeding ratio increases the mineralization of nitrogen compared with a high feeding ratio [43][25].2.3. Role of Earthworms in Soil Carbon Sequestration
Carbon sequestration is a process to fix and store atmospheric carbon dioxide and results in the mitigation of global warming. Soil organic matter (SOM) is formed due to litter decomposition and plays an indispensable role in soil carbon (C) sequestration [39][22]. In the comparison of systems without earthworms and with earthworms, a sequence of events takes place that actually deals with carbon cycling and carbon sequestration. From Figure 3 and Figure 4 it is well understood that the earthworms decrease the potentially mineralizable carbon and increase the readily mineralizable carbon and stabilized carbon. A study reported that earthworms increased the carbon mineralization in straw by employing C13 labeling [46][31] and creates a priming effect by inducing organic matter mineralization [47][32]. There is a report that soil organic C stock and carbon sequestration increased to a small extent due to the addition of vermicompost at a 5 t ha−1 rate [48][33]. This little augmentation in the soil organic C stock may generate large impacts in reducing the concentration of atmospheric C. The presence of earthworms adds vermicast to soil and enhances the soil’s physicochemical and biological properties which favor the growth of plant roots in the deeper layer of soil and gather more C in the soil [48][33]. The increase in organic C in soil maintains a sustainable agriculture system and behaves as a potent sink of atmospheric CO2 [49][34].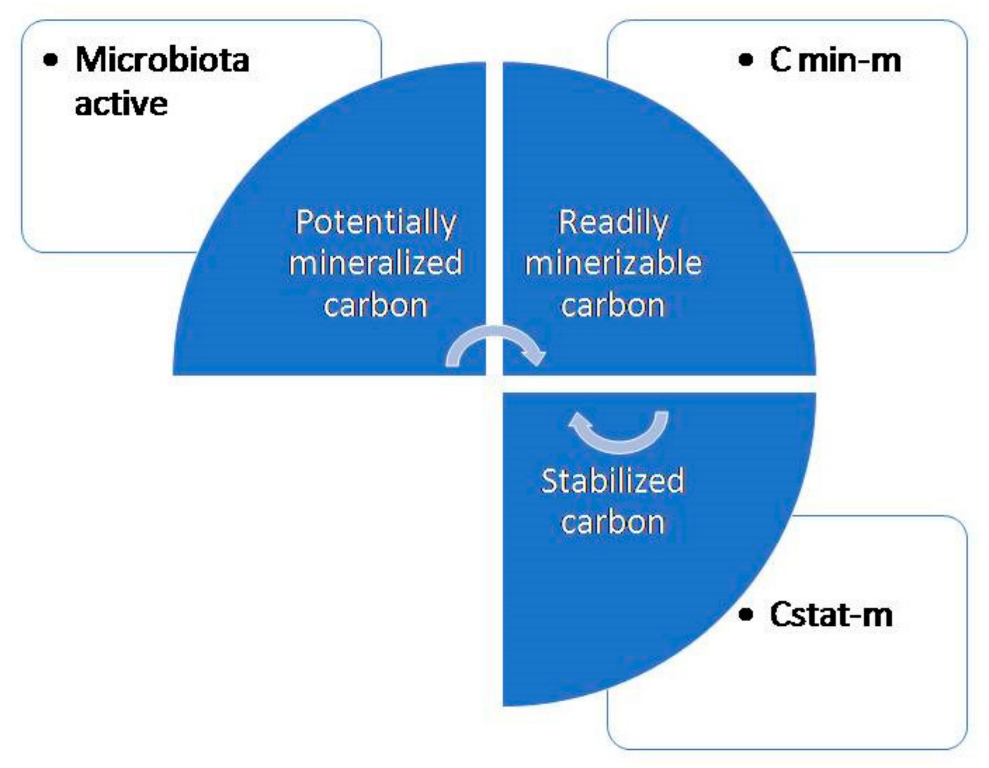
Figure 3. Carbon sequestration in a system without earthworms.
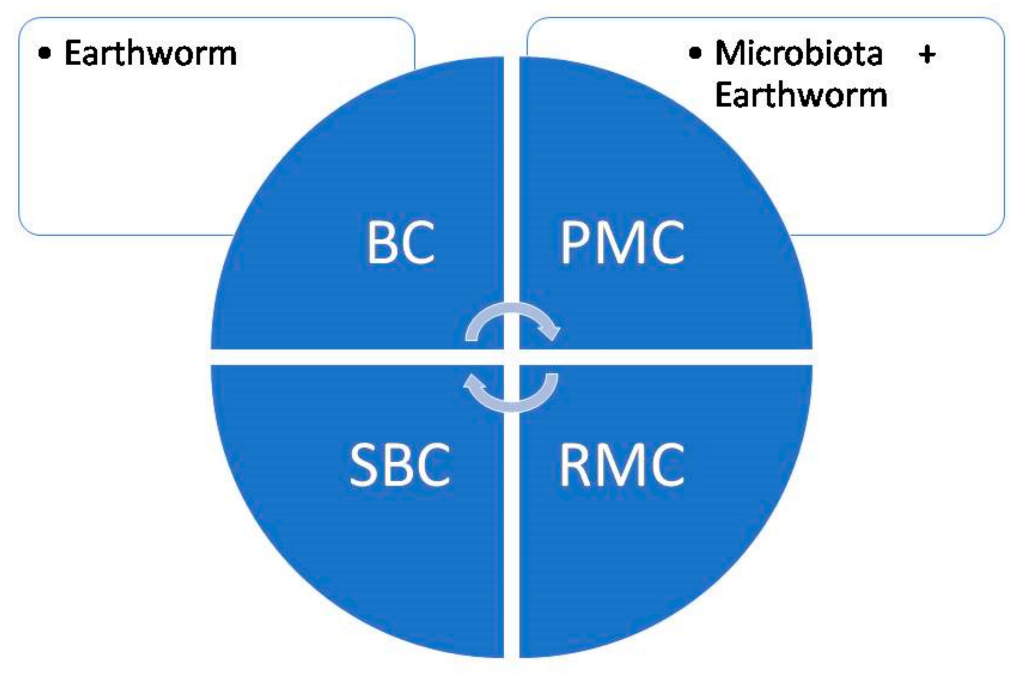
Figure 4. Carbon sequestration in a system with earthworms; BC: basal activated carbon; PMC: potentially mineralizable carbon; SBC: stabilized carbon; and RMC: readily mineralizable carbon.
References
- Mathison, C.; Wiltshire, A.; Dimri, A.P.; Falloon, P.; Jacob, D.; Kumar, P.; Moors, E.; Ridley, J.; Siderius, C.; Stoffel, M.; et al. Regional projections of North Indian climate for adaptation studies. Sci. Total Environ. 2013, 468, S4–S17.
- Biggs, E.M.; Gupta, N.; Saikia, S.D.; Duncan, J.M. The tea landscape of Assam: Multi-stakeholder insights into sustainable livelihoods under a changing climate. Environ. Sci. Pol. 2018, 82, 9–18.
- Rastogi, M.; Singh, S.; Pathak, H. Emission of carbon dioxide from soil. Curr. Sci. 2002, 82, 510–518.
- Smith, K.A.; Ball, T.; Conen, F.; Dobbie, K.E.; Massheder, J.; Rey, A. Exchange of greenhouse gases between soil and atmosphere: Interactions of soil physical factors and biological processes. Eur. J. Soil Sci. 2018, 54, 779–791.
- Frederickson, J.; Howell, G. Large-scale vermicomposting: Emission of nitrous oxide and effects of temperature on earthworm populations: The 7th international symposium on earthworm ecology·Cardiff·Wales. Pedobiologia 2003, 47, 724–730.
- Brown, G.G. How do earthworms affect microfloral and faunal community diversity. Plant Soil. 1995, 170, 209–231.
- Pereira, R.F.; Cardoso, E.J.B.N.; Oliveira, F.C.; Estrada-Bonilla, G.A.; Cerri, C.E.P. A novel way of assessing C dynamics during urban organic waste composting and greenhouse gas emissions in tropical region. Bioresour. Technol. Rep. 2018, 3, 35–42.
- Rogelj, J.; den Elzen, M.; Höhne, N.; Fransen, T.; Fekete, H.; Winkler, H.; Schaeffer, R.; Sha, F.; Riahi, K.; Meinshausen, M. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature 2016, 534, 632–639.
- Boden, T.; Marland, G.; Andres, R.J. National CO2 Emissions from Fossil-Fuel Burning, Cement Manufacture, and Gas Flaring; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy: Oak Ridge, TN, USA, 2017; pp. 1751–2014.
- Yang, F.; Li, G.X.; Yang, Q.Y.; Luo, W.H. Effect of bulking agents on maturity and gaseous emissions during kitchen waste composting. Chemosphere 2013, 93, 1393–1399.
- Barthod, J.; Rumpel, C.; Calabi-Floody, M.; Mora, M.L.; Bolan, N.S.; Dignac, M.F. Adding worms during composting of organic waste with red mud and fly ash reduces CO2 emissions and increases plant available nutrient contents. J. Environ. Manag. 2018, 222, 207–215.
- Mupambwa, H.A.; Mnkeni, P.N.S. Optimizing the vermicomposting of organic wastes amended with inorganic materials for production of nutrient-rich organic fertilizers: A review. Environ. Sci. Pollut. Res. 2018, 25, 10577–10595.
- Maulini-Duran, C.; Artola, A.; Font, X.; Sanchez, A. Gaseous emissions in municipal wastes composting: Effect of the bulking agent. Bioresour. Technol. 2014, 172, 260–268.
- Wang, J.; Hu, Z.; Xu, X.; Jiang, X.; Zheng, B.; Liu, X.; Pan, X.; Kardol, P. Emissions of ammonia and greenhouse gases during combined pre-composting and vermicomposting of duck manure. Waste Manag. 2014, 34, 1546–1552.
- Santos, A.; Bustamante, M.A.; Tortosa, G.; Moral, R.; Bernal, M.P. Gaseous emissions and process development during composting of pig slurry: The influence of the proportion of cotton gin waste. J. Clean. Prod. 2016, 112, 81–90.
- Li, Y.; Hu, S.; Chen, J.; Muller, K.; Li, Y.; Fu, W.; Wang, H. Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: A review. J. Soils Sediments 2018, 18, 546–563.
- Yasmin, N.; Jamuda, M.; Panda, A.K.; Samal, K.; Nayak, J.K. Emission of greenhouse gases (GHGs) during composting and vermicomposting: Measurement, mitigation, and perspectives. Energy Nexus 2022, 7, 100092.
- Samal, K.; Mahapatra, S.; Ali, M.H.; Samal, K. Pharmaceutical wastewater as emerging contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076.
- Dume, B.; Hanc, A.; Svehla, P.; Chane, A.; Nigussie, A. Carbon Dioxide and Methane Emissions during the Composting and Vermicomposting of Sewage Sludge under the Effect of Different Proportions of Straw Pellets. Environ. Sci. Proc. 2021, 8, 7.
- Wust, P.K.; Horn, M.A.; Drake, H.L. In situ hydrogen and nitrous oxide as indicators of concomitant fermentation and denitrification in the alimentary canal of the earthworm Lumbricus terrestris. Appl. Environ. Microbiol. 2009, 75, 1852–1859.
- Wust, P.K.; Horn, M.A.; Henderson, G.; Janssen, P.H.; Rehm, B.H.; Drake, H.L. Gut-associated denitrification and in vivo emission of nitrous oxide by the earthworm families Megascolecidae and Lumbricidae in New Zealand. Appl. Environ. Microbiol. 2009, 75, 3430–3436.
- Angst, G.; John, S.; Mueller, C.W.; Kogel-Knabner, I.; Rethemeyer, J. Tracing the sources and spatial distribution of organic carbon in subsoils using a multi-biomarker approach. Sci. Rep. 2016, 6, 29478.
- Peter, S.D.J.; Siu, M.T.; Marcus, A.H.; Harold, L.D. Emission of nitrous oxide and dinitrogen by diverse earthworm families from Brazil and resolution of associated denitrifying and nitrate-dissimilating taxa. FEMS Microbiol. Ecol. 2013, 83, 375–391.
- Angst, S.; Mueller, C.W.; Cajthaml, T.; Angst, G.; Lhotakova, Z.; Bartuska, M.; Špaldoňová, A.; Frouz, J. Stabilization of soil organic matter by earthworms is connected with physical protection rather than with chemical changes of organic matter. Geoderma 2017, 289, 29–35.
- Ndegwa, P.; Thompson, S.; Das, K. Effects of stocking density and feeding rate on vermicomposting of biosolids. Bioresour. Technol. 2000, 71, 5–12.
- Chan, Y.C.; Sinha, R.K.; Wang, W. Emission of greenhouse gases from home aerobic composting, anaerobic digestion and vermicomposting of household wastes in Brisbane (Australia). Waste Manag. Res. 2011, 29, 540–548.
- Huang, K.; Xia, H. Role of earthworms’ mucus in vermicomposting system: Biodegradation tests based on humification and microbial activity. Sci. Total Environ. 2018, 610, 703–708.
- Luth, R.P.; Germain, P.; Lecomte, M.; Landrain, B.; Li, Y.; Cluzeau, D. Earthworm effects on gaseous emissions during vermifiltration of pig fresh slurry. Bioresour. Technol. 2011, 102, 3679–3686.
- Hobson, A.M.; Frederickson, J.; Dise, N.B. CH4 and N2O from mechanically turned windrow and vermicomposting systems following in-vessel pre-treatment. Waste Manag. 2005, 25, 345–352.
- Lubbers, I.M.; van Groenigen, K.J.; Fonte, S.J.; Six, J.; Brussaard, L.; van Groenigen, J.W. Greenhouse gas emissions from soils increased by earthworms. Nat. Clim. Chang. 2013, 3, 187–194.
- Potthoff, M.; Joergensenb, R.G.; Woltersc, V. Short-term effects of earthworm activity and straw amendment on the microbial C and N turnover in a remoistened arable soil after summer drought. Soil Biol. Biochem. 2001, 33, 583–591.
- Bernard, L.; Chapuis-Lardy, L.; Razafimbelo, T.; Razafindrakoto, M.; Pablo, A.L.; Legname, E.; Poulain, J.; Brüls, T.; O’Donohue, M.; Brauman, A.; et al. Endogeic earthworms shape bacterial functional communities and affect organic matter mineralization in a tropical soil. ISME J. 2012, 6, 213–222.
- Urmi, T.A.; Rahman, M.M.; Islam, M.M.; Islam, M.A.; Jahan, N.A.; Mia, M.A.B.; Siddiqui, M.H.; Kalaji, H.M. Integrated Nutrient Management for Rice Yield, Soil Fertility, and Carbon Sequestration. Plants 2022, 11, 138.
- Gnanavelrajah, N.; Shrestha, R.P.; Schmidt-Vogt, D.; Samarakoon, L. Carbon stock assessment and soil carbon management in agricultural land-uses in Thailand. Land Degrad. Dev. 2008, 19, 242–256.
- Fonte, S.J.; Kong, A.Y.Y.; Van Kessel, C.; Hendrix, P.F.; Six, J. Influence of earthworm activity on aggregate-associated carbon and nitrogen dynamics differs with agroecosystem management. Soil Biol. Biochem. 2007, 39, 1014–1022.
- Hedde, M.; Bureau, F.; Delporte, P.C.; Ecillon, L.; Decaens, T. The effects of earthworm species on soil behaviour depend on land use. Soil Biol. Biochem. 2013, 65, 264e273.
- Bossuyt, H.; Six, J.; Hendrix, P.F. Protection of soil carbon by microaggregates within earthworm cast. Soil Biol. Biochem. 2005, 37, 251–258.
- Zhang, W.; Hendrix, P.F.; Dame, L.E.; Burke, R.A.; Wu, J.; Neher, D.A.; Li, J.; Shao, Y.; Fu, S. Earthworms facilitate carbon sequestration through unequal amplification of carbon stabilization compared with mineralization. Nat. Commun. 2013, 4, 2576.
More