1. Introduction
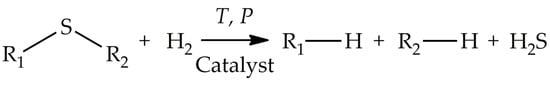
The conventional technology for the removal of SCCs from fuels is hydrodesulphurization (HDS) through catalytic hydrogenation at high temperatures (350–450 °C) and high pressures (30–60 atm). This technique is characterized by a high efficiency in eliminating sulfides, disulfides, and mercaptans by converting them to hydrogen sulfide (
Scheme 1)
[1][5]. The main disadvantages of HDS are high energy consumption due to the harsh reaction conditions and high consumption of costly hydrogen. Moreover, the HDS catalysts (Co or Ni promoted by Mo or W sulfide and supported onto γ-Al
2O
3) are not efficient in the converting of large sulfur-containing molecules (e.g., DBTs and DMDBTs) due to the steric hindrance
[2][6]. To overcome the disadvantages of the conventional HDS technology, alternative approaches, including adsorptive desulfurization (ADS), extractive desulfurization (EDS), biodesulfurization (BDS), and oxidative desulfurization (ODS), have been actively developed.
Scheme 1.
A typical reaction showing hydrodesulfurization (HDS).
ADS involves the removal of SCCs by a physicochemical adsorption process that proceeds at a low temperature and pressure and does not require hydrogen. A wide range of adsorbents based on metal oxides, zeolites, and metal-organic frameworks (MOFs) have been proposed for ADS
[3][4][4,7]. ADS makes it possible to obtain an ultra-high desulfurization efficiency (<10 ppm of S), but the regeneration of adsorbents is rather limited
[5][6][8,9]. EDS is also investigated under mild reaction conditions in the presence of various extractants, including acetonitrile, methanol,
N,
N-dimethylformamide, dimethylsulfoxide, and pyrrolidone
[7][10]. The use of these solvents is associated with emerging environmental and safety issues like wastewater emission and fire hazards. Environmental problems could be solved by using ionic liquids as extractants in EDS
[8][11]. However, their application in large-scale processes is limited by high cost. BDS involves a simple installation process, low energy consumption and operating costs, and mild reaction conditions
[9][12]. The main limitation of this approach is that a noteworthy amount of carbon is mineralized, which reduces the fuel value
[1][5].
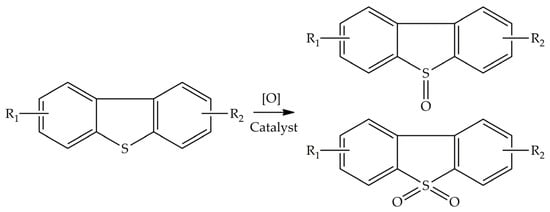
Much attention in recent years has been paid to the ODS technique as an efficient technology for deep desulfurization
[10][11][13,14]. The ODS reaction includes the oxidation of SCCs to corresponding sulfoxides and sulfones in the presence of an oxidizing agent and a catalyst under mild reaction conditions (
Scheme 2). ODS is often combined with EDS to separate the oxidized compounds from the mixture using pure polar solvents (e.g., acetonitrile, methanol
, and so onetc.)
[12][15]. The use of polar solvents makes it possible to obtain a high desulfurization efficiency by the migration of the obtained sulfoxides and sulfones into the polar phase, but it is associated with the environmental issues. The most preferred route is complete mineralization of SCCs to CO
2 and SO
42− without the adding of any extractants into the reaction mixture.
Scheme 2.
A typical reaction showing oxidative desulfurization (ODS).
As one of the ODS methods, photocatalytic oxidation desulfurization (PODS), where reactive oxygen species (ROS)
[13][16] to reduce/oxidize the C−S−C bond are generated, has boosted a surge of scientific interest as one of the most attractive alternative routes to transform naturally abundant, clean, and sustainable solar energy into chemical energy
[14][15][17,18]. The interest in this approach is also proven by the significant increase in the number of publications where PODS is used for deep desulfurization.
HeIn this critical re
inview, the recent achievements in the field of heterogeneous PODS are highlighted, and the conversions of SCCs as well as the mechanisms are discussed.
SectionSection 2 2 is devoted to a brief description of the general principles of the photocatalytic desulfurization, including the main ROS formed during the reaction, the most common photocatalysts for PODS, and the methods for boosting their activity. In the following Section, the current status in the development of highly active photocatalysts for PODS and the main strategies for boosting the activity of semiconductor materials, with their advantages and disadvantages, are described.
It w The influence of operating parameters on the desulfurization efficiency is discussed in Section 4. The a
uthors believe
d that
itthis review will help colleagues in the scientific community who are actively involved in the development of photocatalytic systems for desulfurization.
2. Photocatalysis and Desulfurization
2.1. General Principles
According to the International Union of Pure and Applied Chemistry (IUPAC),
photocatalysis is ‘a change in the rate of a chemical reaction or its initiation under the action of ultraviolet, visible, or infrared radiation in the presence of a substance, i.e., a photocatalyst, which absorbs light and is involved in the chemical transformation of the reaction partners’
[16][19]. The photocatalytic stimulation of a reaction (A
→ B) in the presence of a catalyst (K) in the general form can be written as follows:
when the energy of a photon is higher than the band gap energy (
Eg) of a semiconductor photocatalyst, it can be absorbed, resulting in the promotion of an electron from the valence band (VB) to the conduction band (CB), and leading to the formation of a hole in the VB. After entering the excitation zone, the electron becomes mobile and has a significant reduction potential. Generally, this electron (in the case of its transfer to the semiconductor surface) can be considered a strong one-electron reductant. The formed hole is also very mobile and exhibits the properties of a one-electron oxidizer
[17][18][20,21].
Several ROS can be formed by interaction with the electrons and holes and then reduce/oxidize the C−S−C bond in SCCs
[13][16]. Specifically, O
2 may react with photogenerated electrons to form superoxide radicals (
•O
2−):
The generated
•O
2− could be reduced by photoinduced CB electrons to form hydrogen peroxide (H
2O
2):
Hydroxyl radicals (
•OH) with the highest reactivity among ROS could be formed in several ways: (i) the oxidation of water (Equation (4)), (ii) the reaction of the photogenerated electrons with H
2O
2 (Equation (5)), and (iii) the oxidation of surface hydroxyls by the photogenerated holes (Equation (6)):
It should be noted that in many cases superoxide radicals play a crucial role in the oxidation of SCCs when molecular oxygen or air are used as oxidants
[19][20][22,23], while hydroxyl radicals are involved in PODS when H
2O
2 is added to the reaction mixture for the oxidation of sulfur-containing compounds
[21][22][24,25].
2.2. The Most Common Photocatalysts for Desulfurization
The publication of Fujishima and Honda is considered to be a real breakthrough in photocatalysis
[23][26]. Since then, photocatalysis has been mainly studied in the research fields of pollutant degradation
[24][27], air purification
[25][28], water splitting
[26][29], organic transformations
[27][30], and carbon dioxide reduction
[28][31]. Nowadays, the most common types of semiconductor catalysts used in various photocatalytic reactions are inexpensive and naturally abundant transition metal oxides, especially titanium dioxide (TiO
2).
In 2002, Matsuzawa et al.
[29][32] demonstrated that the conversion of DBT and 4,6-DMDBT in acetonitrile over TiO
2 was about 40% after 10 h under ultraviolet (UV) irradiation. The authors concluded that this method is not applicable for desulfurization of fuels due to low conversion of the substrate. However, the main disadvantage of this method is associated with the use of titanium dioxide as a photocatalyst. It is well-known that TiO
2 crystalline phases (anatase, rutile, and brookite) can only absorb UV light due to their large band gap of 3.0–3.4 eV. In order to ensure efficient solar energy utilization, the development of new photocatalytic materials, which are sensitive to visible light, and methods for shifting the photosensitivity of TiO
2-based catalysts to the visible region (
Eg < 3.0 eV) are important directions in PODS.
In this regard, various ranges of photocatalysts have been proposed for the desulfurization reaction, including visible light-responsive metal oxides
[30][31][33,34], perovskites
[22][32][25,35], graphitic carbon nitride (g-C
3N
4)
[33][34][36,37], and metal-organic frameworks (MOFs)
[35][36][38,39]. However, pure semiconductor photocatalysts are characterized by low activity in PODS due to the rapid recombination of photogenerated electron–hole pairs. To improve their photocatalytic activity under visible light irradiation, various methods have been investigated: (i) band gap engineering, i.e., non-metal doping
[37][40], (ii) deposition of a metal co-catalysts
[38][39][41,42], and (iii) construction of heterojunctions
[20][40][23,43]. Another interesting approach is the combination of PODS with the ADS technique. For this
purpose, a photocatalytic material is supported onto mesoporous materials like MCM-41
[44] or Al-SBA-15 [24].3. Advances in Photocatalytic Desulfurization under Visible Light
This Section is devoted to the current status of the development of highly active photocatalysts for PODS, along with the main advantages and disadvantages of strategies for boosting the activity of semiconductor materials.
Despite the fact that unmodified photocatalysts exhibit relatively low activity in PODS due to the rapid recombination of electron–hole pairs, there have been several reports showing a high efficiency of pure materials. For instance, Dedual and colleagues
[42][45] investigated the effect of operating parameters on the photocatalytic performance in desulfurization of BT and DBT. The authors demonstrated that optimal sulfur removal efficiency (91%) after 3 h occurred with an operating temperature of 40 °C, 0.7% vol% H
2O
2 in a methanol solvent, 6 g·L
−1 TiO
2 loading, a methanol-to-fuel molar ratio of 1, and an initial pH of 4. Although the desulfurization efficiency was relatively high, the use of UV irradiation is not practical in terms of solar light utilization.
It was shown that the Fe
2O
3 photocatalyst is an interesting candidate for the application in PODS
[30][33]. Comparison of the activity of α- and β-Fe
2O
3 showed that the material containing 36.6% β-Fe
2O
3 and 63.4% α-Fe
2O
3 (
Eg = 1.82 eV) exhibited the highest photocatalytic activity (92.3% after 90 min) under visible light. While the stability of this photocatalyst remains unknown, the leaching of iron (ca. 0.49 at% of the used Fe
2O
3) was detected. Thus, it can be assumed that the proposed photocatalyst will lose its activity after each PODS cycle.
It was reported that complex oxides NiCo
2O
4 [43][46], LaVO
4 [44][45][47,48], and Ag
3VO
4 [46][49] were active in the photocatalytic desulfurization under visible light. For instance, a mesoporous Ag
3VO
4 semiconductor, which was synthesized by a hydrothermal approach, exhibited excellent photocatalytic oxidative desulfurization activity up to 92% after 3 h (500 W Xe lamp as a visible light source)
[46][49]. The photocatalyst demonstrated good stability and reusability. The desulfurization activity performance of Ag
3VO
4 was decreased slightly to 91% after six cycles. A comparable SCC removal rate (88%) was obtained using mesoporous lanthanum vanadate LaVO
4 [44][47]. An important advantage of the Ag
3VO
4 and LaVO
4 photocatalysts is the ability to oxidize sulfur-containing compounds in the presence of air.
3.1. Band Gap Engineering (Non-Metal Doping)
Band gap engineering is of great importance for the creation of materials sensitive to visible light and their subsequent application in the photocatalysis. There are three ways of reducing the band gap: shifting the CB minimum, shifting the VB maximum, and introducing impurity levels in the band gap
[47][48][50,51]. The most common method for band gap engineering is the doping of metal oxides, especially TiO
2, into anionic positions with nitrogen, carbon, sulfur, and phosphorus. This approach leads to the formation of a new isolated impurity level, i.e., the N 2p band above the O 2p valence band, which eventually decreases the
Eg of the material and shifts the optical absorption to the visible light region. The main disadvantage of the non-metal doping is associated with the leaching of the dopant
[49][52].
Following this methodology, Kalantari et al.
[37][40] employed an N–TiO
2 photocatalyst for the photocatalytic desulfurization under ambient conditions without any solvents using air as an oxidant. The light absorption extended to the visible region with a red shift in absorption edge for the N-TiO
2 nanoparticles, which resulted in the enhancement of the visible light absorption and photocatalytic activity. As a result, the conversion of DBT using N-TiO
2 was higher than that of the TiO
2-P25 by a factor of 4.7 times. Unfortunately, the desulfurization degree in the presence of the N-doped photocatalyst was low and did not exceed 40% after 4 h. The doping with nitrogen of 2D CeO
2-TiO
2 nanosheets allowed obtaining a much higher conversion of DBT of 94% after 3 h
[50][53]. The enhancement of photocatalytic activity could be attributed to the unique nano thin layer structure of the material, self-doping of biological nitrogen, porous structure, and high surface area. However, a comparison of the photocatalytic activity of the N–TiO
2 nanoparticles
[37][40] and N–CeO
2–TiO
2 nanosheets
[50][53] is quite difficult, since the PODS processes were performed in different reaction conditions, namely sulfur content, nature of oxidant, and light source.
There are several investigations in which graphene was used as a visible light-responsive photocatalyst for PODS
[51][52][54,55]. Graphene is a very promising nanomaterial that has piqued the curiosity of scientists due to its unique optical, electrical, and physicochemical properties
[53][54][56,57]. Ma and colleagues prepared core-shell N-doped graphene nanosphere-anchored bimetallic single atoms and investigated them in PODS using H
2O
2 as an oxidant, methanol as a solvent, and a 500 W Xe lamp as a visible light source
[52][55]. The authors found that the hollow core-shell N-doped graphene decorated with Ni/Cu had a high photocatalytic removal ratio (>99.1%). The photocatalyst demonstrated high activity even after ten cycles because of the synergism of single Ni/Cu atoms and the hollow core-shell N-doping-graphene under visible light. The main disadvantage of the N-doped graphene nanospheres decorated with Ni/Cu is the multi-stage and complex nature of the preparation method, which makes it difficult to use the photocatalyst in a large-scale process.
Despite the fact that the incorporation of non-metals is one of the common approaches for the enhancement of the photocatalytic activity of various materials, this method is often accompanied with leaching of the dopant. The most promising method for obtaining stable and active photocatalysts for PODS is considered to be doping with metals.
3.2. Metal Doping
In the case of the doping strategy, many metals have been employed for improving the photocatalytic activity, including transition and inner transition metals (Er, Zn, Mn, Ce, Nd, Pr, Sm, Sn, Al, Ti, Ni, Fe) as well as noble metals (Pt, Au, Ag)
[55][58]. The use of a noble metal co-catalyst is the most followed approach, which has been widely used for the modification of TiO
2 [56][57][59,60], ZnO
[58][61], g-C
3N
4 [59][62], CdS
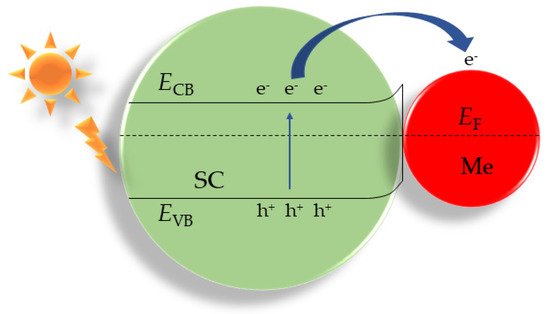
[60][63], and so onetc. In the presence of contact with noble metal nanoparticles, the charge distribution in composite materials is determined by the position of the Fermi level of the metal relative to the band edges in the semiconductor (
Figure 13). The Fermi levels in the contacting metal and semiconductor particles are aligned due to the thermionic emission currents, which leads to the formation of a space charge at the metal–semiconductor contact. If the work function of the metal is greater than the Fermi level of the semiconductor, the Schottky barrier is formed.
In Hereinthis case, the positive charges (h
+) are localized on the semiconductor particle, and the negative charges (e
−) are located on the metal particle. This spatial separation of electrons and holes reduces the recombination of electron–hole pairs, leading to an increase in the photocatalytic activity. Metal nanoparticles also introduce changes in the absorption spectrum of hybrid materials due to the localized surface plasmon resonance (LSPR)
[61][62][64,65].
Figure 13. Band structure of the Schottky barrier between metal nanoparticles and a semiconductor: EF is the Fermi level; SC is the semiconductor; Me are metal nanoparticles.
In the case of the doping strategy, many metals have been employed for improving the activity materials in PODS. The most common approach is doping with noble metals
[2][21][38][39][63][64][65][6,24,41,42,66,67,68]. For instance, Wang et al.
[63][66] demonstrated that the activity of Ag-loaded Bi
2WO
6 in PODS is greatly enhanced compared with the pure Bi
2WO
6. The authors concluded that the incorporation of the Ag nanoparticles led to the efficient separation and preventing of the recombination of electron–hole pairs. Similar observations were found for the Ag/Fe
3O
4/graphene ternary nanocomposite
[65][68]. In the Ag/Fe
3O
4/graphene ternary nanocomposite, Fe
3O
4 adsorbs the visible light photons and generates the electrons and holes on its CB and VB, respectively. The Ag nanoparticles and graphene can act as acceptors for the induced electrons and holes of Fe
3O
4, respectively, to facilitate the separation of electron–hole pairs and interfacial charge transfer. In turn, the LSPR phenomena of the Ag nanoparticles promotes the visible-light absorption of the ternary nanocomposite. Thus, the synergism arising from the separation of electron–hole pairs and LSPR led to a significant increase in the photocatalytic activity of Ag/Fe
3O
4/graphene (95%) compared to Ag/graphene, Fe
3O
4/graphene, and other samples.
A green approach was proposed by Chen and colleagues
[39][42], who synthesized the Ag–TiO
2 photocatalyst supported on porous glass and used it in the desulfurization of model fuel containing 50 ppm of DBT or BT without adding external oxidants such as O
2 and H
2O
2. It is well known that the coexistence of fuel and O
2 or H
2O
2 could trigger an explosion accident when applied in industrial applications. The authors proposed to use ethanol, which serves as an electron acceptor by consuming valance band holes to suppress the recombination of photogenerated electron–hole pairs
[66][69] and acts as a source of highly active hydroxyl radicals. As a result, the desulfurization efficiency was about 84% after 80 min. The prepared photocatalyst was also active in the photodegradation of Rhodamine B, methylene blue, and methyl orange, with the rate constant of 0.14, 0.18, and 0.055 min
−1, respectively. The development of efficient photocatalysts for the removal of dyes is also at the forefront of catalysis science, because organic dyes, which remain in the effluents of the textile industry, are usually persistent and difficult to degrade by conventional wastewater treatment techniques
[67][68][70,71].
There are several recent reports where it is suggested to use rare metals as dopants
[69][70][71][72,73,74]. Despite the fact the Ir/Pr–N–CQDs–TiO
2 [69][72], Pr/Ce–N–TiO
2 [70][73], and Er/W–N–TiO
2 [71][74] photocatalysts provide a high degree of SCC removal, rare metals are characterized by a high price. Noble metals also have a high price; therefore, investigations should be shifted towards using Earth-abundant metals such as Cu
[72][75], Ni
[73][76], Na
[74][77], and Mo
[22][25] as co-catalysts. For instance, Zhang et al.
[74][77] prepared Na-doped g-C
3N
4 for the photocatalytic denitrogenation and desulfurization for fuels under visible light irradiation using molecular O
2 as an oxidant to substitute for the expensive H
2O
2. They demonstrated that a moderate amount of Na in g-C
3N
4 generated the highly dispersed and porous nanosheets, which further improved the surface energy and reduced the recombination rate of electron–hole pairs. Moreover, the synthesized photocatalysts were characterized by the ability to absorb more visible light since the calculated band gaps of g-C
3N
4 were 2.70 eV, while Na-doped g-C
3N
4 were in the range of 2.02–2.44 eV, depending on the amount of the dopant. An important advantage of the proposed method is the complete conversion of N- and S-containing organic compounds into CO
2 without the formation of intermediate oxygenate products. The complete mineralization of SCCs was also detected by Belousov and colleagues
[22][25], who employed nanosized Bi
2W
xMo
1−xO
6 solid solutions with various compositions as photocatalysts for PODS. The implementation of complete oxidation will not lead to the additional separation of the oxidation product from the hydrocarbon mixture by extraction methods with toxic solvents.
3.3. Creation of Heterojunctions
As mentioned earlier, doping with various metals allows the separation of the photogenerated charge carriers to be obtained. However, the most common dopants, namely noble metals, are characterized by a high price. Moreover, in some cases, the leaching of a dopant was observed. Thus, the stability of a photocatalyst remains unclear.
For the spatial separation of electron–hole pairs in photocatalysts, the creation of heterojunctions has been proven to be one of the most promising ways for the preparation of advanced materials
[75][76][77][78][78,79,80,81]. There are three conventional types of heterojunction photocatalysts, namely with a straddling gap (type-I), with a staggered gap (type-II), and with a broken gap (type-III). Among them, the type-II heterostructures, which can be constructed using various semiconductors, are the most useful in the field of photocatalysis because type-I and -III heterostructures do not provide an effective separation of the electron–hole pairs
[68][75][71,78]. On the other hand, the main limitation for the type-II heterojunction is that the oxidation and reduction abilities of the transferred e
− and h
+ decrease because of the electrons migrating to the CB of the semiconductor 1 (SC1), with lower reduction potential and holes accumulating on the VB of the semiconductor 2 (SC2) with lower oxidation potential
[79][82]. Therefore, in addition to the type-II, the construction of Z- and S-scheme heterostructures is a promising direction in photocatalysis, since their development should address the disadvantages of the type-II heterojunctions. In the Z-scheme heterostructures, the photogenerated electrons from the CB of the SC1 with a lower reduction potential migrate to the VB of the SC2 with a lower oxidation potential owing to the electrostatic attraction between the electrons and holes. The S-scheme (Step-scheme) heterojunctions are characterized by a superior redox ability, and their efficiency has been proven in environmental remediation
[80][81][82][83][83,84,85,86], water splitting
[84][85][86][87,88,89], and CO
2 reduction
[87][88][89][90,91,92]. The heterojunction approach may become the most promising route for the preparation of highly active materials for PODS, since a wide range of semiconductors (metal oxides, g-C
3N
4, complex oxides, MOFs
, and so onetc.) can be coupled and then used in the reaction. This allows for combining the different favorable properties of each compound, extending their absorption range, improving their chemical stability towards photocorrosion, and decreasing the recombination of electron–hole pairs
[49][68][90][52,71,93].
There are several examples where the heterojunction strategy was used for the preparation of photocatalysts for desulfurization. The main efforts are aimed to obtain type-II and Z-scheme heterostructures.
3.4. Supported Photocatalysts
Many materials used for PODS have a tendency toward severe particle agglomeration, which reduces the photocatalytic activity as well as the number of surface active sites. To overcome these problems, support materials can be used to disperse and immobilize nanoparticles. The performance of supported photocatalysts has been recently reviewed in detail by Hitam et al.
[1][5] and Zhou et al.
[6][9]. On the other hand, since 2020, there have been several reports related to the supported photocatalytic materials, which should be mentioned.
It was shown that the most popular photocatalyst, TiO
2, can be immobilized onto SBA-15
[91][147] and porous glass
[92][148]. For the photocatalytic oxidation of DBT, Guo and colleagues
[91][147] prepared TiO
2@SBA-15 composites and sensitized these with organic dye (2,9-dichloroquinacridone, DCQ) to extend the spectral response range of the photocatalyst from UV light to visible light. The authors found that the DCQ-TiO
2@SBA-15 photocatalyst has a better performance than the unsensitized TiO
2@SBA-15, and the desulfurization rate can reach up to 96% within 90 min. Although photocatalyst sensitization has been a research hotspot in PODS, there is still a need to reduce the cost of this technology and improve the stability of the process. From the point of view of the practical application of the PODS technology, the most attractive is the use of materials that are sensitive to visible light without sensitization with organic dyes.
For instance, the core-shell MoS
2–g-C
3N
4–BiOBr@MCM-41 photocatalyst adsorbents with different percentages of MoS
2 (1, 3, and 5 wt%) were investigated in a one-step photooxidative-adsorptive desulfurization under simulated solar light
[41][44]. Among the different samples, the sample with 5 wt% of MoS
2 had the highest catalytic activity in 75 min due to strong interaction between components, highest coverage of MCM-41 with active phases, high capability of light absorption, and low recombination of charge carriers. The authors revealed the direct Z-scheme transfer of the photogenerated charges in this dual heterojunction.
It could be assumed that a suitable selection of support materials with a high surface area might play crucial roles in enhancing the PODS performance. The support materials can not only disperse and immobilize photocatalyst nanoparticles, preventing their agglomeration, but are also capable of improving the adsorption capacity towards SCCs.