Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Conner Chen and Version 5 by Conner Chen.
The resistance of microorganisms has appeared since the first antimicrobial was used. Antimicrobial resistance is the ability of microorganisms (such as bacteria, viruses, fungi, or parasites) to resist the action of an antimicrobial agent.
- antimicrobial resistance
- microorganisms
- bacteria
1. Antimicrobial Resistance
The resistance of microorganisms has appeared since the first antimicrobial was used [1]. Antimicrobial resistance is the ability of microorganisms (such as bacteria, viruses, fungi, or parasites) to resist the action of an antimicrobial agent. Antimicrobial resistance may be due to intrinsic resistance (when microorganisms are naturally resistant to the action of certain antibiotics) or acquired (due to the adaptation of microorganisms through genetic modification) [2][3][4][5].
It is essential to find out which mechanism underpins the resistance to learn how to combat this threatening phenomenon. Additionally, knowledge of the mechanisms involved helps in the design of new molecules of antimicrobials to overcome resistance. The general mechanisms of antimicrobial resistance are genetic (transfer of genes), mutations, target-mediated mechanisms, inactivation or modification of antimicrobial molecules, reduced uptake of antimicrobials, active efflux, and biofilms [6][7][8][9]. Another important aspect is the prudent use of antimicrobials by avoiding their misuse or overuse [10][11]. Research shows that antibiotic resistance may also occur independently of antibiotic exposure [12][13].
2. Highlights of the Most Resistant Bacteria Worldwide
According to the World Health Organization (WHO), the most resistant bacteria currently existing are divided into three categories according to how urgent the need to discover new antibiotics is (Table S3, Supplementary Materials) [14]. Out of these pathogens, some are resistant to fluoroquinolones (FQNs). The mechanisms by which bacteria develop resistance to FQNs are alterations in target enzymes, altered drug permeation (both in Gram-positive and Gram-negative bacteria), and plasmid acquisition [15]. Over time, resistance to FQNs developed alongside researchers’ efforts to improve the molecules of this class [16][17][18].
3. The Development of Antibacterial Resistance over Time
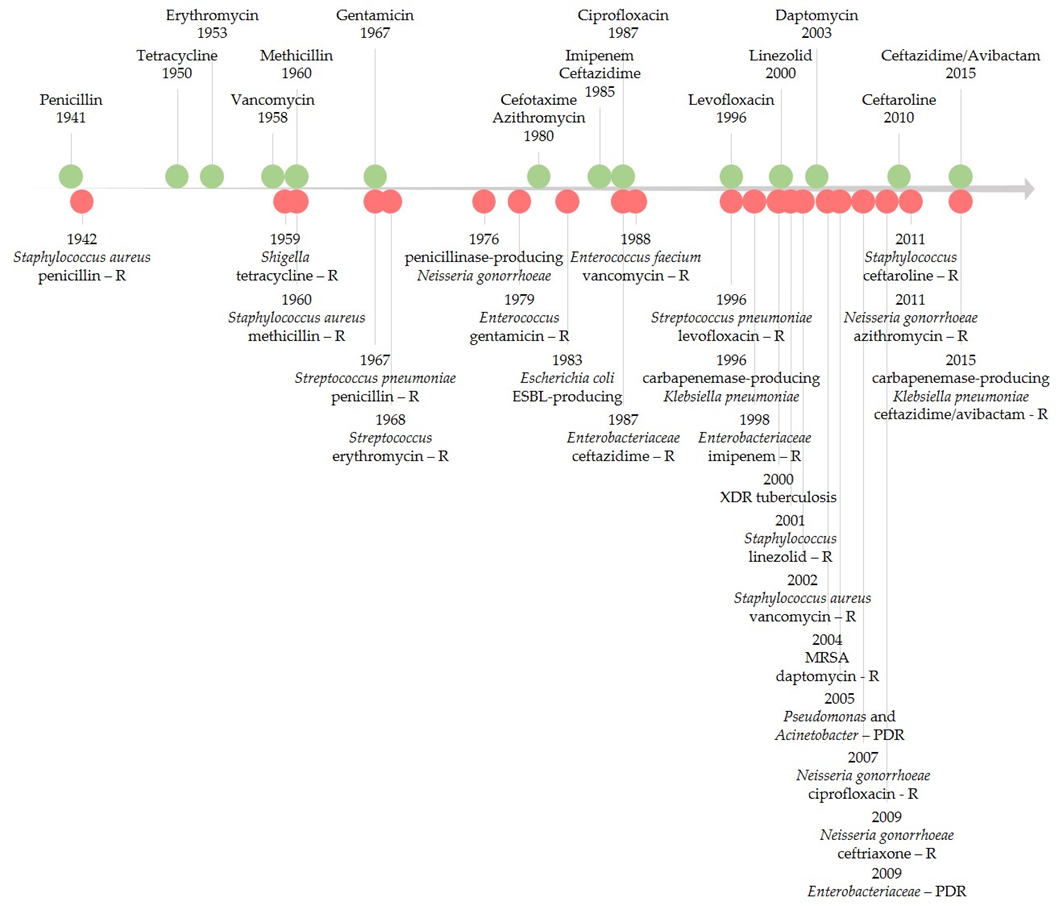
Since the introduction of the first antibiotic in therapy, there have been different levels of interest in the antibiotic resistance phenomenon. Podolsky (2018) described five eras of response to antibiotic resistance. Between 1945 and 1963, when antibiotic resistance appeared to be controlled by the pharmaceutical industry, little effort was undertaken to combat this threat, mainly on a local scale. During 1963–1981, a growing concern arose, fueled by the discovery of bacterial resistance spread across strains or species through what people now know as plasmids [19][20]. Then, from 1981–1992, this threat was beginning to be approached from a more global perspective, raising awareness of the misuse of antibiotics on multiple levels. From 1992–2013, concerns over antibiotic resistance increased; this is a shared global problem that requires interventions spread across various sectors. Finally, from 2013 to the present, the burden of antibiotic resistance is still viewed with great concern while emerging infections with resistant pathogens continue to spread globally [21].
Figure 1 illustrates the timeline of key points of antibiotic resistance occurrences based on early literature reports of resistance and reports of healthcare transmission or outbreaks [22][23][24]. FQNs were no exception for the development of antibacterial resistance [18][25][26]. Resistance to FQNs has arisen after widespread use in humans and animals [27][28]. Between 2001 and 2006, FQN-resistant E. coli isolates dramatically increased in the United Kingdom (from 6% to 20%). By 2010, it decreased to 17%, a phenomenon possibly linked to changes in prescribing [29]. For Enterobacteriaceae (e.g., E. coli), even higher quinolones (QNs) resistance rates were recorded worldwide. In 2015, in the US, reports showed the problematic fact that up to 30% of community-associated isolates were FQN non-susceptible [30]. As the figure highlights, antibiotic resistance is a never-ending phenomenon, unfortunately directly linked to the number of used antibiotics [31].
4. The Emergence of Resistance to Antibiotics Relatively Recently Introduced in Therapy
There have also been reports of resistance or possible mechanisms of resistance development to antibiotics relatively recently introduced in therapy (Table S4, Supplementary Materials) [32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56]. The leading causes of antibiotic resistance’s rapid emergence are overuse, inappropriate prescribing, and extensive agricultural use. Concerning the availability of new antibiotics, the economic and regulatory obstacles are mainly incriminated in hindering the development of these substances [32][57][58][59][60][61]. Improper or excessive use of antimicrobial agents accelerates the natural process of resistance [62]. Without effective antibiotics, the possibility of treating infectious diseases is endangered. Additionally, various medical procedures such as organ transplantation or major surgery could become even riskier. Antimicrobial resistance also impacts rising costs due to extended hospital stays and the need for longer-term intensive care [4].
5. New Mechanisms for Bacterial Resistance
Bacteria are constantly gaining resistance due to their genetic plasticity, suffering mutations frequently. They include new genes in their DNA relatively easily through transformation, transduction, and conjugation. These processes allow sharing of the resistance genes from a “gene carrier” bacteria to another. These mutations lead to multiple modifications in the cell and, in the end, to a form of resistance [2][63][64].
A good example is the resistance of Bacteroides fragilis to metronidazole. Bacteroides fragilis is an anaerobic colon resident, but it was found in many extraintestinal infections such as foot, brain, and abdominal infections. The resistance of Bacteroides fragilis is mainly correlated with nim genes in the chromosome or plasmid and multi-drug efflux pumps [65].
A complex mechanism of resistance is bacteria-forming biofilms. For example, Pseudomonas aeruginosa is a dangerous pathogen that manifests adaptive antibiotic resistance in addition to its existing resistance mechanisms such as efflux systems, antibiotic-inactivating enzymes, and decreased outer membrane permeability. Adaptive resistance is a response to environmental conditions, and it consists in forming a biofilm and existing in the form of persisting cells that tolerate the antibiotic. The biofilm is an aggregate of bacteria in a polymeric material. Living bacteria in the biofilm are more resistant to antibiotics due to the decreased permeability. In addition, the persisting cells in the biofilm are incapable of replicating in the presence of the antibiotic. Moreover, when the antibiotic is no longer present, they repopulate the biofilm and are responsible for the reactivation of chronic infections [66]. In this regard, some ciprofloxacin-nitroxide hybrids synthesized by Verderosa, A.D. et al. (2017) demonstrated the potential to overcome the resistance of biofilms to antimicrobials in two ways: stimulation of biofilm dispersal or direct cell killing [67].
On the other hand, the persistence of antibiotics is less understood nowadays. Eisenreich W. et al. (2022) addressed this phenomenon in a recently published review article. They proposed a new theory related to the persistence state of bacteria. So, in this state, bacteria become more susceptible to mutation-based antibiotic resistance [68].
6. Resistance to FQNs
There are a few reasons why bacterial resistance to FQNs develops. The dose and duration of administration of the drug are two essential factors. In addition, repetitive exposure and administration of low doses of FQN can enhance bacterial resistance, causing multiple mutations. Therefore, a critical aspect of avoiding bacterial resistance is maintaining a proper schedule of drug administration to ensure that the serum concentrations of FQN are higher than the minimum inhibitory concentration (MIC). Additionally, repeated use of the same agent should be avoided [69].
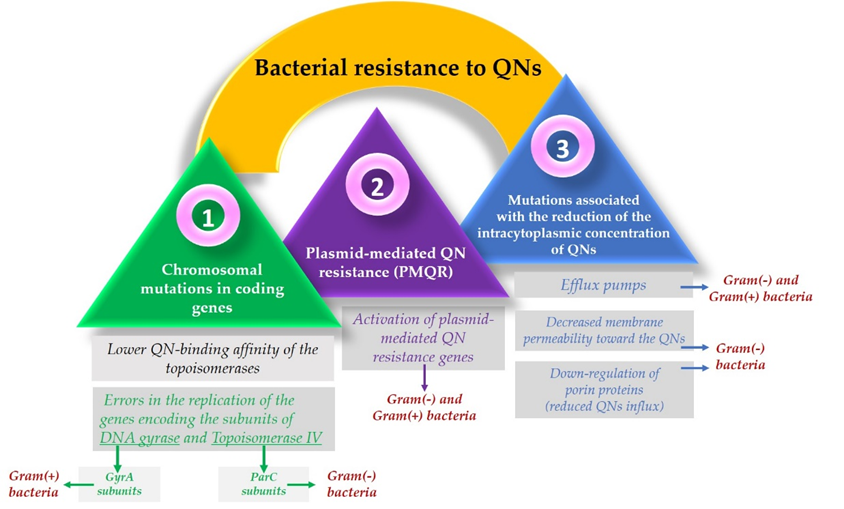
Resistance to FQNs occurs because bacteria use multiple mechanisms to adapt and survive when interacting with the drug [70][71]. One of the most used resistance mechanisms is the mutation of the genes that encode the type II Topoisomerases. This mechanism focuses on the alteration of the target site known as the quinolone resistance-determining region (QRDR) [72] and leads to a lower quinolone-binding affinity of the Topoisomerase enzymes [73]. Usually, concerning Gram-negative bacteria, FQNs affect the gyrase while in Gram-positive bacteria, FQNs target the Topoisomerase IV [69]. These mutations allow the bacteria to adapt after contact with the FQN [25]. So, as a result, it is considered that in Gram-negative bacteria, resistance occurs due to alterations in the DNA gyrase. In contrast, in Gram-positive bacteria, it is due to Topoisomerase IV mutations [74].
As DNA gyrase and Topoisomerase IV are cytoplasmic enzymes, achieving low cytoplasmic FQN concentrations is considered another bacteria solution that confers resistance [15]. Another mechanism includes mutations that reduce drug accumulation [69], such as downregulation of chromosome-encoded porins or increased drug elimination, by multi-drug efflux pumps [73]. The maintenance of low concentrations of FQN in the bacterial cells of Gram-positive bacteria results from the action of three efflux pumps, members of the major facilitator superfamily (MFS) of transporters [75]. One of them (NorA) is involved in the resistance development of hydrophilic FQN (e.g., norfloxacin). At the same time, the other two (NorB and NorC) are responsible for the resistance to hydrophilic and hydrophobic QNs (e.g., moxifloxacin, sparfloxacin). The efflux pumps are also present in Gram-negative bacteria, part of the transporters’ resistance nodulation-division (RND) superfamily [15].
However, unlike Gram-positive bacteria, where resistance results from active efflux transporters [76], Gram-negative bacteria have a structural advantage conferred by their double-membrane structure. The cell wall of Gram-negative bacteria acts as a barrier for hydrophilic molecules since the ability to infiltrate through the outer membrane is conditioned by the presence of porin proteins. Mutations that result in the downregulation of these proteins reduce cellular FQN accumulation as a consequence, especially that of the hydrophilic molecules [69][72][74].
Resistance mechanisms can also be encoded in mobile genes called plasmids [69], known as plasmid-mediated quinolone resistance (PMQR) genes [77]. Some of them encode transporters that can export drugs such as FQNs. Plasmids’ efflux pumps are essential in supporting the resistance to FQNs because they can remove the drug from the bacterial cell [72]. Additionally, to protect the bacterial cell from the FQN effect, they can encode topoisomerase-binding proteins or a modified enzyme that decreases FQN activity [73].
The activity of older FQNs has been studied to enhance the properties of new compounds regarding the installation of bacterial resistance. It was concluded that with newer FQNs, the bacterial resistance installs less rapidly because of their dual activity against DNA gyrase and Topoisomerase IV [72][78]. Furthermore, since both targets are equally affected, it would be less likely to elicit mutational resistance [69].
Specific structural changes to FQNs have been made to achieve this more complex targeting. This is the example of some fourth-generation representatives of FQNs; they are the result of improving the old FQN’s structure by adding a methoxy radical at the C8 position. This structural change can be found in moxifloxacin and gatifloxacin. In addition to this modification, gatifloxacin has a methyl group on the piperazinyl ring and moxifloxacin has a bicyclic ring in position C7. These structural changes were thought to be responsible for the mechanism of action targeting both DNA gyrase and Topoisomerase IV in Gram-positive bacteria. However, the exact reason these compounds act like this is still unclear. Initially, it was considered that their C8 methoxy group was the trigger for this action. Moreover, it was concluded that this type of targeting was not just the result of the methoxy group because delafloxacin, another FQN, does not possess this radical and is also responsible for the exact targeting [25][74]. Furthermore, delafloxacin is a more acidic FQN and is consequently more susceptible to deprotonation at a neutral pH. Therefore, as a consequence, delafloxacin shows an improved cellular uptake in acidic conditions [25]. The mechanisms involved in the development of bacterial resistance to QNs are illustrated in Figure 2.
References
- Abushaheen, M.A.; Muzaheed; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; et al. Antimicrobial Resistance, Mechanisms and Its Clinical Significance. Dis. Mon. 2020, 66, 100971.
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 481–511.
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic Resistance. J. Infect. Public Health 2017, 10, 369–378.
- Antimicrobial Resistance. Available online: https://www.who.int/en/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 16 June 2021).
- Antimicrobial Resistance Information from FDA. Available online: https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/antimicrobial-resistance-information-fda (accessed on 21 July 2021).
- Marston, H.D.; Dixon, D.M.; Knisely, J.M.; Palmore, T.N.; Fauci, A.S. Antimicrobial Resistance. JAMA 2016, 316, 1193–1204.
- Mayers, D.L.; Sobel, J.D.; Ouellette, M.; Kaye, K.S.; Marchaim, D. (Eds.) Antimicrobial Drug Resistance: Clinical and Epidemiological Aspects, 2nd ed.; Springer International Publishing: Berlin, Germany, 2017; Volume 2, ISBN 978-3-319-47264-5.
- Petchiappan, A.; Chatterji, D. Antibiotic Resistance: Current Perspectives. ACS Omega 2017, 2, 7400–7409.
- Schaenzer, A.J.; Wright, G.D. Antibiotic Resistance by Enzymatic Modification of Antibiotic Targets. Trends Mol. Med. 2020, 26, 768–782.
- McClelland, S.; Lamoureux, B.; Larson, E. Trends in Antimicrobial Resistance Legislation 2011-2019: A Review of the US Policy Response to the Antimicrobial Resistance Threat and Its Public Health Impact. Am. J. Infect. Control 2021, 49, 813–817.
- Bulteel, A.J.B.; Larson, E.L.; Getahun, H. Identifying Global Research Gaps to Mitigate Antimicrobial Resistance: A Scoping Review. Am. J. Infect. Control 2021, 49, 818–824.
- Hershberg, R. Antibiotic-Independent Adaptive Effects of Antibiotic Resistance Mutations. Trends Genet. 2017, 33, 521–528.
- Knöppel, A.; Näsvall, J.; Andersson, D.I. Evolution of Antibiotic Resistance without Antibiotic Exposure. Antimicrob. Agents Chemother. 2017, 61, e01495-17.
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 3 July 2021).
- Hooper, D.C.; Jacoby, G.A. Mechanisms of Drug Resistance: Quinolone Resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31.
- Rusu, A.; Lungu, I.-A.; Moldovan, O.-L.; Tanase, C.; Hancu, G. Structural Characterization of the Millennial Antibacterial (Fluoro)Quinolones—Shaping the Fifth Generation. Pharmaceutics 2021, 13, 1289.
- Owens, R.C.; Ambrose, P.G. Clinical use of the fluoroquinolones. Med. Clin. N. Am. 2000, 84, 1447–1469.
- Kim, E.S.; Hooper, D.C. Clinical Importance and Epidemiology of Quinolone Resistance. Infect. Chemother. 2014, 46, 226–238.
- Watanabe, T. Infective heredity of multiple drug resistance in bacteria. Bacteriol. Rev. 1963, 27, 87–115.
- Wax, R.G.; Lewis, K.; Salyers, A.A.; Taber, H. Bacterial Resistance to Antimicrobials; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-1-4200-0875-3.
- Podolsky, S.H. The Evolving Response to Antibiotic Resistance (1945–2018). Palgrave Commun. 2018, 4, 1–8.
- Antibiotic Resistance Threats in the United States (Ar-Threats-2013-508.Pdf) 2013. Available online: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on 4 July 2021).
- Centers for Disease Control and Prevention (U.S.). Antibiotic Resistance Threats in the United States, 2019; Centers for Disease Control and Prevention (U.S.): Atlanta, GA, USA, 2019.
- Johnson, A.P. Surveillance of Antibiotic Resistance. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140080.
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662.
- Dalhoff, A. Global Fluoroquinolone Resistance Epidemiology and Implictions for Clinical Use. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 976273.
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Med. Chem. 2014, 6, 25–64.
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. MMBR 2010, 74, 417–433.
- Livermore, D.M.; Hope, R.; Reynolds, R.; Blackburn, R.; Johnson, A.P.; Woodford, N. Declining Cephalosporin and Fluoroquinolone Non-Susceptibility among Bloodstream Enterobacteriaceae from the UK: Links to Prescribing Change? J. Antimicrob. Chemother. 2013, 68, 2667–2674.
- Spellberg, B.; Doi, Y. The Rise of Fluoroquinolone-Resistant Escherichia Coli in the Community: Scarier Than We Thought. J. Infect. Dis. 2015, 212, 1853–1855.
- Carlet, J. World alliance against antibiotic resistance: The WAAAR declaration against antibiotic resistance. Med. Intensiva 2015, 39, 34–39.
- Fernandes, P.; Martens, E. Antibiotics in Late Clinical Development. Biochem. Pharmacol. 2017, 133, 152–163.
- TYGACIL® (Tigecycline) for Injection for Intravenous Use-Prescribing Information (021821s021lbl.Pdf). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021821s021lbl.pdf (accessed on 4 July 2021).
- Wang, L.; Liu, D.; Lv, Y.; Cui, L.; Li, Y.; Li, T.; Song, H.; Hao, Y.; Shen, J.; Wang, Y.; et al. Novel Plasmid-Mediated Tet(X5) Gene Conferring Resistance to Tigecycline, Eravacycline, and Omadacycline in a Clinical Acinetobacter Baumannii Isolate. Antimicrob. Agents Chemother. 2019, 64, e01326-19.
- ZERBAXA® (Ceftolozane and Tazobactam) for Injection, for Intravenous Use-Prescribing Information (Zerbaxa_pi.Pdf). Available online: https://www.merck.com/product/usa/pi_circulars/z/zerbaxa/zerbaxa_pi.pdf (accessed on 4 July 2021).
- Wi, Y.M.; Greenwood-Quaintance, K.E.; Schuetz, A.N.; Ko, K.S.; Peck, K.R.; Song, J.-H.; Patel, R. Activity of Ceftolozane-Tazobactam against Carbapenem-Resistant, Non-Carbapenemase-Producing Pseudomonas Aeruginosa and Associated Resistance Mechanisms. Antimicrob. Agents Chemother. 2017, 62, e01970-17.
- AVYCAZ (Ceftazidime and Avibactam) for Injection, for Intravenous Use-Prescribing Information (Avycaz_Final_PI_CBE-0_10_2019.Pdf). Available online: https://media.allergan.com/actavis/actavis/media/allergan-pdf-documents/product-prescribing/Avycaz_Final_PI_CBE-0_10_2019.pdf (accessed on 4 July 2021).
- Wang, Y.; Wang, J.; Wang, R.; Cai, Y. Resistance to Ceftazidime–Avibactam and Underlying Mechanisms. J. Glob. Antimicrob. Resist. 2020, 22, 18–27.
- Wise, M.G.; Estabrook, M.A.; Sahm, D.F.; Stone, G.G.; Kazmierczak, K.M. Prevalence of Mcr-Type Genes among Colistin-Resistant Enterobacteriaceae Collected in 2014-2016 as Part of the INFORM Global Surveillance Program. PLoS ONE 2018, 13, e0195281.
- de Jonge, B.L.M.; Karlowsky, J.A.; Kazmierczak, K.M.; Biedenbach, D.J.; Sahm, D.F.; Nichols, W.W. In Vitro Susceptibility to Ceftazidime-Avibactam of Carbapenem-Nonsusceptible Enterobacteriaceae Isolates Collected during the INFORM Global Surveillance Study (2012 to 2014). Antimicrob. Agents Chemother. 2016, 60, 3163–3169.
- Kazmierczak, K.M.; Bradford, P.A.; Stone, G.G.; de Jonge, B.L.M.; Sahm, D.F. In Vitro Activity of Ceftazidime-Avibactam and Aztreonam-Avibactam against OXA-48-Carrying Enterobacteriaceae Isolated as Part of the International Network for Optimal Resistance Monitoring (INFORM) Global Surveillance Program from 2012 to 2015. Antimicrob. Agents Chemother. 2018, 62, e00592-18.
- Kazmierczak, K.M.; de Jonge, B.L.M.; Stone, G.G.; Sahm, D.F. In Vitro Activity of Ceftazidime/Avibactam against Isolates of Enterobacteriaceae Collected in European Countries: INFORM Global Surveillance 2012-15. J. Antimicrob. Chemother. 2018, 73, 2782–2788.
- Sader, H.S.; Castanheira, M.; Shortridge, D.; Mendes, R.E.; Flamm, R.K. Antimicrobial Activity of Ceftazidime-Avibactam Tested against Multidrug-Resistant Enterobacteriaceae and Pseudomonas Aeruginosa Isolates from U.S. Medical Centers, 2013 to 2016. Antimicrob. Agents Chemother. 2017, 61, e01045-17.
- Sader, H.S.; Castanheira, M.; Mendes, R.E.; Flamm, R.K. Frequency and Antimicrobial Susceptibility of Gram-Negative Bacteria Isolated from Patients with Pneumonia Hospitalized in ICUs of US Medical Centres (2015–17). J. Antimicrob. Chemother. 2018, 73, 3053–3059.
- Senchyna, F.; Gaur, R.L.; Sandlund, J.; Truong, C.; Tremintin, G.; Kültz, D.; Gomez, C.A.; Tamburini, F.B.; Andermann, T.; Bhatt, A.; et al. Diversity of Resistance Mechanisms in Carbapenem-Resistant Enterobacteriaceae at a Health Care System in Northern California, from 2013 to 2016. Diagn. Microbiol. Infect. Dis. 2019, 93, 250–257.
- Yin, D.; Wu, S.; Yang, Y.; Shi, Q.; Dong, D.; Zhu, D.; Hu, F. China Antimicrobial Surveillance Network (CHINET) Study Group Results from the China Antimicrobial Surveillance Network (CHINET) in 2017 of the In Vitro Activities of Ceftazidime-Avibactam and Ceftolozane-Tazobactam against Clinical Isolates of Enterobacteriaceae and Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2019, 63, e02431-18.
- Karlowsky, J.A.; Kazmierczak, K.M.; Bouchillon, S.K.; de Jonge, B.L.M.; Stone, G.G.; Sahm, D.F. In Vitro Activity of Ceftazidime-Avibactam against Clinical Isolates of Enterobacteriaceae and Pseudomonas Aeruginosa Collected in Asia-Pacific Countries: Results from the INFORM Global Surveillance Program, 2012 to 2015. Antimicrob. Agents Chemother. 2018, 62, e02569-17.
- Hackel, M.; Kazmierczak, K.M.; Hoban, D.J.; Biedenbach, D.J.; Bouchillon, S.K.; de Jonge, B.L.M.; Stone, G.G. Assessment of the In Vitro Activity of Ceftazidime-Avibactam against Multidrug-Resistant Klebsiella Spp. Collected in the INFORM Global Surveillance Study, 2012 to 2014. Antimicrob. Agents Chemother. 2016, 60, 4677–4683.
- Flamm, R.K.; Nichols, W.W.; Sader, H.S.; Farrell, D.J.; Jones, R.N. In Vitro Activity of Ceftazidime/Avibactam against Gram-Negative Pathogens Isolated from Pneumonia in Hospitalised Patients, Including Ventilated Patients. Int. J. Antimicrob. Agents 2016, 47, 235–242.
- Wilson, W.R.; Kline, E.G.; Jones, C.E.; Morder, K.T.; Mettus, R.T.; Doi, Y.; Nguyen, M.H.; Clancy, C.J.; Shields, R.K. Effects of KPC Variant and Porin Genotype on the In Vitro Activity of Meropenem-Vaborbactam against Carbapenem-Resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2019, 63, e02048-18.
- Gonzalez, M.D.; McMullen, A.R.; Wallace, M.A.; Crotty, M.P.; Ritchie, D.J.; Burnham, C.A.D. Susceptibility of Ceftolozane-Tazobactam and Ceftazidime-Avibactam Against a Collection of β-Lactam-Resistant Gram-Negative Bacteria. Ann. Lab. Med. 2017, 37, 174–176.
- Kazmierczak, K.M.; de Jonge, B.L.M.; Stone, G.G.; Sahm, D.F. In Vitro Activity of Ceftazidime/Avibactam against Isolates of Pseudomonas Aeruginosa Collected in European Countries: INFORM Global Surveillance 2012-15. J. Antimicrob. Chemother. 2018, 73, 2777–2781.
- Sader, H.S.; Castanheira, M.; Flamm, R.K.; Mendes, R.E.; Farrell, D.J.; Jones, R.N. Ceftazidime/Avibactam Tested against Gram-Negative Bacteria from Intensive Care Unit (ICU) and Non-ICU Patients, Including Those with Ventilator-Associated Pneumonia. Int. J. Antimicrob. Agents 2015, 46, 53–59.
- Hachem, R.; Reitzel, R.; Rolston, K.; Chaftari, A.-M.; Raad, I. Antimicrobial Activities of Ceftazidime-Avibactam and Comparator Agents against Clinical Bacteria Isolated from Patients with Cancer. Antimicrob. Agents Chemother. 2017, 61, e02106-16.
- FETROJA (Cefiderocol) for Injection, for Intravenous Use-Prescribing Information (Fetroja.Pdf). Available online: https://www.shionogi.com/content/dam/shionogi/si/products/pdf/fetroja.pdf (accessed on 4 July 2021).
- Malik, S.; Kaminski, M.; Landman, D.; Quale, J. Cefiderocol Resistance in Acinetobacter Baumannii: Roles of β-Lactamases, Siderophore Receptors, and Penicillin Binding Protein 3. Antimicrob. Agents Chemother. 2020, 64, e01221-20.
- Ventola, C.L. The Antibiotic Resistance Crisis. Pharm. Ther. 2015, 40, 277–283.
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global Contributors to Antibiotic Resistance. J. Glob. Infect. Dis. 2019, 11, 36–42.
- Livermore, D.M. Microbiology Society. 8 November 2016. Available online: https://microbiologysociety.org/ (accessed on 26 July 2022).
- Nisnevitch, M. Antibiotic Resistance and Antibiotic Alternatives: Looking towards the Future. Sci. Prog. 2016, 99, 92–96.
- Skandalis, N.; Maeusli, M.; Papafotis, D.; Miller, S.; Lee, B.; Theologidis, I.; Luna, B. Environmental Spread of Antibiotic Resistance. Antibiotics 2021, 10, 640.
- Cunha, C.B.; Opal, S.M. Antibiotic Stewardship: Strategies to Minimize Antibiotic Resistance While Maximizing Antibiotic Effectiveness. Med. Clin. N. Am. 2018, 102, 831–843.
- Alós, J.-I. Resistencia bacteriana a los antibióticos: Una crisis global. Enferm. Infecc. Microbiol. Clínica 2015, 33, 692–699.
- Bhardwaj, S.; Mehra, P.; Dhanjal, D.S.; Sharma, P.; Sharma, V.; Singh, R.; Nepovimova, E.; Chopra, C.; Kuča, K. Antibiotics and Antibiotic Resistance-Flipsides of the Same Coin. Curr. Pharm. Des. 2022.
- Ghotaslou, R.; Bannazadeh Baghi, H.; Alizadeh, N.; Yekani, M.; Arbabi, S.; Memar, M.Y. Mechanisms of Bacteroides Fragilis Resistance to Metronidazole. Infect. Genet. Evol. 2018, 64, 156–163.
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic Resistance in Pseudomonas Aeruginosa: Mechanisms and Alternative Therapeutic Strategies. Biotechnol. Adv. 2019, 37, 177–192.
- Verderosa, A.D.; de la Fuente-Núñez, C.; Mansour, S.C.; Cao, J.; Lu, T.K.; Hancock, R.E.W.; Fairfull-Smith, K.E. Ciprofloxacin-Nitroxide Hybrids with Potential for Biofilm Control. Eur. J. Med. Chem. 2017, 138, 590–601.
- Eisenreich, W.; Rudel, T.; Heesemann, J.; Goebel, W. Link Between Antibiotic Persistence and Antibiotic Resistance in Bacterial Pathogens. Front. Cell. Infect. Microbiol. 2022, 12, 900848.
- Jacoby, G.A. Mechanisms of Resistance to Quinolones. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005, 41 (Suppl. 2), S120–S126.
- Zhang, Y.; Li, X.; Mi, K. Mechanisms of Fluoroquinolone Resistance in Mycobacterium Tuberculosis. Yi Chuan Hered. 2016, 38, 918–927.
- Sandra Georgina Solano-Gálvez Mechanisms of Resistance to Quinolones. In Antimicrobial Resistance; Valencia-Segrove, M.F. (Ed.) IntechOpen: Rijeka, Croatia, 2020; p. Ch. 2. ISBN 978-1-83962-433-9.
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J.V. Fluoroquinolone Resistance: Mechanisms, Impact on Bacteria, and Role in Evolutionary Success. Trends Microbiol. 2014, 22, 438–445.
- Cuypers, W.L.; Jacobs, J.; Wong, V.; Klemm, E.J.; Deborggraeve, S.; Van Puyvelde, S. Fluoroquinolone Resistance in Salmonella: Insights by Whole-Genome Sequencing. Microb. Genom. 2018, 4, e000195.
- Roychoudhury, S.; Makin, K.; Twinem, T.; Leunk, R.; Hsu, M.C. In Vitro Resistance Development to Nemonoxacin in Streptococcus Pneumoniae: A Unique Profile for a Novel Nonfluorinated Quinolone. Microb. Drug Resist. 2016, 22, 578–584.
- Phillips-Jones, M.K.; Harding, S.E. Antimicrobial Resistance (AMR) Nanomachines-Mechanisms for Fluoroquinolone and Glycopeptide Recognition, Efflux and/or Deactivation. Biophys. Rev. 2018, 10, 347–362.
- Hooper, D.C.; Jacoby, G.A. Topoisomerase Inhibitors: Fluoroquinolone Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025320.
- Wei, M.; Tu, N.; Yang, K. Resistance Mechanism of Carbapenem-Resistant Enterobacteriaceae to Quinolones. Clin. Lab. 2021, 67.
- Emmerson, A.M.; Jones, A.M. The Quinolones: Decades of Development and Use. J. Antimicrob. Chemother. 2003, 51 (Suppl. 1), 13–20.
- Correia, S.; Poeta, P.; Hébraud, M.; Capelo, J.L.; Igrejas, G. Mechanisms of Quinolone Action and Resistance: Where Do We Stand? J. Med. Microbiol. 2017, 66, 551–559.
More