Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Gao Zhang.
Heat shock protein (HSP90), a highly conserved molecular chaperon, is indispensable for the maturation of newly synthesized poly-peptides and provides a shelter for the turnover of misfolded or denatured proteins.
- heat shock protein 90
- INHIBITORS
- cancer therapeutics
1. Introduction
The efficient and accurate control of the cellular protein pool is crucial for homeostasis within the crowded environment of a single cell [1]. HSP90 is one of the heat shock protein members. When functionally upregulated under environmental stress, HSP90 protects cells from detrimental effects [1]. HSP90 is evolutionarily conserved and ubiquitously expressed across species, which accounts for 1–2% of total cellular proteins in the unstressed condition [2]. It can be further increased to about 4–6% in the stressed condition [3]. Due to its abundance and adhesive properties, HSP90 has been likened to “molecular glue” [4]. The structure of HSP90 comprises three domains: the N-terminal domain (NTD) with ATPase activity, the middle domain (MD) that binds to the client protein, and the primary dimerization C-terminal domain (CTD) [1,5][1][5]. HSP90 utilizes ATP to keep its “closed” conformation for the binding of client proteins. The immature client proteins proceed to fold, accompanied by ATP hydrolyzation and energy release. After that, HSP90 releases the matured product and is transformed into an “open” conformation. Co-chaperones are also required for the intricate control of ATP hydrolysis rates and the certain conformational states [1,6,7][1][6][7].
Over 20 co-chaperones of HSP90 have been documented in eukaryotic cells, which modulate the molecular functions of HSP90 in four major ways: (1) coordinate the interplay between HSP90 and other chaperone systems, such as HSP70; (2) stimulate or inhibit the ATPase activity of HSP90, i.e., AHA1 for stimulation and CDC37 for inhibition; (3) recruit specific classes of clients to HSP90; (4) contribute to various aspects of the chaperone cycle through their enzymatic activities (Figure 1). The co-chaperones containing the TPR domain (i.e., HOP) facilitate the cooperative and successive action of the HSP90-HSP70-HSP40 complex to achieve the maturation of client proteins. Separately, the co-chaperones that inhibit the ATPase activity are more likely to be involved in client loading or the formation of mature HSP90 complexes, whereas those that enhance the ATPase activity are regarded as activators of the HSP90 conformational cycle [1].
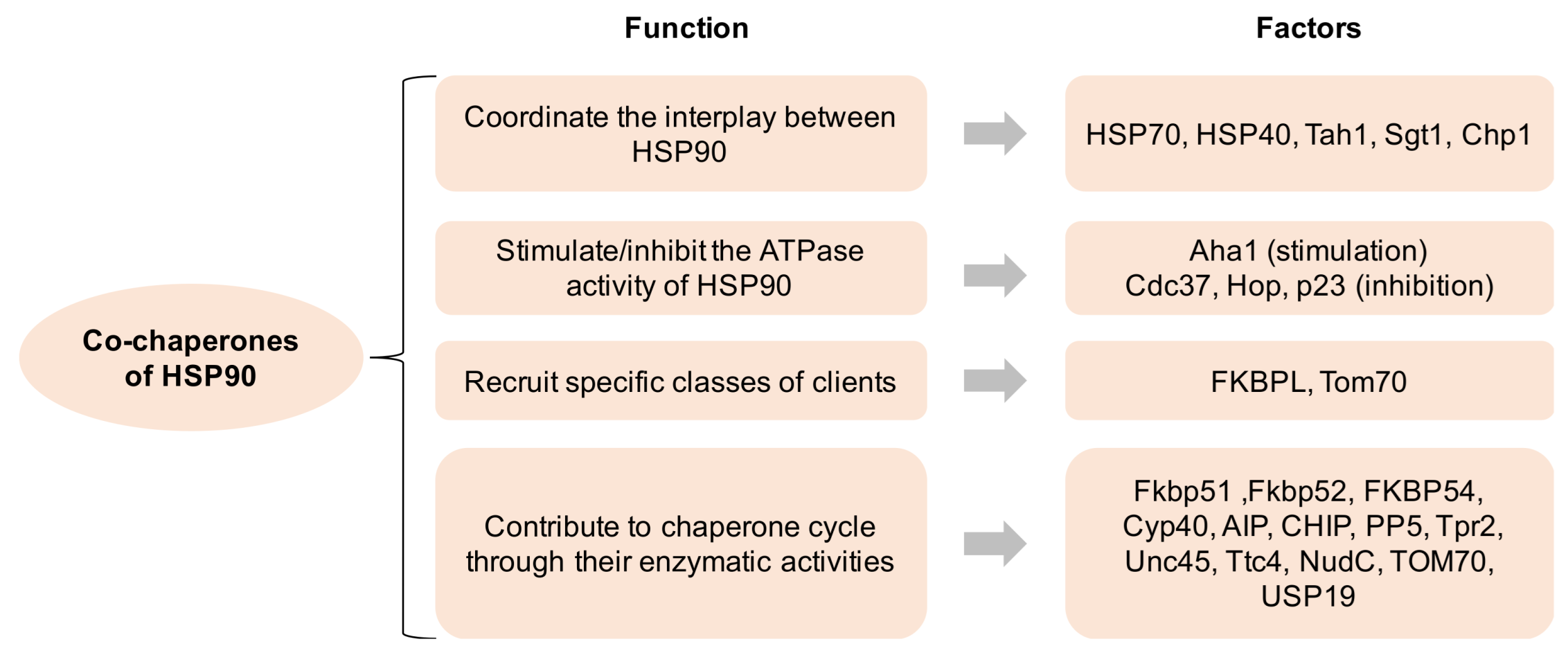
Figure 1. Co-chaperones of HSP90 and its function. HSP90 comprises four isoforms: (1) HSP90α (an inducible form) and HSP90β (a constitutive form) are mainly located in the cellular cytosol; (2) the glucose-regulated protein (GRP94) is localized in the endoplasmic reticulum; (3) Hsp75/tumor necrosis factor receptor associated protein 1 (TRAP-1, also known as mitochondrial HSP90) is located on the mitochondria [7,8][7][8]. All HSP90 isoforms play critical roles in cancer, neurodegenerative disorders, and other disease states, indicating that their pharmacological targeting may have profound implications for the treatment of these illnesses [9].
2. Subcellular Localization of HSP90
HSP90 is a highly conserved molecular chaperone on the evolutionary level and is constitutively expressed in most organs and tissues [20][10]. HSP90 assists in the proper folding, intracellular disposition and proteolytic turnover of many key regulators of cellular homeostasis with its ATPase activity [12,21,22,23][11][12][13][14]. HSP90 is mainly located in cytoplasm under normal conditions, where polypeptides are synthesized, and aberrantly folded proteins are produced. By using an in vivo PET tracer or in vitro organelle fractionation assay, HSP90 was found to be expressed ‘everywhere’, including the nucleus, mitochondrion, and plasma membrane, as well as also being secreted into the extracellular matrix [24,25,26][15][16][17]. The differential expression of sub-cellular HSP90 may exert distinctive functions in multiple biological processes, especially tumorigenesis [25][16]. Interestingly, the differential expression pattern of HSP90 between normal cells and cancer cells has already been documented, with the discrepancies selectively existing in the mitochondria or extracellular matrix [26,27][17][18].
2.1. Cytosolic Localization of HSP90
HSP90, as a critical homodimer chaperone machinery, was expressed within the cytosol component [28][19]. By constructing various truncated forms of HSP90 and using a confocal microscopy, Passinen S. et al., found that the C-terminal half of HSP90 (amino acids between 333 and 664) is responsible for the cytoplasmic localization [22][13]. Nonetheless, by fusing with the nuclear localization signal sequence (NLS), NLS-HSP90 was preferentially expressed in the nucleus [22,28][13][19].
Previous studies have focused on the discrepancy of HSP90 expression between normal cells and cancer cells and demonstrated that a higher protein level of HSP90 is expressed in the latter. In these studies, the majority of HSP90 was also localized in the cytoplasm [23,27][14][18]. Perhaps the cytosolic HSP90 largely contributes to the folding/refolding of critical oncogenic drivers and pro-survival regulators by supplying a co-chaperone buffering system. Additionally, cytosolic HSP90 has also been termed “molecular glue”, highlighting its abundance and buffering properties [4]. Most of the client proteins of HSP90 are located in the cytoplasm, exerting a variety of functions, including signaling transduction, post-transcriptional modification, metabolic rewiring and cytoskeleton remodeling. Recently, HSP90 was found to transiently crosslink actin filaments in vitro, and this dynamic interaction between HSP90 and almost all cytoplasmic filamentous structures highlights its role in modulating actin filament bundling behavior [4,29][4][20]. HSP90 was also associated with tubulin and probably protects it from heat denaturation [30][21]. Likewise, it was shown that HSP90 protects myosin from heat stress [31][22]. Therefore, HSP90 is functionally involved in the management of the cytoskeleton.
2.2. Nuclear Localization of HSP90
A low expression of HSP90 (5–10% of total cellular HSP90) was detected in the nucleus of normal cells [32][23]. Recent data, however, have shown that the elevated expression of nuclear HSP90 could be detected in breast cancer and non-small cell lung cancer (NSCLC) [33,34,35][24][25][26]. For example, a nuclear accumulation of heat shock protein 90 might predict the poor survival of patients with NSCLC. Furthermore, the nuclear staining of HSP90 was also positively correlated with the age and smoking status of patients with NSCLC [34][25]. Interestingly, in quiescent Saccharomyces cerevisiae cells, Hsp90 and its co-chaperones were found to accumulate in the nucleus with the requirement of the α/β importin system, which was enhanced during periods of relative metabolic inactivity [36][27]. In fact, the HSP90 protein comprises sequences that are homologous to the recognized traditional or alternative nuclear import and export signals: the nuclear localization sequence [21][12].
Importantly, HSP90 is associated with multiple nuclear chaperone clients including nucleic acid, histone, transcription factors, and epigenetic regulators [4]. HSP90 also modulates multiple biological functions in the nucleus including RNA synthesis, processing, and multiple telomerase activities [4,37][4][28]. The nuclear translocation of HSP90 is governed by FKBP52, steroid receptors, and kinases [21,38][12][29]. Zinc finger proteins, helix-loop-helix proteins, MyoD1, E12, HIF1α, HSF1, and glucocorticoid receptor interact with HSP90 [4,39,40][4][30][31]. The transfection of 3T3 cells with HSP90 fused with EGFP revealed that HSP90 was located in the nuclear membrane upon exposure to an elevated temperature [41][32]. Many small chemical molecules enter the cytoplasm through penetrating the plasma membrane, but it was shown that none of them could enter the nucleus, rendering their efficacy limited [42][33]. However, HSP90 could be translocated from cytoplasm to nucleus, which protects cancer cells from therapeutic pressure [43][34]. This suggests that nuclear-directed HSP90 inhibitors should be taken into consideration.
2.3. Mitochondrial Localization of HSP90
The mitochondrial expression of HSP90 was unexplored until Kang B. et al., first demonstrated the high expression of HSP90 in tumoral mitochondrion, but barely in normal tissues [26][17]. Based on this ground-breaking work, substantial research took place, which focused on the development of small molecular drugs with the organelle-specific targeting of HSP90. Collectively, the goal of these inhibitors is to trigger a sudden collapse of mitochondrial integrity and apoptosis that would selectively occur in tumor cells. Several inhibitors that specifically target mitochondrion have emerged and shown intriguing effects in multiple cancer types, including pancreatic cancer, breast cancer, colon cancer, NSCLC, melanoma, glioblastoma, prostate cancer, lymphoma, and leukemia (Table 1) [26,44,45,46,47,48,49,50,51][17][35][36][37][38][39][40][41][42]. By investigating the metabolic network in the tumoral mitochondrion regulated by HSP90, Chae Y. et al., highlighted that mitochondrial HSP90 (hereafter mtHSP90), but not cytosolic HSP90, binds and stabilizes the electron transport chain complex II subunit succinate dehydrogenase-B (SDHB), which maintains cellular respiration under low-nutrient conditions and contributes to HIF1α-mediated tumorigenesis in patients carrying SDHB mutations [52][43]. Cryo-EM data also confirmed the dynamic interplay between mitochondrial HSP90 and SDHB folding intermediates [53][44].
Table 1.
HSP90 clients are associated with hallmarks of cancer.
| Phenotype | Clients | References |
|---|---|---|
| Uncontrolled proliferation | EGFR, HER2, RAF1, CDK4, Akt, BCR-ABL, v-Src, c-Src, FAK, CKII, CHK1, eIF-2α kinase | [6,16,59,60,61,62][6][17,45][54,46][55,4756,[48][4957,]58,][50][51][52][53][54][55] |
| Anti-apoptosis | p53, Akt, Survivin, IKK, NF-κB, PLK, WEE1, Myc, CDK4, CDK6 | [6,42,55,63,64,65,66,67,68][6][33][48][56][57][58][59][60][61] |
| Angiogenesis | HIF1α, Akt, EGFR, HRE2, FLT3, VEGFR2 | [19,69,[70,63][71,64][72,73][62]65][66][67] |
| Immortalization | Telomerase | [18][68] |
| Invasion/Metastasis | MMP2, MMP9, c-MET | [19,34,74,75][25][62][69][70] |
| Others | Glucocorticoid receptor, Mineralocorticoid receptor, Progesterone receptor, Estrogen receptor, Androgen receptor, Oestrogen receptor, Nitric oxide synthase, Centrin/centrosome, Calmodulin, MDM2, UHRF1, BRCA2, OCT4, Nanog, STAT3, Calcineurin, CFTR, NLR proteins, RAD51/RAD52, Tau, HCK, JAK1 and/or JAK2 | [76,94][7177,]78,79,[72]80,[81,82,73][74][75][76][7783,84,85,86,87,88,][7889,][7990,][80][81][91,82][84][85]92,93,][83[86][87][88][89] |
It is worth mentioning that TRAP1 has been regarded as another version of HSP90—namely, mitochondrial HSP90—because it shares 60% of its sequence with HSP90 and contains the same domains: NTD, ND, and CTD [32,95][23][90]. Interestingly, Kang B. et al., also established that TRAP1 was consistently elevated in primary tumors, whereas it was nearly undetectable in normal tissues [26][17]. Moreover, one of the important client proteins of TRAP1 was cyclophilin D, a mitochondrial residential protein, which maintains mitochondrial integrity by preserving cells from apoptosis. Furthermore, the crystal structures of HSP90 and TRAP1 provide further molecular insights that can be exploited for the development of novel inhibitors [96,97][91][92]. Taken together, TARP1 is another attractive target for developing cancer drugs [98][93].
2.4. Membrane and Extracellular Localization of HSP90
HSP90 was first viewed as an artifact when it was found on the cell surface by a functional screening, due to its abundant expression within the cells. After cautious verification, HSP90 is no longer considered as being exclusively located within the cell [75][70]. HSP90 can also be secreted into the extracellular matrix. Accordingly, the cell surface expression of HSP90 is higher on cancer cells than that on normal cells, which correlates with the malignant stage of the tumor. Previous work has also shown that the cell surface of HSP90 could strengthen the migration potential of cancer cells that is distinct from the function of the intracellular HSP90 pool [99,100][94][95]. Thus, the cell surface of HSP90 is also an attractive therapeutic target in terms of inhibiting tumor invasion and metastasis [75,101][70][96].
The first evidence for the detection of HSP90 in the extracellular matrix was implicated in 1986 when Barrott J. et al., found a mouse tumor-specific antigen that was identified as a heat shock protein, which is now recognized as HSP90 [102][97]. Since the ATP level in the extracellular environment is low due to the lack of energy source, the extracellular HSP90 (hereafter eHSP90) may function independently of ATP. The secretion of eHSP90 is induced by environmental stresses and growth factors [64[57][98],103], and it is affected by post-translational modifications to the chaperone, including phosphorylation and acetylation [104][99]. Recent work has also shown that HSP90α is released by invasive cancer cells via exosomes, which contributes to their invasive nature by interacting with plasmin [69][63]. Another study found that both HSP90α and HSP90β are secreted by cancer cells to interact with MMP2 and MMP9 to enhance the invasive capacity of tumor cells. Similarly, eHSP90 was detected in normal cells only in response to stress, while cancer cells consecutively secrete HSP90 [105,106][100][101]. Interestingly, eHSP90 interacts with a series of receptors such as EGFR/HER2/LPR1 to promote the downstream signal transduction associated with tumor growth and metastasis, which resembles the EMT phenotype [107,108,109][102][103][104]. Further, eHSP90 expression correlates with an increase in metastatic potential and a decrease in the immune response in multiple cancer types [100][95]. Although the specific inhibition of eHSP90 does not affect cancer cell growth in vitro or tumour xenograft progression in vivo [99][94], the inhibition of eHSP90 is effective in conquering metastasis with minor side effects, highlighting the clinical potential of eHSP90 inhibitors [49,101,106,110,111][40][96][101][105][106].
References
- Taipale, M.; Jarosz, D.F.; Lindquist, S. HSP90 at the hub of protein homeostasis: Emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 515–528.
- Lai, B.; Chin, N.; Stanek, A.; Keh, W.; Lanks, K. Quantitation and intracellular localization of the 85K heat shock protein by using monoclonal and polyclonal antibodies. Mol. Cell. Biol. 1984, 4, 2802–2810.
- Borkovich, K.; Farrelly, F.; Finkelstein, D.; Taulien, J.; Lindquist, S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol. Cell. Biol. 1989, 9, 3919–3930.
- Csermely, P.; Schnaider, T.; Soti, C.; Prohaszka, Z.; Nardai, G. The 90-kDa molecular chaperone family: Structure, function, and clinical applications. A comprehensive review. Pharmacol. Ther. 1998, 79, 129–168.
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 2011, 475, 324–332.
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360.
- Buchner, J.; Li, J. Structure, Function and Regulation of the Hsp90 Machinery. Biomed. J. 2013, 36, 106–117.
- Blagg, B.S.J.; Kerr, T.D. Hsp90 inhibitors: Small molecules that transform the Hsp90 protein folding machinery into a catalyst for protein degradation. Med. Res. Rev. 2006, 26, 310–338.
- Sanchez, J.; Carter, T.R.; Cohen, M.S.; Blagg, B.S.J. Old and New Approaches to Target the Hsp90 Chaperone. Curr. Cancer Drug Targets 2020, 20, 253–270.
- Hong, D.S.; Banerji, U.; Tavana, B.; George, G.C.; Aaron, J.; Kurzrock, R. Targeting the molecular chaperone heat shock protein 90 (HSP90): Lessons learned and future directions. Cancer Treat. Rev. 2012, 39, 375–387.
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Cancer 2005, 5, 761–772.
- Nardai, G.; Schnaider, T.; Söti, C.; Ryan, M.; Hoj, P.B.; Somogyi, J.; Csermely, P. Characterization of the 90 kDa heat shock protein (HSP90)-associated ATP/GTPase. J. Biosci. 1996, 21, 179–190.
- Passinen, S.; Valkila, J.; Manninen, T.; Syvälä, H.; Ylikomi, T. The C-terminal half of Hsp90 is responsible for its cytoplasmic localization. JBIC J. Biol. Inorg. Chem. 2001, 268, 5337–5342.
- Young, J.C.; Agashe, V.R.; Siegers, K.; Hartl, F.U. Pathways of chaperone-mediated protein folding in the cytosol. Nat. Rev. Mol. Cell Biol. 2004, 5, 781–791.
- Vermeulen, K.; Naus, E.; Ahamed, M.; Attili, B.; Siemons, M.; Luyten, K.; Celen, S.; Schymkowitz, J.; Rousseau, F.; Bormans, G. Evaluation of NMS-E973 as a PET tracer for in vivo visualisation of HSP90. Theranostics 2019, 9, 554–572.
- Milicevic, Z.; Bogojevic, D.; Mihailovic, M.; Petrovic, M.; Krivokapic, Z. Molecular characterization of hsp90 isoforms in colorectal cancer cells and its association with tumour progression. Int. J. Oncol. 2008, 32, 1169–1178.
- Kang, B.H.; Plescia, J.; Dohi, T.; Rosa, J.; Doxsey, S.J.; Altieri, D.C. Regulation of Tumor Cell Mitochondrial Homeostasis by an Organelle-Specific Hsp90 Chaperone Network. Cell 2007, 131, 257–270.
- Ferrarini, M.; Heltai, S.; Zocchi, M.R.; Rugarli, C. Unusual expression and localization of heat-shock proteins in human tumor cells. Int. J. Cancer 1992, 51, 613–619.
- Kang, K.I.; Devin, J.; Cadepond, F.; Jibard, N.; Guiochon-Mantel, A.; Baulieu, E.E.; Catelli, M.G. In vivo functional protein-protein interaction: Nuclear targeted hsp90 shifts cytoplasmic steroid receptor mutants into the nucleus. Proc. Natl. Acad. Sci. USA 1994, 91, 340–344.
- Koyasu, S.; Nishida, E.; Kadowaki, T.; Matsuzaki, F.; Iida, K.; Harada, F.; Kasuga, M.; Sakai, H.; Yahara, I. Two mammalian heat shock proteins, HSP90 and HSP100, are actin-binding proteins. Proc. Natl. Acad. Sci. USA 1986, 83, 8054–8058.
- Weis, F.; Moullintraffort, L.; Heichette, C.; Chrétien, D.; Garnier, C. The 90-kDa Heat Shock Protein Hsp90 Protects Tubulin against Thermal Denaturation. J. Biol. Chem. 2010, 285, 9525–9534.
- Etard, C.; Roostalu, U.; Strähle, U. Shuttling of the chaperones Unc45b and Hsp90a between the A band and the Z line of the myofibril. J. Cell Biol. 2008, 180, 1163–1175.
- Hoter, A.; El-Sabban, M.E.; Naim, H.Y. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int J. Mol. Sci. 2018, 19, 2560.
- Diehl, M.C.; Idowu, M.O.; Kimmelshue, K.; York, T.P.; Elmore, L.W.; Holt, S.E. Elevated expression of nuclear Hsp90 in invasive breast tumors. Cancer Biol. Ther. 2009, 8, 1952–1961.
- Su, J.-M.; Hsu, Y.-Y.; Lin, P.; Chang, H. Nuclear Accumulation of Heat-shock Protein 90 Is Associated with Poor Survival and Metastasis in Patients with Non-small Cell Lung Cancer. Anticancer Res. 2016, 36, 2197–2203.
- Lee, J.H.; Chung, I.K. Curcumin inhibits nuclear localization of telomerase by dissociating the Hsp90 co-chaperone p23 from hTERT. Cancer Lett. 2010, 290, 76–86.
- Tapia, H.; Morano, K.A. Hsp90 Nuclear Accumulation in Quiescence Is Linked to Chaperone Function and Spore Development in Yeast. Mol. Biol. Cell 2010, 21, 63–72.
- Toogun, O.A.; DeZwaan, D.C.; Freeman, B.C. The Hsp90 Molecular Chaperone Modulates Multiple Telomerase Activities. Mol. Cell. Biol. 2008, 28, 457–467.
- Galigniana, M.D.; Echeverría, P.C.; Erlejman, A.G.; Piwien-Pilipuk, G. Role of molecular chaperones and TPR-domain proteins in the cytoplasmic transport of steroid receptors and their passage through the nuclear pore. Nucleus 2010, 1, 299–308.
- Kim, H.L.; Cassone, M.; Otvos, L., Jr.; Vogiatzi, P. HIF-1α and STAT3 client proteins interacting with the cancer chaperone Hsp90: Therapeutic considerations. Cancer Biol. Ther. 2008, 7, 10–14.
- Echeverria, P.C.; Mazaira, G.; Erlejman, A.; Gomez-Sanchez, C.; Piwien Pilipuk, G.; Galigniana, M.D. Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin beta. Mol. Cell Biol. 2009, 29, 4788–4797.
- Langer, T.; Rosmus, S.; Fasold, H. Intracellular localization of the 90 kDA heat shock protein (HSP90α) determined by expression of a EGFP-HSP90α-fusion protein in unstressed and heat stressed 3T3 cells. Cell Biol. Int. 2003, 27, 47–52.
- Garg, G.; Khandelwal, A.; Blagg, B.S. Anticancer Inhibitors of Hsp90 Function: Beyond the Usual Suspects. Adv. Cancer Res. 2016, 129, 51–88.
- Moser, C.; Lang, S.A.; Stoeltzing, O. Heat-shock protein 90 (Hsp90) as a molecular target for therapy of gastrointestinal cancer. Anticancer Res. 2009, 29, 2031–2042.
- Kang, B.H.; Altieri, D.C. Compartmentalized cancer drug discovery targeting mitochondrial Hsp90 chaperones. Oncogene 2009, 28, 3681–3688.
- Altieri, D.C. Mitochondrial Hsp90 chaperones as novel molecular targets in prostate cancer. Futur. Oncol. 2010, 6, 487–489.
- Kang, B.H.; Tavecchio, M.; Goel, H.L.; Hsieh, C.-C.; Garlick, D.S.; Raskett, C.M.; Lian, J.B.; Stein, G.S.; Languino, L.R.; Altieri, D.C. Targeted inhibition of mitochondrial Hsp90 suppresses localised and metastatic prostate cancer growth in a genetic mouse model of disease. Br. J. Cancer 2011, 104, 629–634.
- Siegelin, M.D.; Dohi, T.; Raskett, C.M.; Orlowski, G.M.; Powers, C.M.; Gilbert, C.A.; Ross, A.H.; Plescia, J.; Altieri, D.C. Exploiting the mitochondrial unfolded protein response for cancer therapy in mice and human cells. J. Clin. Investig. 2011, 121, 1349–1360.
- Siegelin, M.D. Inhibition of the mitochondrial Hsp90 chaperone network: A novel, efficient treatment strategy for cancer? Cancer Lett. 2013, 333, 133–146.
- Seo, Y.H. Organelle-specific Hsp90 inhibitors. Arch. Pharmacal Res. 2015, 38, 1582–1590.
- Bryant, K.G.; Chae, Y.C.; Martinez, R.L.; Gordon, J.C.; Elokeley, K.M.; Kossenkov, A.V.; Grant, S.; Childers, W.E.; Abou-Gharbia, M.; Altieri, D.C. A Mitochondrial-targeted purine-based HSP90 antagonist for leukemia therapy. Oncotarget 2017, 8, 112184–112198.
- Zhang, G.; Frederick, D.T.; Wu, L.; Wei, Z.; Krepler, C.; Srinivasan, S.; Chae, Y.C.; Xu, X.; Choi, H.; Dimwamwa, E.; et al. Targeting mitochondrial biogenesis to overcome drug resistance to MAPK inhibitors. J. Clin. Investig. 2016, 126, 1834–1856.
- Chae, Y.C.; Angelin, A.; Lisanti, S.; Kossenkov, A.V.; Speicher, K.D.; Wang, H.; Powers, J.; Tischler, A.; Pacak, K.; Fliedner, S.; et al. Landscape of the mitochondrial Hsp90 metabolome in tumours. Nat. Commun. 2013, 4, 2139.
- Liu, Y.; Elnatan, D.; Sun, M.; Myasnikov, A.G.; Agard, D.A. Cryo-EM reveals the dynamic interplay between mitochondrial Hsp90 and SdhB folding intermediates. BioRxiv 2020.
- Münster, P.N.; Marchion, D.C.; Basso, A.D.; Rosen, N. Degradation of HER2 by ansamycins induces growth arrest and apoptosis in cells with HER2 overexpression via a HER3, phosphatidylinositol 3′-kinase-AKT-dependent pathway. Cancer Res. 2002, 62, 3132–3137.
- Terasawa, K.; Minami, M.; Minami, Y. Constantly Updated Knowledge of Hsp90. J. Biochem. 2005, 137, 443–447.
- Schulte, T.W.; Blagosklonny, M.V.; Romanova, L.; Mushinski, J.F.; Monia, B.P.; Johnston, J.F.; Nguyen, P.; Trepel, J.; Neckers, L.M. Destabilization of Raf-1 by geldanamycin leads to disruption of the Raf-1-MEK-mitogen-activated protein kinase signalling pathway. Mol. Cell. Biol. 1996, 16, 5839–5845.
- Stepanova, L.; Leng, X.; Parker, S.B.; Harper, J.W. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 1996, 10, 1491–1502.
- Shiotsu, Y.; Soga, S.; Akinaga, S. Heat Shock Protein 90-antagonist Destabilizes Bcr-Abl/HSP90 Chaperone Complex. Leuk. Lymphoma 2002, 43, 961–968.
- Xu, Y.; Lindquist, S. Heat-shock protein hsp90 governs the activity of pp60v-src kinase. Proc. Natl. Acad. Sci. USA 1993, 90, 7074–7078.
- Xu, Y.; Singer, M.A.; Lindquist, S. Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc. Natl. Acad. Sci. USA 1999, 96, 109–114.
- Martins, A.S.; Davies, F.; Workman, P. Inhibiting the molecular evolution of cancer through HSP90. Oncotarget 2012, 3, 1054–1056.
- Miyata, Y.; Yahara, I. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J. Biol. Chem. 1992, 267, 7042–7047.
- Arlander, S.J.; Eapen, A.K.; Vroman, B.T.; McDonald, R.J.; Toft, D.O.; Karnitz, L.M. Hsp90 Inhibition Depletes Chk1 and Sensitizes Tumor Cells to Replication Stress. J. Biol. Chem. 2003, 278, 52572–52577.
- Uma, S.; Hartson, S.D.; Chen, J.-J.; Matts, R.L. Hsp90 Is Obligatory for the Heme-regulated eIF-2α Kinase to Acquire and Maintain an Activable Conformation. J. Biol. Chem. 1997, 272, 11648–11656.
- Fortugno, P.; Beltrami, E.; Plescia, J.; Fontana, J.; Pradhan, D.; Marchisio, P.C.; Sessa, W.C.; Altieri, D.C. Regulation of survivin function by Hsp90. Proc. Natl. Acad. Sci. USA 2003, 100, 13791–13796.
- Cheng, C.F.; Fan, J.; Fedesco, M.; Guan, S.; Li, Y.; Bandyopadhyay, B.; Bright, A.M.; Yerushalmi, D.; Liang, M.; Chen, M.; et al. Transforming growth factor alpha (TGFalpha)-stimulated secretion of HSP90alpha: Using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFbeta-rich environment during wound healing. Mol. Cell Biol. 2008, 28, 3344–3358.
- Broemer, M.; Krappmann, D.; Scheidereit, C. Requirement of Hsp90 activity for IkappaB kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-kappaB activation. Oncogene 2004, 23, 5378–5386.
- Chen, L.; Li, J.; Farah, E.; Sarkar, S.; Ahmad, N.; Gupta, S.; Larner, J.; Liu, X. Cotargeting HSP90 and Its Client Proteins for Treatment of Prostate Cancer. Mol. Cancer Ther. 2016, 15, 2107–2118.
- Aligue, R.; Akhavan-Niak, H.; Russell, P. A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90. EMBO J. 1994, 13, 6099–6106.
- Tang, X.X.; Regan, P.L.; Jacobs, J.; Wang, G.; Torres, J.; Edo, R.; Friedmann, J. Tang Hsp90 inhibition increases p53 expression and destabilizes MYCN and MYC in neuroblastoma. Int. J. Oncol. 2010, 38, 105–112.
- Tsutsumi, S.; Beebe, K.; Neckers, L. Impact of heat-shock protein 90 on cancer metastasis. Futur. Oncol. 2009, 5, 679–688.
- McCready, J.; Sims, J.D.; Chan, D.; Jay, D.G. Secretion of extracellular hsp90α via exosomes increases cancer cell motility: A role for plasminogen activation. BMC Cancer 2010, 10, 294.
- Smith-Jones, P.M.; Solit, D.B.; Akhurst, T.; Afroze, F.; Rosen, N.; Larson, S.M. Imaging the pharmacodynamics of HER2 degradation in response to Hsp90 inhibitors. Nat. Biotechnol. 2004, 22, 701–706.
- Han, S.-Y. Small Molecule Induced FLT3 Degradation. Pharmaceuticals 2022, 15, 320.
- Masson-Gadais, B.; Houle, F.; Laferriere, J.; Huot, J. Integrin alphavbeta3, requirement for VEGFR2-mediated activation of SAPK2/p38 and for Hsp90-dependent phosphorylation of focal adhesion kinase in endothelial cells activated by VEGF. Cell Stress Chaperones. 2003, 8, 37–52.
- Basso, A.D.; Solit, D.B.; Chiosis, G.; Giri, B.; Tsichlis, P.; Rosen, N. Akt Forms an Intracellular Complex with Heat Shock Protein 90 (Hsp90) and Cdc37 and Is Destabilized by Inhibitors of Hsp90 Function. J. Biol. Chem. 2002, 277, 39858–39866.
- Holt, S.E.; Aisner, D.L.; Baur, J.; Tesmer, V.M.; Dy, M.; Ouellette, M.; Trager, J.B.; Morin, G.B.; Toft, D.O.; Shay, J.W.; et al. Functional requirement of p23 and Hsp90 in telomerase complexes. Genes Dev. 1999, 13, 817–826.
- Wang, S.; Pashtan, I.; Tsutsumi, S.; Xu, W.; Neckers, L. Cancer cells harboring MET gene amplification activate alternative signaling pathways to escape MET inhibition but remain sensitive to Hsp90 inhibitors. Cell Cycle 2009, 8, 2050–2056.
- Eustace, B.K.; Sakurai, T.; Stewart, J.K.; Yimlamai, D.; Unger, C.; Zehetmeier, C.; Lain, B.; Torella, C.; Henning, S.W.; Beste, G.; et al. Functional proteomic screens reveal an essential extracellular role for hsp90α in cancer cell invasiveness. Nat. Cell Biol. 2004, 6, 507–514.
- Zhang, P.C.; Liu, X.; Li, M.M.; Ma, Y.Y.; Sun, H.T.; Tian, X.Y.; Wang, Y.; Liu, M.; Fu, L.S.; Wang, Y.F.; et al. AT-533, a novel Hsp90 inhibitor, inhibits breast cancer growth and HIF-1alpha/VEGF/VEGFR-2-mediated angiogenesis in vitro and in vivo. Biochem. Pharmacol. 2020, 172, 113771.
- Howard, K.J.; Holley, S.J.; Yamamoto, K.R.; Distelhorst, C.W. Mapping the HSP90 binding region of the glucocorticoid receptor. J. Biol. Chem. 1990, 265, 11928–11935.
- Kovacs, J.J.; Murphy, P.J.M.; Gaillard, S.; Zhao, X.; Wu, J.-T.; Nicchitta, C.V.; Yoshida, M.; Toft, D.O.; Pratt, W.B.; Yao, T.-P. HDAC6 Regulates Hsp90 Acetylation and Chaperone-Dependent Activation of Glucocorticoid Receptor. Mol. Cell 2005, 18, 601–607.
- Smith, D.F.; Whitesell, L.; Nair, S.C.; Chen, S.; Prapapanich, V.; Rimerman, A.R. Progesterone receptor structure and function altered by geldanamycin, an hsp90-binding agent. Mol. Cell. Biol. 1995, 15, 6804–6812.
- Sabbah, M.; Radanyi, C.; Redeuilh, G.; Baulieu, E.-E. The 90 kDa heat-shock protein (hsp90) modulates the binding of the oestrogen receptor to its cognate DNA. Biochem. J. 1996, 314, 205–213.
- Knoblauch, R.; Garabedian, M.J. Role for Hsp90-Associated Cochaperone p23 in Estrogen Receptor Signal Transduction. Mol. Cell. Biol. 1999, 19, 3748–3759.
- Fliss, A.E.; Benzeno, S.; Rao, J.; Caplan, A.J. Control of estrogen receptor ligand binding by Hsp90. J. Steroid Biochem. Mol. Biol. 2000, 72, 223–230.
- Ni, L.; Yang, C.-S.; Gioeli, D.; Frierson, H.; Toft, D.O.; Paschal, B.M. FKBP51 Promotes Assembly of the Hsp90 Chaperone Complex and Regulates Androgen Receptor Signaling in Prostate Cancer Cells. Mol. Cell. Biol. 2010, 30, 1243–1253.
- Loo, M.A.; Jensen, T.J.; Cui, L.; Hou, Y.-X.; Chang, X.-B.; Riordan, J.R. Perturbation of Hsp90 interaction with nascent CFTR prevents its maturation and accelerates its degradation by the proteasome. EMBO J. 1998, 17, 6879–6887.
- García-Cardeña, G.; Fan, R.; Shah, V.; Sorrentino, R.; Cirino, G.; Papapetropoulos, A.; Sessa, W.C. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature 1998, 392, 821–824.
- Ding, G.; Chen, P.; Zhang, H.; Huang, X.; Zang, Y.; Li, J.; Li, J.; Wong, J. Regulation of Ubiquitin-like with Plant Homeodomain and RING Finger Domain 1 (UHRF1) Protein Stability by Heat Shock Protein 90 Chaperone Machinery. J. Biol. Chem. 2016, 291, 20125–20135.
- Noguchi, M.; Yu, D.; Hirayama, R.; Ninomiya, Y.; Sekine, E.; Kubota, N.; Ando, K.; Okayasu, R. Inhibition of homologous recombination repair in irradiated tumor cells pretreated with Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Biochem. Biophys. Res. Commun. 2006, 351, 658–663.
- Bradley, E.; Bieberich, E.; Mivechi, N.F.; Tangpisuthipongsa, D.; Wang, G. Regulation of Embryonic Stem Cell Pluripotency by Heat Shock Protein 90. Stem Cells 2012, 30, 1624–1633.
- Roby, J.A.; Esser-Nobis, K.; Dewey-Verstelle, E.C.; Fairgrieve, M.R.; Schwerk, J.; Lu, A.Y.; Soveg, F.W.; Hemann, E.A.; Hatfield, L.D.; Keller, B.C.; et al. Flavivirus Nonstructural Protein NS5 Dysregulates HSP90 to Broadly Inhibit JAK/STAT Signaling. Cells 2020, 9, 899.
- Liu, Z.; Li, H.; He, L.; Xiang, Y.; Tian, C.; Li, C.; Tan, P.; Jing, J.; Tian, Y.; Du, L.; et al. Discovery of Small-Molecule Inhibitors of the HSP90-Calcineurin-NFAT Pathway against Glioblastoma. Cell Chem. Biol. 2019, 26, 352–365.e7.
- Salminen, A.; Ojala, J.; Kaarniranta, K.; Hiltunen, M.; Soininen, H. Hsp90 regulates tau pathology through co-chaperone complexes in Alzheimer’s disease. Prog. Neurobiol. 2011, 93, 99–110.
- Kadota, Y.; Shirasu, K. The HSP90 complex of plants. Biochim. Biophys. Acta Bioenergy 2012, 1823, 689–697.
- Peng, Y.; Chen, L.; Li, C.; Lu, W.; Chen, J. Inhibition of MDM2 by hsp90 Contributes to Mutant p53 Stabilization. J. Biol. Chem. 2001, 276, 40583–40590.
- Li, D.; Marchenko, N.D.; Schulz, R.; Fischer, V.; Velasco-Hernandez, T.; Talos, F.; Moll, U.M. Functional Inactivation of Endogenous MDM2 and CHIP by HSP90 Causes Aberrant Stabilization of Mutant p53 in Human Cancer Cells. Mol. Cancer Res. 2011, 9, 577–588.
- Altieri, D.C.; Stein, G.S.; Lian, J.B.; Languino, L.R. TRAP-1, the mitochondrial Hsp90. Biochim. Biophys. Acta 2012, 1823, 767–773.
- Lee, C.; Park, H.-K.; Jeong, H.; Lim, J.; Lee, A.-J.; Cheon, K.Y.; Kim, C.-S.; Thomas, A.P.; Bae, B.; Kim, N.D.; et al. Development of a Mitochondria-Targeted Hsp90 Inhibitor Based on the Crystal Structures of Human TRAP1. J. Am. Chem. Soc. 2015, 137, 4358–4367.
- Sung, N.; Lee, J.; Kim, J.-H.; Chang, C.; Joachimiak, A.; Lee, S.; Tsai, F.T.F. Mitochondrial Hsp90 is a ligand-activated molecular chaperone coupling ATP binding to dimer closure through a coiled-coil intermediate. Proc. Natl. Acad. Sci. USA 2016, 113, 2952–2957.
- Condelli, V.; Crispo, F.; Pietrafesa, M.; Lettini, G.; Matassa, D.S.; Esposito, F.; Landriscina, M.; Maddalena, F. HSP90 Molecular Chaperones, Metabolic Rewiring, and Epigenetics: Impact on Tumor Progression and Perspective for Anticancer Therapy. Cells 2019, 8, 532.
- Tsutsumi, S.; Scroggins, B.; Koga, F.; Lee, M.J.; Trepel, J.; Felts, S.; Carreras, C.; Neckers, L. A small molecule cell-impermeant Hsp90 antagonist inhibits tumor cell motility and invasion. Oncogene 2008, 27, 2478–2487.
- Tsutsumi, S.; Neckers, L. Extracellular heat shock protein 90: A role for a molecular chaperone in cell motility and cancer metastasis. Cancer Sci. 2007, 98, 1536–1539.
- Barrott, J.J.; Haystead, T.A.J. Hsp90, an unlikely ally in the war on cancer. FEBS J. 2013, 280, 1381–1396.
- Ullrich, S.J.; Robinson, A.E.; Law, L.W.; Willingham, M.; Appella, E. A mouse tumor-specific transplantation antigen is a heat shock-related protein. Proc. Natl. Acad. Sci. USA 1986, 83, 3121–3125.
- Li, W.; Li, Y.; Guan, S.; Fan, J.; Cheng, C.-F.; Bright, A.M.; Chinn, C.; Chen, M.; Woodley, D.T. Extracellular heat shock protein-90α: Linking hypoxia to skin cell motility and wound healing. EMBO J. 2007, 26, 1221–1233.
- Yang, Y.; Rao, R.; Shen, J.; Tang, Y.; Fiskus, W.; Nechtman, J.; Atadja, P.; Bhalla, K. Role of Acetylation and Extracellular Location of Heat Shock Protein 90α in Tumor Cell Invasion. Cancer Res. 2008, 68, 4833–4842.
- Eustace, B.K.; Jay, D.G. Extracellular roles for the molecular chaperone, hsp90. Cell Cycle 2004, 3, 1096–1098.
- Stellas, D.; El Hamidieh, A.; Patsavoudi, E. Monoclonal antibody 4C5 prevents activation of MMP2 and MMP9 by disrupting their interaction with extracellular HSP90 and inhibits formation of metastatic breast cancer cell deposits. BMC Cell Biol. 2010, 11, 51.
- Sidera, K.; Gaitanou, M.; Stellas, D.; Matsas, R.; Patsavoudi, E. A Critical Role for HSP90 in Cancer Cell Invasion Involves Interaction with the Extracellular Domain of HER-2. J. Biol. Chem. 2008, 283, 2031–2041.
- Woodley, D.T.; Fan, J.; Cheng, C.-F.; Li, Y.; Chen, M.; Bu, G.; Li, W. Participation of the lipoprotein receptor LRP1 in hypoxia-HSP90α autocrine signaling to promote keratinocyte migration. J. Cell Sci. 2009, 122, 1495–1498.
- Hance, M.W.; Dole, K.; Gopal, U.; Bohonowych, J.E.; Jezierska-Drutel, A.; Neumann, C.A.; Liu, H.; Garraway, I.P.; Isaacs, J.S. Secreted Hsp90 Is a Novel Regulator of the Epithelial to Mesenchymal Transition (EMT) in Prostate Cancer. J. Biol. Chem. 2012, 287, 37732–37744.
- Bishop, S.; Burlison, J.; Blagg, B.J. Hsp90: A Novel Target for the Disruption of Multiple Signaling Cascades. Curr. Cancer Drug Targets 2007, 7, 369–388.
- Smith, J.R.; Workman, P. Targeting the cancer chaperone HSP90. Drug Discov. Today. Ther. Strateg. 2007, 4, 219–227.
More
