Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Gao Zhang and Version 2 by Camila Xu.
Heat shock protein (HSP90), a highly conserved molecular chaperon, is indispensable for the maturation of newly synthesized poly-peptides and provides a shelter for the turnover of misfolded or denatured proteins.
- heat shock protein 90
- INHIBITORS
- cancer therapeutics
1. Introduction
The efficient and accurate control of the cellular protein pool is crucial for homeostasis within the crowded environment of a single cell [1]. HSP90 is one of the heat shock protein members. When functionally upregulated under environmental stress, HSP90 protects cells from detrimental effects [1]. HSP90 is evolutionarily conserved and ubiquitously expressed across species, which accounts for 1–2% of total cellular proteins in the unstressed condition [2]. It can be further increased to about 4–6% in the stressed condition [3]. Due to its abundance and adhesive properties, HSP90 has been likened to “molecular glue” [4]. The structure of HSP90 comprises three domains: the N-terminal domain (NTD) with ATPase activity, the middle domain (MD) that binds to the client protein, and the primary dimerization C-terminal domain (CTD) [1][5][1,5]. HSP90 utilizes ATP to keep its “closed” conformation for the binding of client proteins. The immature client proteins proceed to fold, accompanied by ATP hydrolyzation and energy release. After that, HSP90 releases the matured product and is transformed into an “open” conformation. Co-chaperones are also required for the intricate control of ATP hydrolysis rates and the certain conformational states [1][6][7][1,6,7].
Over 20 co-chaperones of HSP90 have been documented in eukaryotic cells, which modulate the molecular functions of HSP90 in four major ways: (1) coordinate the interplay between HSP90 and other chaperone systems, such as HSP70; (2) stimulate or inhibit the ATPase activity of HSP90, i.e., AHA1 for stimulation and CDC37 for inhibition; (3) recruit specific classes of clients to HSP90; (4) contribute to various aspects of the chaperone cycle through their enzymatic activities (Figure 1). The co-chaperones containing the TPR domain (i.e., HOP) facilitate the cooperative and successive action of the HSP90-HSP70-HSP40 complex to achieve the maturation of client proteins. Separately, the co-chaperones that inhibit the ATPase activity are more likely to be involved in client loading or the formation of mature HSP90 complexes, whereas those that enhance the ATPase activity are regarded as activators of the HSP90 conformational cycle [1].
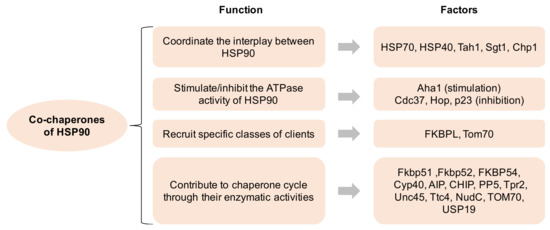
Figure 1. Co-chaperones of HSP90 and its function. HSP90 comprises four isoforms: (1) HSP90α (an inducible form) and HSP90β (a constitutive form) are mainly located in the cellular cytosol; (2) the glucose-regulated protein (GRP94) is localized in the endoplasmic reticulum; (3) Hsp75/tumor necrosis factor receptor associated protein 1 (TRAP-1, also known as mitochondrial HSP90) is located on the mitochondria [7][8][7,8]. All HSP90 isoforms play critical roles in cancer, neurodegenerative disorders, and other disease states, indicating that their pharmacological targeting may have profound implications for the treatment of these illnesses [9].
2. Subcellular Localization of HSP90
HSP90 is a highly conserved molecular chaperone on the evolutionary level and is constitutively expressed in most organs and tissues [10][20]. HSP90 assists in the proper folding, intracellular disposition and proteolytic turnover of many key regulators of cellular homeostasis with its ATPase activity [11][12][13][14][12,21,22,23]. HSP90 is mainly located in cytoplasm under normal conditions, where polypeptides are synthesized, and aberrantly folded proteins are produced. By using an in vivo PET tracer or in vitro organelle fractionation assay, HSP90 was found to be expressed ‘everywhere’, including the nucleus, mitochondrion, and plasma membrane, as well as also being secreted into the extracellular matrix [15][16][17][24,25,26]. The differential expression of sub-cellular HSP90 may exert distinctive functions in multiple biological processes, especially tumorigenesis [16][25]. Interestingly, the differential expression pattern of HSP90 between normal cells and cancer cells has already been documented, with the discrepancies selectively existing in the mitochondria or extracellular matrix [17][18][26,27].
2.1. Cytosolic Localization of HSP90
HSP90, as a critical homodimer chaperone machinery, was expressed within the cytosol component [19][28]. By constructing various truncated forms of HSP90 and using a confocal microscopy, Passinen S. et al., found that the C-terminal half of HSP90 (amino acids between 333 and 664) is responsible for the cytoplasmic localization [13][22]. Nonetheless, by fusing with the nuclear localization signal sequence (NLS), NLS-HSP90 was preferentially expressed in the nucleus [13][19][22,28].
Previous studies have focused on the discrepancy of HSP90 expression between normal cells and cancer cells and demonstrated that a higher protein level of HSP90 is expressed in the latter. In these studies, the majority of HSP90 was also localized in the cytoplasm [14][18][23,27]. Perhaps the cytosolic HSP90 largely contributes to the folding/refolding of critical oncogenic drivers and pro-survival regulators by supplying a co-chaperone buffering system. Additionally, cytosolic HSP90 has also been termed “molecular glue”, highlighting its abundance and buffering properties [4]. Most of the client proteins of HSP90 are located in the cytoplasm, exerting a variety of functions, including signaling transduction, post-transcriptional modification, metabolic rewiring and cytoskeleton remodeling. Recently, HSP90 was found to transiently crosslink actin filaments in vitro, and this dynamic interaction between HSP90 and almost all cytoplasmic filamentous structures highlights its role in modulating actin filament bundling behavior [4][20][4,29]. HSP90 was also associated with tubulin and probably protects it from heat denaturation [21][30]. Likewise, it was shown that HSP90 protects myosin from heat stress [22][31]. Therefore, HSP90 is functionally involved in the management of the cytoskeleton.
2.2. Nuclear Localization of HSP90
A low expression of HSP90 (5–10% of total cellular HSP90) was detected in the nucleus of normal cells [23][32]. Recent data, however, have shown that the elevated expression of nuclear HSP90 could be detected in breast cancer and non-small cell lung cancer (NSCLC) [24][25][26][33,34,35]. For example, a nuclear accumulation of heat shock protein 90 might predict the poor survival of patients with NSCLC. Furthermore, the nuclear staining of HSP90 was also positively correlated with the age and smoking status of patients with NSCLC [25][34]. Interestingly, in quiescent Saccharomyces cerevisiae cells, Hsp90 and its co-chaperones were found to accumulate in the nucleus with the requirement of the α/β importin system, which was enhanced during periods of relative metabolic inactivity [27][36]. In fact, the HSP90 protein comprises sequences that are homologous to the recognized traditional or alternative nuclear import and export signals: the nuclear localization sequence [12][21].
Importantly, HSP90 is associated with multiple nuclear chaperone clients including nucleic acid, histone, transcription factors, and epigenetic regulators [4]. HSP90 also modulates multiple biological functions in the nucleus including RNA synthesis, processing, and multiple telomerase activities [4][28][4,37]. The nuclear translocation of HSP90 is governed by FKBP52, steroid receptors, and kinases [12][29][21,38]. Zinc finger proteins, helix-loop-helix proteins, MyoD1, E12, HIF1α, HSF1, and glucocorticoid receptor interact with HSP90 [4][30][31][4,39,40]. The transfection of 3T3 cells with HSP90 fused with EGFP revealed that HSP90 was located in the nuclear membrane upon exposure to an elevated temperature [32][41]. Many small chemical molecules enter the cytoplasm through penetrating the plasma membrane, but it was shown that none of them could enter the nucleus, rendering their efficacy limited [33][42]. However, HSP90 could be translocated from cytoplasm to nucleus, which protects cancer cells from therapeutic pressure [34][43]. This suggests that nuclear-directed HSP90 inhibitors should be taken into consideration.
2.3. Mitochondrial Localization of HSP90
The mitochondrial expression of HSP90 was unexplored until Kang B. et al., first demonstrated the high expression of HSP90 in tumoral mitochondrion, but barely in normal tissues [17][26]. Based on this ground-breaking work, substantial research took place, which focused on the development of small molecular drugs with the organelle-specific targeting of HSP90. Collectively, the goal of these inhibitors is to trigger a sudden collapse of mitochondrial integrity and apoptosis that would selectively occur in tumor cells. Several inhibitors that specifically target mitochondrion have emerged and shown intriguing effects in multiple cancer types, including pancreatic cancer, breast cancer, colon cancer, NSCLC, melanoma, glioblastoma, prostate cancer, lymphoma, and leukemia (Table 1) [17][35][36][37][38][39][40][41][42][26,44,45,46,47,48,49,50,51]. By investigating the metabolic network in the tumoral mitochondrion regulated by HSP90, Chae Y. et al., highlighted that mitochondrial HSP90 (hereafter mtHSP90), but not cytosolic HSP90, binds and stabilizes the electron transport chain complex II subunit succinate dehydrogenase-B (SDHB), which maintains cellular respiration under low-nutrient conditions and contributes to HIF1α-mediated tumorigenesis in patients carrying SDHB mutations [43][52]. Cryo-EM data also confirmed the dynamic interplay between mitochondrial HSP90 and SDHB folding intermediates [44][53].
Table 1.
HSP90 clients are associated with hallmarks of cancer.
| Phenotype | Clients | References |
|---|---|---|
| Uncontrolled proliferation | EGFR, HER2, RAF1, CDK4, Akt, BCR-ABL, v-Src, c-Src, FAK, CKII, CHK1, eIF-2α kinase | [6]6[45][46][47][48][49][50][51][52][,1653,17],54[54,55][55][,56,57,58,59,60,61,62] |
| Anti-apoptosis | p53, Akt, Survivin, IKK, NF-κB, PLK, WEE1, Myc, CDK4, CDK6 | [6][33][48][56][57][,4258,55],63,64[,6559][60][61][6,66,67,68] |
| Angiogenesis | HIF1α, Akt, EGFR, HRE2, FLT3, VEGFR2 | [62][63][64][65][66][67][19,69,70,71,72,73] |
| Immortalization | Telomerase | [68][18] |
| Invasion/Metastasis | MMP2, MMP9, c-MET | [25][62][69][70][19,34,74,75] |
| Others | Glucocorticoid receptor, Mineralocorticoid receptor, Progesterone receptor, Estrogen receptor, Androgen receptor, Oestrogen receptor, Nitric oxide synthase, Centrin/centrosome, Calmodulin, MDM2, UHRF1, BRCA2, OCT4, Nanog, STAT3, Calcineurin, CFTR, NLR proteins, RAD51/RAD52, Tau, HCK, JAK1 and/or JAK2 | [71][72][73][74][7683,77[75][76][77][78][79],78],79[80][81][82][[84,80][85,81][86,82],83[,84,86,87,88,89,9087][88][89,85],91,92,93,94] |
It is worth mentioning that TRAP1 has been regarded as another version of HSP90—namely, mitochondrial HSP90—because it shares 60% of its sequence with HSP90 and contains the same domains: NTD, ND, and CTD [23][90][32,95]. Interestingly, Kang B. et al., also established that TRAP1 was consistently elevated in primary tumors, whereas it was nearly undetectable in normal tissues [17][26]. Moreover, one of the important client proteins of TRAP1 was cyclophilin D, a mitochondrial residential protein, which maintains mitochondrial integrity by preserving cells from apoptosis. Furthermore, the crystal structures of HSP90 and TRAP1 provide further molecular insights that can be exploited for the development of novel inhibitors [91][92][96,97]. Taken together, TARP1 is another attractive target for developing cancer drugs [93][98].
2.4. Membrane and Extracellular Localization of HSP90
HSP90 was first viewed as an artifact when it was found on the cell surface by a functional screening, due to its abundant expression within the cells. After cautious verification, HSP90 is no longer considered as being exclusively located within the cell [70][75]. HSP90 can also be secreted into the extracellular matrix. Accordingly, the cell surface expression of HSP90 is higher on cancer cells than that on normal cells, which correlates with the malignant stage of the tumor. Previous work has also shown that the cell surface of HSP90 could strengthen the migration potential of cancer cells that is distinct from the function of the intracellular HSP90 pool [94][95][99,100]. Thus, the cell surface of HSP90 is also an attractive therapeutic target in terms of inhibiting tumor invasion and metastasis [70][96][75,101].
The first evidence for the detection of HSP90 in the extracellular matrix was implicated in 1986 when Barrott J. et al., found a mouse tumor-specific antigen that was identified as a heat shock protein, which is now recognized as HSP90 [97][102]. Since the ATP level in the extracellular environment is low due to the lack of energy source, the extracellular HSP90 (hereafter eHSP90) may function independently of ATP. The secretion of eHSP90 is induced by environmental stresses and growth factors [57][98][64,103], and it is affected by post-translational modifications to the chaperone, including phosphorylation and acetylation [99][104]. Recent work has also shown that HSP90α is released by invasive cancer cells via exosomes, which contributes to their invasive nature by interacting with plasmin [63][69]. Another study found that both HSP90α and HSP90β are secreted by cancer cells to interact with MMP2 and MMP9 to enhance the invasive capacity of tumor cells. Similarly, eHSP90 was detected in normal cells only in response to stress, while cancer cells consecutively secrete HSP90 [100][101][105,106]. Interestingly, eHSP90 interacts with a series of receptors such as EGFR/HER2/LPR1 to promote the downstream signal transduction associated with tumor growth and metastasis, which resembles the EMT phenotype [102][103][104][107,108,109]. Further, eHSP90 expression correlates with an increase in metastatic potential and a decrease in the immune response in multiple cancer types [95][100]. Although the specific inhibition of eHSP90 does not affect cancer cell growth in vitro or tumour xenograft progression in vivo [94][99], the inhibition of eHSP90 is effective in conquering metastasis with minor side effects, highlighting the clinical potential of eHSP90 inhibitors [40][96][101][105][106][49,101,106,110,111].
