Aging is a progressive decline of an organism over time. In contrast, senescence occurs throughout an organism’s lifespan. It is a cell-cycle arrest preventing the proliferation of damaged cells. Cellular and molecular senescence timing is crucial for the pace of aging and disease development and progression. The accumulation of senescent cells during a lifespan leads to organismal senescence. Senescent multinucleated giant cells are present in many age-related diseases and cancer. Although senescence was assumed to be irreversible, studies now show that senescent multinucleated giant cells overcome senescence in various cancers, becoming the source of highly aggressive mononucleated stem-like cells, which divide and initiate tumor development and progression.
1. Introduction
Aging Versus Senescence
Although the terms “aging” and “senescence” are often used interchangeably, this is incorrect because they describe entirely different processes. Aging is a progressive decline by accumulating detrimental changes in an organism over time. In contrast, senescence occurs throughout an organism’s lifespan. It refers to cell-cycle arrest, preventing the proliferation of damaged cells and failure to resume the cell cycle in response to mitogenic stimuli. There is also a difference between cellular and organismal senescence. Senescent cells accumulate during the lifespan, leading to the aging of whole organisms, i.e., organismal senescence. Senescence is also different from quiescence, a reversible cell-cycle arrest
[1]. Cellular and molecular senescence timing determines the pace of aging and disease development. Senescence was first described by Hayflick and Moorhead
[2] in cells at the end of their replicative lifespan affected by telomere shortening
[3]. In addition to telomere shortening and disorder, various stimuli, such as DNA damage, oxidative stress, oncogenes, and viral and bacterial infection, induce senescence
[4][5][6][7][8][4,5,6,7,8]. It is a common belief that senescence evolved as a tumor-suppressive mechanism preventing the malignant transformation of damaged cells
[9][10][9,10]. However, developmentally programmed senescence, regulating embryonic development and patterning (for example, limbs, kidneys, and neural system), suggests that non-pathological senescence must be evolutionarily older.
Recent studies show that approximately 60% of in vitro fertilized human embryos are arrested at the 3–8 cell stage due to a senescence-like state. These embryos have lower ribosomes, histones, MYC, and p53 activity. Some of them also have decreased glycolysis or higher- or lower-than-normal oxidative phosphorylation. Embryos treated with the SIRT agonists, resveratrol or nicotinamide riboside (NR) change their metabolism, partially reversing senescence
[11].
Senescence is one of the causative agents of aging. Senescent cell numbers increase with age, thus contributing to organismal senescence and aging-related disorders. However, senescence is also beneficial as it is crucial for embryogenesis, tissue homeostasis, remodeling, and wound healing
[4][12][4,12]. Although, by definition, senescence permanently arrests the cell cycle, recent studies indicate that this is not necessarily true and that, in specific biological/pathological contexts, senescence may be reversible
[13][14][15][13,14,15]. Experimental data show that eliminating senescent cells by pharmacologic or genetic methods improves health and prolongs life. The main factors driving senescence during aging are: epigenetic, telomere attrition, DNA and mitochondrial damage, changes in protein synthesis and response to nutrient signaling, exhaustion of stem cells, and chronic inflammation
[16].
Senescent cells have changed morphology, organelle functions, chromatin organization, gene expression, and metabolism, resulting in the acquisition of a pro-inflammatory phenotype, known as the senescence-associated secretory phenotype (SASP), characterized by the overexpression of interleukins IL-1a, IL-6, IL-15, and IL-8, and GRO-a, MIP-1a, IFN γ, VEGF, ICAM-1, and GM-CSE chemokines, signaling molecules, and growth factors
[15][16][17][18][19][20][15,16,17,18,19,20].
Senescent cells are often flattened and highly enlarged. Such an increase in cytoplasm volume and the cytoplasm to DNA ratio results in the dilution of transcription-required factors leading to the cell-cycle arrest and senescence
[21][22][21,22]. Senescent cells often form giant multinucleated cells (GMCs), containing many nuclei and vacuoles. Sometimes these giant cells have many large lysosomes and increased senescence-associated beta-galactosidase (SA-βgal) activity
[22][23][22,23]. The integrity of the nuclear membrane is compromised in giant cells, and nuclear and chromatin architecture is changed because of loss or under-expression of Lamin B1. Lamin is an essential structural component of the nuclear lamina, a network of filaments underlying the nuclear envelope
[24][25][24,25]. The gene transcription depends on the architecture and condensation status of chromatin. In senescent cells, to silence the transcription of the proliferation-promoting genes, the chromatin condenses into dense senescence-associated heterochromatin foci (SAHFs)
[22][26][22,26]. The loss of nuclear membrane integrity causes the release of chromatin fragments into the cytoplasm. This activates the DNA-sensing receptor cyclic GMP–AMP synthase (cGAS) and its downstream effector stimulator of the interferon genes (STING) pathway, fueling the development of the SASP phenotype
[22][27][28][22,27,28].
Other hallmarks of the senescence phenotype are the changes in the number, size, cristae structure, metabolic properties of mitochondria, and mitostasis
[29]. Because of the reduction in mitophagy, the senescent cells have more mitochondria with deteriorated mitochondrial oxidative phosphorylation (OXPHOS). The dysfunction of mitochondria increases the production of reactive oxygen species (ROS), which enhance DNA damage and DNA-damage response pathway (DDR), ultimately aggravating SASP
[29][30][31][29,30,31]. Mitochondria of senescent cells are also resistant to apoptosis through the upregulation of the antiapoptotic Bcl-2 proteins and pro-survival pathways
[32][33][32,33].
Cell-cycle arrest during senescence depends on the decreased phosphorylation of Retinoblastoma protein (pRB). The hypo-phosphorylated RB binds the transcription factor E2F, preventing its binding to the promoters of genes regulating the G1/S phase transition and entry into the S phase of mitosis, ultimately halting the cell cycle. The pRB function, and thus cell-cycle arrest, is regulated by the p16Ink4a/RB and p53/p21CIP1 pathways, whose final downstream effector is the pRB
[15][22][15,22].
2. Giant Multinucleated Cell Formation
Giant cells form through the fusion of individual mononuclear cells. The fusing cells can be of the same or of different origin (homotypic or heterotypic fusion, respectively). Because macrophages are intrinsically fusogenic, they are the primary source of the giant multinuclear cells observed in aging. They derive from either the homotypic or heterotypic fusion of macrophages with other cell types. Cell fusion is a multi-step endeavor. First, cells must be fusion-competent; approach each other; contact, adhere, and merge the cell membranes to become a single, functional entity. The molecules and pathways involved in each step of cell fusion are described in detail by Helming and Gordon
[34] and Kloc et al., 2022
[35].
3. Giant Cells in Aging Arteries
Arteriosclerosis and giant cell arteritis (GCA) are typical examples of vascular aging. Vascular aging depends on immune cells and inflammaging, a low-grade inflammation resulting from long-term stimulation of the innate immune system
[36]. GCA is an inflammatory vasculopathy of large- and medium-sized arteries
[36][37][38][36,37,38]. Mitochondrial dysfunction, causing the excessive production of ROS, is a significant contributor to vascular aging. ROS induces senescence of vessel endothelial and smooth muscle cells, which acquire the SASP phenotype
[37][39][40][37,39,40]. The first immune cells responding to SASP and its pro-inflammatory secretome are vascular dendritic cells (vasDCs), located in the vessel wall. The cytokines released from the activated DCs recruit T cells and monocytes. Monocytes differentiate into macrophages, which phagocyte cell debris and release various signaling molecules, including VEGF, further enhancing vessel inflammation and over-proliferation of the myofibroblasts in the vessel wall intima
[37][41][37,41]. Subsequently, the activated macrophages fuse into giant multinucleated cells (GMCs), which produce a massive amount of matrix metalloprotease-9 (MMP-9) and other proteolytic enzymes. The resulting excessive digestion of the extracellular matrix disrupts the vessel wall, allowing infiltration by the T cells, and furthering the inflammation and the formation of the inflammation foci (granulomas). The ultimate result is a collapse of the inner elastic membrane and occlusion of the vessel lumen by overgrown muscle cells (
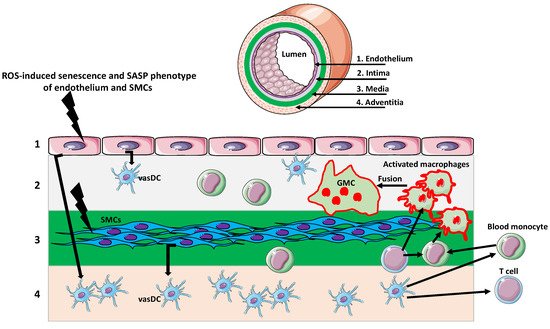
Figure 1)
[37][38][42][43][37,38,42,43].
Figure 1.
Giant multinucleated cells in giant cell arteritis (GCA).
The artery wall consists of several layers. The endothelial layer (1) faces the vessel lumen and abuts the intima (2), which contains vascular dendritic cells (vasDCs). Below is the media (3) containing smooth muscle cells (SMCs). The outermost layer is adventitia (4) composed of collagenous and elastic fibers matrix and various immune cells, including vasDCs. Different signals originating from age-related cellular, organellar, and metabolic dysfunctions, such as excessive reactive oxygen species (ROS) production, induce senescence and senescence-associated secretory phenotype (SASP) of endothelium and SMCs. The secretion of various senescence factors activates the vasDCs, which produce signals recruiting T cells and monocytes to the vessel wall. Monocytes, activated by the inflammatory environment and T cell signaling, differentiate into macrophages. The further activation of macrophages, by the inflammatory environment/T cells, induces their fusion and the formation of multinuclear giant cells (GMCs). The GMCs over-produce proteolytic enzymes, which digest the extracellular matrix and disrupt the vessel wall integrity.
4. Giant Multinucleated Cells in Aging Gonads
In mammals, the first system showing signs of physiological aging is the female reproductive system. Although an age-related decline in fertility is mainly attributable to the declining quality of oocytes, the inflammaging of the ovarian tissue plays a vital role in this process. The oocyte environment consists of a stromal extracellular matrix (ECM), smooth muscle cells, endothelial cells, fibroblasts, and various immune cells. Studies of the ovaries from reproductively old mice showed excessive fibrosis of the ovarian stroma, which correlated with the overexpression of inflammatory cytokines and factors, as well as the presence of macrophage-derived multinucleated giant cells in the ovarian stroma
[44][45][44,45]. It is known that macrophage fusion into giant cells is a hallmark of chronic inflammation. Thus, an inflammatory environment in the ovarian stroma may induce macrophage fusion. Macrophage fusion enhances phagocytosis and the degradation of large extracellular targets
[34]. Although the exact function of giant cells in aged ovaries remains unknown, they may be involved in the degradation of large fibrotic regions or the removal of cellular debris accumulating in the ovary because of multiple ovulatory cycles and atresia of the follicles during the reproductive life span
[44][45][44,45]. Recently, Foley et al.
[45] suggested that macrophage-derived giant cells, absent in reproductively young mice, and their secretomes, are the markers and sources of inflammation in the aging ovary. They can also be a potential target in treatments promoting reproductive longevity.