Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Kelly Anzola and Version 2 by Rita Xu.
Sjögren syndrome (SS) belongs to the family of rheumatic autoimmune diseases characterized by systemic compromise with exocrine glands as target organs that are affected by chronic inflammation and immune-mediated destruction of the tissue, leading to severe dryness of the mouth and eyes. Extra-glandular symptoms are frequent and include fatigue, polyarthralgias, myositis, polyneuropathy, and gammaglobulinopathies, among others.
- Sjögren syndrome
- nuclear medicine
- radiopharmaceuticals
1. Introduction
Since there is evidence of the presence of common pathophysiologic mechanisms shared by SS and thyroiditis, it is also common to find thyroid involvement in patients with SS as part of the peri-epithelial extra salivary manifestations of the disease [1][2]. Systemic complications regarding extra-glandular tissues are associated with vascular, respiratory, gastrointestinal, renal, and neurological systems, possibly affecting one-third of these patients [2][1].
SS may be present as a primary condition or accompanying other autoimmune diseases as secondary SS. In addition, SS has a predominant clinical presentation in females, with a female:male ratio of 9:1, higher than for all other autoimmune diseases [2][1]. The clinical phenotype of SS varies from benign conditions such as mild exocrinopathies to severe systemic manifestations such as B-cell non-Hodgkin’s lymphoma (NHL) [3][4][3,4]. These lymphomas arise predominantly from memory B cells in the marginal zone of lymphoid tissue, with mucosa-associated lymphoid tissue (MALT) lymphomas being the most frequent type [5] and the parotid and minor salivary glands being the most frequent sites of involvement [6]. Patients at high risk of developing NHL are male and have clinical manifestations of severe systemic disease, such as vasculitis, splenomegaly, lymphadenopathy, glomerulonephritis, haematologic manifestations, high expression of autoantibodies, cryoglobulins and hypergammaglobulinemia, high biopsy focus scores, and presence of ectopic germinal centers, among others [7].
2. Role of New Molecular Image Strategies in Sjogren’s Syndrome
The goal of SS is to be able to stratify pSS through the integration of clinical, laboratory, histopathology, and imaging data; thus, it could be possible to identify which patients have clinical manifestations related to inflammatory activity or sequelae; the possibility to identify which patients are on the risk of lymphoma development it is also important. Because new treatment strategies are emerging, there is also evident the need for new probes for a more reliable treatment response evaluation [8][43]. The potential for diagnosis and classification of pSS of the salivary glands ultrasound (SGUS) has been pointed out previously by different authors [9][44]; nowadays this technique is emerging as a complementary tool for biopsy purposes. In a recent study the authors reported that by adding SGUS as a minor item to ACR/EULAR criteria, the sensitivity could rise to 95.6% [10][45]. Other authors have reported that a combined positivity of SGUS and anti-SSA antibodies provides a high predictive value for the diagnosis of pSS [11][46] The main limitation of SGUS is the lack of a standardized scoring system, however, as an initiative to overcome this issue, the Outcome Measures in Rheumatology Clinical Trials (OMERACT) proposed a four-grade semiquantitative score with good intra and interobserver agreement results [12][47]. Last generation ultra-high resolution ultrasound (UHFUS) transducers, which produce frequencies up to 70 MHz with resolution up to 30 um, offer new possibilities to visualize labial salivary glands and to guide diagnostic biopsy procedures [13][48]. Recently, Baldini et al. demonstrated that the mean labial glandular surface area obtained by the high-resolution ultrasound-guided procedure was significantly higher than the area obtained by the traditional biopsy procedure. This procedure could facilitate the assessment of the focus score [14][49]. Promising innovations are ultrasound elastography (with algorithms to evaluate quantitatively the tissue stiffness of the salivary gland tissue [15][50], the application of artificial intelligence to automatically score for biopsy purposes [16][51], and the ultrasound-guided core needle biopsy of major salivary glands which could represent an alternative to surgical biopsy [17][52]. MRI does not belong to the common standard tools used for the diagnostic approach of pSS. Because of its high spatial resolution the major impact in such patients is for local staging of pSS associated with salivary glands lymphomas [18][53]. Salivary gland scintigraphy is a nuclear medicine technique that through the administration intravenously of 99mTc-04 allows for to evaluate of the function of major salivary glands (perfusion, concentration, and elimination characteristics) [19][54]. Sialoscintigraphy is no longer part of the recent classification criteria for pSS [20][40]; the reported diagnostic approach shows a sensitivity of up to 90% with specificity of around 50%, making this tool not able to distinguish the functional compromise of SS and other salivary glands pathologies. It is possible that this technique could have some potential indication in the future as part of the tools available for the follow-up of patients [19][54]. Molecular nuclear medicine imaging has emerged with several biomarkers with potential clinical impact for its contribution to diagnosing, assessing the inflammatory status, and assessing disease progression. These images allow in vivo the characterization of cells and the phenomena involved in inflammatory diseases. For this purpose, by using radiolabeled molecules (administered in nanomolar amounts), that participate in the biochemical and pathophysiological process of chronic inflammation, it is possible to make a qualitative and quantitative assessment of the inflammatory burden in vivo. Recent advances in the development of target-specific imaging agents, allow reusearchers to perform a non-invasive evaluation of various molecular events such as angiogenesis, apoptosis, and cell trafficking in living organisms. Cellular and molecular changes occur a long time before structural changes, therefore, non-invasive visualization and quantification of molecular processes facilitate the early detection of the disease, establish a prognosis, and could potentially estimate the potential impact of biologic therapies.- a.
-
Somatostatin Receptor Imaging
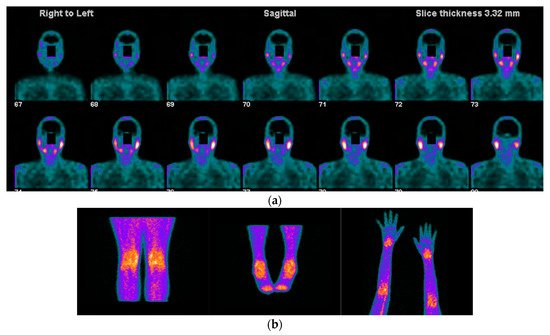 Figure 1. Images acquired after 99mTc-HYNIC-TOC administration i.v in a patient with SS, sicca symptoms, and painful joints. In (a) high abnormal uptake in parotids and submandibular salivary glands. In (b) shows high abnormal uptake in knees, ankles, elbows, and carpal joints.One systematic review [22][56] reported promising results of radiolabelled somatostatin analogs as diagnostic tools for localizing and identifying sites of active inflammation in joints as well as extra-articular involvement, such as the salivary glands, in patients with chronic inflammatory diseases (Figure 1a,b and Figure 2a,b).
Figure 1. Images acquired after 99mTc-HYNIC-TOC administration i.v in a patient with SS, sicca symptoms, and painful joints. In (a) high abnormal uptake in parotids and submandibular salivary glands. In (b) shows high abnormal uptake in knees, ankles, elbows, and carpal joints.One systematic review [22][56] reported promising results of radiolabelled somatostatin analogs as diagnostic tools for localizing and identifying sites of active inflammation in joints as well as extra-articular involvement, such as the salivary glands, in patients with chronic inflammatory diseases (Figure 1a,b and Figure 2a,b).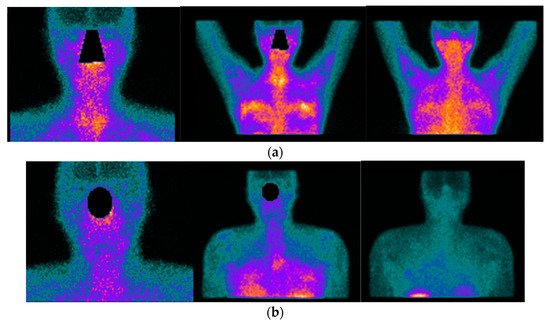 Figure 2. 42-year-old female patient with the previous history of bilateral mammoplasty; four years later she complained of dryness (VAS 5/10), fatigue (VAS 10/10), pain (VAS:8/10) ESSPRI: 23, and thyroiditis. She was diagnosed with SS under ACR-EULAR criteria and she started treatment with DMARDS for two years without relieving symptoms. She asked her surgeon to remove the implants and six months after the removal of the implants there was an improvement in her clinical condition, dryness (VAS: 2/10), fatigue (VAS:6/10), pain (VAS: 5/10) post-surgery ESSPRI: 13. In (a) 99mTc HYNIC-TOC images show high abnormal uptake of the radiotracer in the thyroid gland, submandibular glands, with a high abnormal bilateral mammary periprosthetic distribution of the radiotracer. SS was diagnosed under ACR-EULAR criteria. In (b) 99mTc HYNIC-TOC images 6 months post removal of prosthesis show a significant decrease in uptake of the radiotracer in submandibular glands and thyroid gland. There was a concern that silicone could be associated with SS.
Figure 2. 42-year-old female patient with the previous history of bilateral mammoplasty; four years later she complained of dryness (VAS 5/10), fatigue (VAS 10/10), pain (VAS:8/10) ESSPRI: 23, and thyroiditis. She was diagnosed with SS under ACR-EULAR criteria and she started treatment with DMARDS for two years without relieving symptoms. She asked her surgeon to remove the implants and six months after the removal of the implants there was an improvement in her clinical condition, dryness (VAS: 2/10), fatigue (VAS:6/10), pain (VAS: 5/10) post-surgery ESSPRI: 13. In (a) 99mTc HYNIC-TOC images show high abnormal uptake of the radiotracer in the thyroid gland, submandibular glands, with a high abnormal bilateral mammary periprosthetic distribution of the radiotracer. SS was diagnosed under ACR-EULAR criteria. In (b) 99mTc HYNIC-TOC images 6 months post removal of prosthesis show a significant decrease in uptake of the radiotracer in submandibular glands and thyroid gland. There was a concern that silicone could be associated with SS.- b.
-
B-lymphocyte Imaging in SS
- c.
-
T-lymphocyte Imaging in SSNew radiotracers, such as 18F-fluorbenzoyl interleukin-2, have also been developed for PET systems, allowing detection of activated T lymphocytes for the same purposes. In a preclinical study, Di Gialleonardo et al. [44][78] identified activated T lymphocytes in inflamed tissues, highlighting the potential of the probe for detecting activated T lymphocytes in autoimmune diseases such as SS. Because T lymphocytes are also mainly implicated in the pathogenesis of SS, molecular images for detecting T lymphocyte activity would play an important role in diagnostic approaches [9][44].
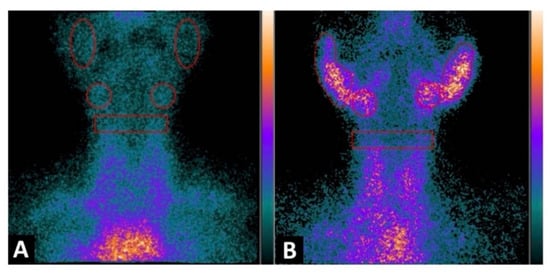 Figure 3. Planar image of the neck was obtained 1h after 99mTc-IL2 injection in a control subject (A) and in a patient with Sjögren syndrome at the time of diagnosis (B). In (A) the scan shows no 99mTc-IL2 uptake by the salivary glands. In (B) an evident accumulation of 99mTc-IL2 can be observed in both parotids and submandibular glands, indicating the presence of activated lymphocytes. The calculated parotid to background (P/B) ratios are 1.35 and 1.30 in right and left glands, respectively, and the submandibular gland to background (S/B) ratios are 1.57 and 1.64 in right and left glands, respectively. Reprinted with permission from Imaging Activated-T-Lymphocytes in the Salivary Glands of Patients with Sjögren’s Syndrome by 99mTc-Interleukin-2: Diagnostic and Therapeutic Implications Campagna, G.; Anzola, L. K.; Varani, M.; Lauri, C.; Gentiloni Silveri, G.; Chiurchioni, L.; et al. J. Clin. Med. 2022, 11 (15), 4368. [43][77].
Figure 3. Planar image of the neck was obtained 1h after 99mTc-IL2 injection in a control subject (A) and in a patient with Sjögren syndrome at the time of diagnosis (B). In (A) the scan shows no 99mTc-IL2 uptake by the salivary glands. In (B) an evident accumulation of 99mTc-IL2 can be observed in both parotids and submandibular glands, indicating the presence of activated lymphocytes. The calculated parotid to background (P/B) ratios are 1.35 and 1.30 in right and left glands, respectively, and the submandibular gland to background (S/B) ratios are 1.57 and 1.64 in right and left glands, respectively. Reprinted with permission from Imaging Activated-T-Lymphocytes in the Salivary Glands of Patients with Sjögren’s Syndrome by 99mTc-Interleukin-2: Diagnostic and Therapeutic Implications Campagna, G.; Anzola, L. K.; Varani, M.; Lauri, C.; Gentiloni Silveri, G.; Chiurchioni, L.; et al. J. Clin. Med. 2022, 11 (15), 4368. [43][77].- d.
-
Other PET Radiopharmaceuticals Used in SS
