Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Margarita L. Martinez-Fierro and Version 8 by Conner Chen.
Several viral diseases oftenusually affect the oral cavity; for example, human immunodeficiency virus (HIV) infection may initially present with oral lesions, human papillomavirus (HPV) infection often increases the risk of developing oral squamous cell carcinoma, and oral damage has been documented during hepatitis B and C virus infections.
- COVID-19
- cavity buccal
- oral lesion
1. Introducctióon
CThe coronavirus disease 2019 (COVID-19) is the pathology caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is a virus that containsing in its genetic material a single strand of RNA [1] [1]. Some of the most common clinical signs and symptoms of COVID-19 are fever, sore throat, headache, shortness of breath, dry cough, stomachbelly pain, vomiting, and sometimes diarrhea [2] [2]. Angiotensin-converting enzyme receptor 2 (ACE2) is one of the majin knorwn receptors known for SARS-CoV-2 to enter the cells inof the lungs, liver, kidneys, gastrointestinal system, and even on the endotheliuma of dermal papillary vessels and eon the epithelium.al surfaces of sweat gland surfacess [ 3 ][3]. A vVariety ofous skin manifestations have been described in patients with COVID-19, including pseudomumps-chilblain, varicelliform lesions, erythema multiforme-like lesions, urticaria form, maculopapular, purpura and petechiae, mottling, and livedo reticularis-like lesions [ 4 ] [ 5 ] [4,5]. In the oral cavity, ACEA2 is expressed in the oral mucosa, especially and in greater quantity oin the lingual surface and in saliva-producing glands in relativeon to the mucosa of the mouth or palate [ 6 ] [6]. Dysgeusia is the first recognized oral symptom of COVID-19 reported in 38% of patients, especially in North Americans and Europeans and in patients with mild to –moderate disease severity [ 4 ][4]. Since the first oral manifestations associated with COVID-19 were described, several reports have been published describing a wide variety of lesions, where the most frequent oral lesion manifestation is ulceration [ 7 ] [7], in addition to white plaques, petechiae, geographic tongue, macules, nodules, bullous angina, necrotizing periodontal disease, blisters, and erythema -multiforme-like lesions [ 8 ] [ 9 ][8,9]. It has been reported that Lloss of taste and/or smell has been reported to persistremains for up to 14 days and to progressses more rapidly in older patients; recovery of oralfrom mouth lesions occurs at the same time as patients recover from COVID-19, representing an association between the manifestations of all clinical lesions appearing in the mouth and patients' SARS-CoV-2 infection. of [ 10 ]patients [10].
SGinceven that the clinical manifestations of COVID-19 beyond lung damage caused by inflammation are still nonot yet adequately understood, the oral health conditions associated with COVID-19 continue to be studied to gain a better appreciation of the oral manifestations. The crisis that the world continues to face from COVID-19 has highlighted the importance of understanding the implicit conditions that lead to COVID-19-related outcomes, primarily mortality, as well as positivity and severity [ 11 ] [ 12 ] [ 13 ] [ 14 ] [11,12,13,14].
2. The oOral cCavity and its rIts Role in iImmunity
The oral cavity has three major host defenses against microbial invasion: the oral mucosa, nonspecific (innate) immunity, and adaptive (acquired) immunity. The oral mucosa consists of a layer of interconnected epithelial cells containing mainly keratinocytes resting on a basal membrane and provides a physical barrier that protects the underlying tissues from microorganisms and environmental threats in the oral cavity [15][16][17][15,16,17]. Cells of the immune system and oral keratinocytes in the lamina propria of the oral mucosa detect some pathogen-associated molecular patterns that have been conserved by evolution in specific classes of microorganisms [18][19][18,19]. Pattern recognition receptors distinguish between different molecular structures of microorganisms and thus prevent the generation of immunoinflammatory responses against these microorganisms [20][21][20,21]. Pattern recognition receptor families have been described in some reports, in which have been included for example the toll-like receptor family (TLR-1 to TLR-10) and the C-type lectin receptor family (Dectin-1, Dectin-2, dendritic cell specific intercellular adhesion molecule 3-grabbing nonintegrin) [22]. The initiation and determination of the type of specific adaptive immune responses induced by pattern recognition receptors also dictate the magnitude and duration of the responses and whether or not memory T cells are activated [20]. The specificity, type, and sensitivity of pattern recognition receptor-mediated adaptive immune responses are determined by the nature of the infectious agent, for example, if they are viruses, bacteria, fungi, and/or protozoan [23]. By the microenvironment characteristics, the type of cells expressing each family of pattern recognition receptors, the anatomical site where these cells are located, and the combination of interactions that occur between these factors [24]. Warning signals generated by some tissue-damaging factors, including hypoxia, radiation, and trauma to some extent affect the speed and magnitude of the immune response [25].
3. Oral Cavity during Viral and Bacterial Infections
Several viral diseases usually affect the oral cavity; for example, human immunodeficiency virus (HIV) infection may initially present with oral lesions, human papillomavirus (HPV) infection often increases the risk of developing oral squamous cell carcinoma, and oral damage has been documented during hepatitis B and C virus infections [26][27][28][29][26,27,28,29].
There is a combination of proteins with complementary dynamics in virus and/or bacterial infection in the oral cavity. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) has been shown to be significantly stimulated in response to infections that are primarily viral in origin [30]. Interferon gamma-induced protein-10 (IP-10) levels are generally slightly elevated in patients with bacterial infections and highly elevated in patients with viral infections. It is also known that C-reactive protein (CRP) levels are commonly found to be increased in patients who have developed infections of bacterial origin and that CRP levels are less elevated in patients who have developed infections caused by viruses (Figure 1) [31].
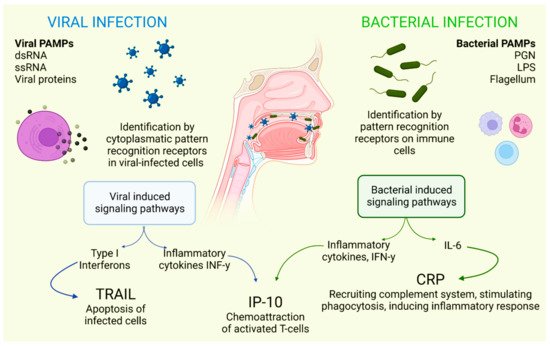
Figure 1. Selective host response against bacterial and viral infections. Different molecules and signaling pathways are dynamically involved in complementing the response to virus and bacterial infections (including CRP, IP-10 and TRAIL). IL-6: interleukin-6; LPS: lipopolysaccharide; PAMPs: pathogen-associated molecular patterns; PGN: peptidoglycan; ssRNA: single-stranded RNA; dsRNA: double-stranded RNA. Adapted with permission from Oved, K. et al. (2015) [32], which was distributed under the terms of the Creative Commons Attribution License https://creativecommons.org/licenses/by/4.0/; accessed date 28 November 2021.
Some drugs used in the treatment of viral infections can also contribute to damage to the oral cavity. High doses of corticosteroids may trigger fungal infections such as oral candidiasis; antiviral drugs can cause dry mouth, aphthous ulcers, and stomatitis; and the use of antiviral drugs can cause dry mouth [33]. Additionally, many patients have been prescribed antibiotics that are effective against Gram-negative and Gram-positive bacteria, which usually has a direct impact on the homeostasis of the mouth and all microorganisms found in this cavity [34].
Oral Cavity and SARS-CoV-2 Infection
As mentioned before, SARS-CoV-2 is an RNA-positive virus with an icosahedral morphology that possesses S proteins that are the binding site for ACE2 in humans [1], which, in addition to the lungs, pancreas, adipose tissue, liver, or kidney, this receptor is also expressed in salivary glands [35]. The oral cavity is a gateway for many pathogens, and SARS-CoV-2 is not the exception. This virus is detected in the saliva of all COVID-19 patients even with more sensitivity than that of nasopharyngeal testing [36].
When the ACE2 protein of the host cell and the S protein of SARS-CoV-2 bind, an interaction occurs that allows the coronavirus to use the machinery of these host cells to replicate and subsequently destroy these same cells, triggering the oral symptoms and signs [37]. In addition to this mechanism, which explains the cause of several manifestations of oral lesions caused by COVID-19, it is also possible that these lesions are the result of opportunistic infections that facilitate immune system alterations and may also be facilitated by possible systemic damage and adverse effects that can be triggered by treatment [38]. Figure 2 represents the location of ACE2 in different tissues and structures of the oral cavity, as well as those interacting with SARS-CoV-2.
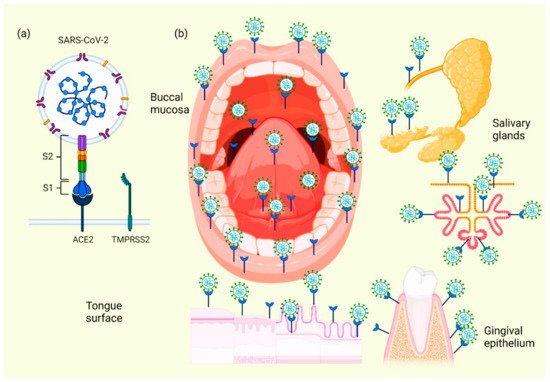
Figure 2. Location of ACE2 on oral cavity and its interaction with the SARS-CoV-2. Binding between the SARS-CoV-2 S protein and the ACE2 protein allows entry of the coronavirus, which subsequently allows its replication and the immediate activation of a possible innate immune response against the virus, including the infiltration of a myriad of immune cells and the subsequent production of many proinflammatory cytokines. This triggers the manifestation of symptoms and signs in the oral cavity of patients with COVID-19 (a). Various spaces and surfaces in the oral cavity where the virus and its receptors are detected, such as the oral mucosa, periodontal tissues, salivary glands, and tongue (b).
Cellular analyses of ACE2 expression as a SARS-CoV-2 entry factor revealed that no oral epithelial subpopulations are at particular risk. The ACE2 receptor was detected in nine oral epithelial cell groups, including basal 1–3, basal cyclic, salivary gland ducts, serous salivary glands, and mucous salivary glands, indicating that multiple oral epithelial cell subpopulations are prone to infection [39]. Coexpression of the most important entry factors ACE2 and TMPRSS2 in mucosal and salivary gland epithelial cells was rare in salivary gland acini and ducts [40]. The clinical development of chemosensitivity disorders usually occurs at the beginning of the infection phase in which the first symptoms are already present, usually within the first three days [41]. Two theories have been described on the pathophysiological factors causing dysgeusia and loss of olfaction in the course of COVID-19 infection: the first theory indicates that SARS-CoV-2 infects neurons using active cellular transport to gain access to the central nervous system [42]. The second theory suggests that these dysfunctions are due to the inhibition of the ACE2 receptor; it hasnhibitors of this protein have already been reported that inhibitors of this protein ino induce ageusia through a complex mechanism involving the sodium channel present in the taste buds and the G -protein-coupled receptor; upon interaction between SARS-CoV-2 and hthe host cell receptors, the latter are inactivated, resulting in the loss of chemical taste signaling in their action potentials and, therefore, of the correct sensory perception of taste [ 41 ] [41].
