1. Introduction
Energy plays a vital role in ensuring national security. It becomes a concern because the demand is growing rapidly along with economic development, population growth, and technological development. Currently, energy resources rely on non-renewable energy, such as fossil fuels (coal, natural gas, and oil). Meanwhile, the availability of these resources is finite and will be vanished if they continue to be exploited unwisely. On the other hand, fossil fuel combustion produces CO2 that will exacerbate global warming.
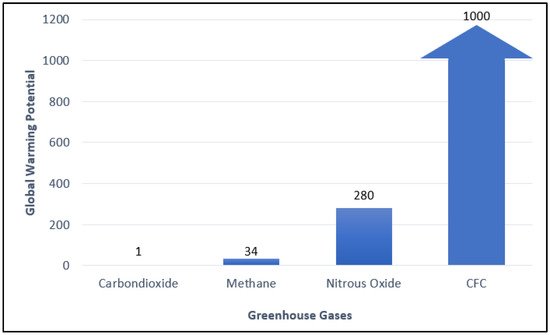
The global warming issue has attracted a lot of attention. Through the 2015 Paris Agreement, many countries have committed to contributing to climate change mitigation and reducing greenhouse gas emissions. According to Environmental Protection Agency (EPA), methane is the second-largest contributor to climate change accounting for about 10% of all greenhouse gasses emitted in 2019. The Intergovernmental Panel on Climate Change (IPCC) states that comparatively, it has 34 times greater Global Warming Potential (GWP) than carbon dioxide (Figure 1). Methane is emitted from natural sources (wetlands, termites, marine, freshwater, and CH4 hydrate) and human activities (energy, industry, agriculture, land use, and waste management). Globally, 50–65 percent of total methane in the atmosphere emits from human activities.
Figure 1. Global Warming Potential (GWP) of various types of greenhouse gases
[1].
Methane is the simplest hydrocarbon with a symmetric tetrahedral structure leading methane to have low polarizability and low electron and proton affinity
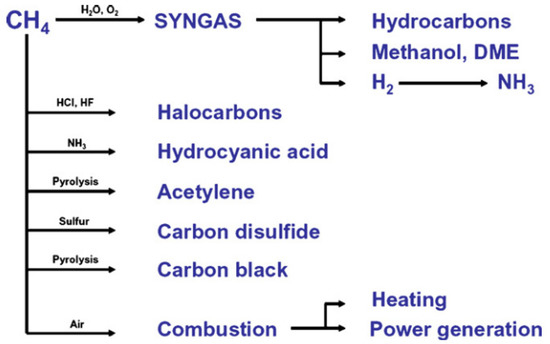
[2]. Methane is a very stable and non-reactive substance. Therefore, it is difficult to activate the C-H bond; hence, high pressure and temperature are required. As the main component of natural gas, methane has a high caloric value and is classified as a good hydrogen source. It is converted into various chemicals such as syngas for ammonia production, methanol, hydrocarbon, acetylene, and carbon disulfide. It can also be used directly as fuel by direct combustion (
Figure 2).
Figure 2. Utilization of methane in industries
[3]. Reproduced with permission from Holmen. Copyright © 2022 Elsevier.
Methanol and ammonia are the most critical methane conversion products required in large quantities
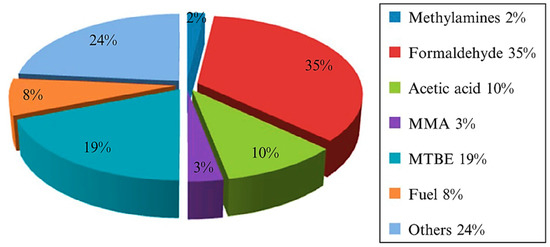
[3]. Liquefaction of methane to methanol is preferred over gaseous methane because the liquid state is easier to handle, store, and transport. Methanol is also vital in the industry since it is used as feedstock for textiles, pharmaceuticals, plastics, and biodiesel. In addition, methanol is clean energy and intermediate source for a wide range of industrial applications (
Figure 3). Therefore, converting methane into higher value-added chemicals such as methanol is necessary to develop sustainable energy sources and address global warming issues.
Figure 3. Products of Methanol in the industry
[4]. Reproduced with permission from Dalena et.al. Copyright © 2022 Elsevier.
Methanol can be produced by selective oxidation through indirect or direct methods
[5]. Industrially, indirect methods are employed for the production of methanol. The process produces intermediate syngas (CO and H
2) through steam or dry reforming, then hydrogenated to obtain methanol. However, the syngas process is highly endothermic, requiring a large external energy supply. Therefore, this process still has many drawbacks, such as high energy consumption, complicated operation, catalyst deactivation, and reactor stability
[6][7]. The direct method of methanol production is desirable to reduce production costs because the process bypasses syngas production. However, the target product is still unsatisfactory due to the low selectivity of methanol.
Zakaria and Kamarudin
[8] have summarized several relevant technologies to convert methane into methanol. These include conventional catalytic processes, plasma technology, supercritical water, biological (membrane), and photocatalytic technology. However, some drawbacks are still inevitable, including (i) the relatively slow selectivity of methanol formation for conventional catalytic technology; (ii) low conversion productivity and limited by the amount of methane that can be dissolved in water for plasma technology; (iii) reactor design and high corrosion rate when treating effluents containing halogen for supercritical water processes; (iv) membrane incompatibility due to methane permeability, which can cause dissolution, swelling, or breakage of the membrane; and (v) limitations of investigation of photocatalysts and suitable photoreactors for photocatalytic technology
[8].
It is desirable to synthesize methane at a lower temperature to reduce energy consumption and maintain catalyst stability. Among several technologies, photocatalytic technology seems to be a promising method for the conversion of methane conversion to methanol, as it offers an effective way to produce methanol using renewable solar energy under ambient conditions. Furthermore, photon energy that exceeds activation energy for a specific chemical reaction promotes the redox reaction. Therefore, the involvement of solar energy to facilitate photocatalytic reaction exhibits more environmentally friendly, sustainable, and renewable than fossil fuel-based energy. This technology can also reduce production costs and increase economic benefits. In addition, catalyst deactivation will be significantly reduced due to the low temperature and pressure used (under ambient conditions) in the photocatalytic process.
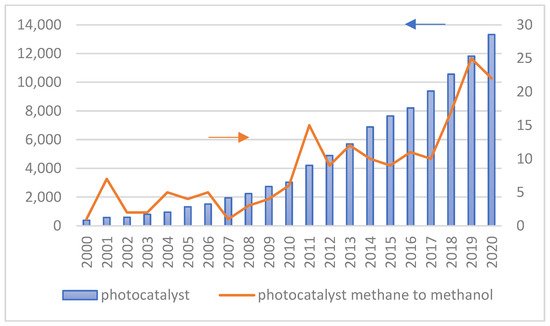
Figure 4 shows the number of publications on the utilization of photocatalytic technology in general and specifically for converting methane to methanol from 2000 to 2020. The increasing number of scientific publications on photocatalytic technology for methane to methanol conversion provides clear evidence that this topic is significant. In addition, many researchers studied the effect of different structure engineering and nanomaterials on the performance of photocatalysts since their energy conversion efficiency is principally influenced by the physicochemical properties of photocatalysts and operating conditions during the photocatalytic reaction.
Figure 4. The number of research Scopus with TITLE–ABS–KEY photocatalyst–photocatalytic and photocatalyst–methane–methanol.
2. Heterogeneous Photocatalyst for Photo-Oxidation Methane to Methanol
Photocatalysis is commonly referred to as artificial photosynthesis since the photosynthetic system inspires the process. The essence of natural photosynthesis is to drive chemical reactions using optical photon energy. Upon illumination of sunlight, oxygen (O
2) is produced from the oxidation of water (H
2O) and carbon dioxide (CO
2), which further transform to generate hydrocarbons with the help of chlorophylls to absorb and transfer photon energy
[9]. The broad definition of photocatalysis can also be adopted from IUPAC, which can be defined as a “Change in the rate of a chemical reaction or its initiation under the action of ultraviolet, visible or infrared radiation in the presence of a substance—the photocatalyst—that absorbs light and is involved in the chemical transformation of the reaction partners.”
Currently, photocatalytic technology has been widely applied to solve problems related to air pollution
[10] and environmental remediation of water contaminants
[11], including dyes degradation
[12], organic pollutants removal
[13][14][15], heavy metals removal
[16], and oil degradation in wastewater
[17]. In addition, photocatalytic technology provides alternative ways to produce renewable energy, including water splitting, CO
2 reduction, N
2 fixation, and, in particular, CH
4 oxidation to methanol
[9].
2.1. Thermodynamics for Methane to Methanol Conversion
Methane has very stable and strong C-H bonds in the CH4 molecules, which remain challenging to convert methane into more useful hydrocarbon and hydrogen.
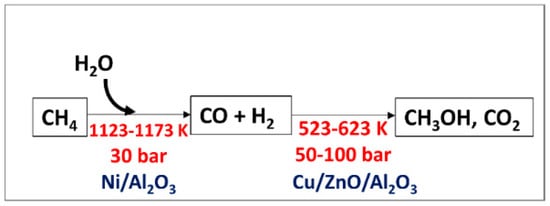
Methanol from methane in the industry is produced indirectly from a steam reforming process using a nickel-based catalyst followed by high-pressure catalytic conversion of the synthetic gas to methanol (
Figure 5). Steam reforming is an unfavorable thermodynamic reaction and can only be proceeded at high temperature and pressure. Nevertheless, this process can produce H
2 with high purity. However, there are still challenges, such as high energy consumption due to the formation of highly endothermic syngas
[5], low catalyst activity, which still needs further development
[18], and complex treatment to prevent heat transfer problems
[18].
Figure 5. Indirect process of methanol production (Sharma et al., 2020). Reproduced with permission from Sharma et al. Copyright © 2022, MDPI.
Methanol can also be obtained from the direct conversion of methane with water. Thermodynamically, converting methane into methanol using water as a reactant is an endothermic process (ΔH0298 = 77 kJ/mol). However, this process is thermodynamically unfavorable at any temperature, as reflected by the Gibbs free energy data at a temperature range of 273 K to 1500 K. Furthermore, photocatalytic reaction offers an alternative way to facilitate these unfavorable reactions under ambient conditions. The process includes methane and water as the reactant and light as the driving force.
2.2. Proposed Mechanism of Photocatalytic Conversion of Methane to Methanol
The development of the conversion of methane to methanol by photocatalytic technology was initiated by Ogura & Kataoka
[19]. The reaction involves water vapor at temperatures below 100 °C, methane gas, and UV irradiation at atmospheric pressure. First, methane gas continuously flowed into the water surface to obtain a mixture of water vapor and methane. Then, the mixture of water vapor and methane reacted and produced methanol as the main product. The brief mechanism of photochemical methane conversion in the research conducted by Ogura and Kataoka
[19] is shown in Equations (1)–(4).
The reaction begins with the photolysis of water to produce a hydroxyl radical (•OH). The hydroxyl radical then reacts with methane to produce methyl radicals (•CH
3). Finally, the methyl radical reacts with other water molecules to produce methanol and hydrogen. Furthermore, Noceti et al.
[20] combined two reactions, including water splitting and methane conversion, to produce methanol and hydrogen using La/WO
2-based photocatalyst, as shown briefly in Equations (5)–(10). The reaction was carried out at a temperature of 94 °C, atmospheric pressure, and with visible light irradiation has succeeded in oxidizing methane with water as an oxidizing agent. As a result, the reaction produces methanol and hydrogen with a methane conversion of 4%.
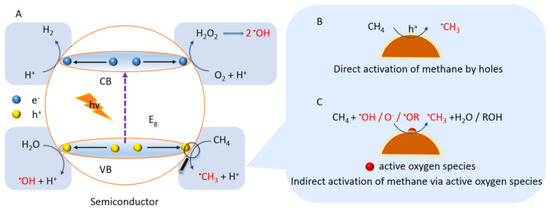
However, the first obstacle in the photocatalytic conversion of methane to methanol is how to activate the highly stable methane molecule. There are two possible scenarios for activating methane using a semiconductor-based catalyst
[21]. Methane can be activated directly by photogenerated holes in the VB where •OH is produced by reduction of H
2O
2 and oxidation of H
2O while •O
2− produced during the reaction by H
2O
2 and •OH. Methanol can be obtained by: (i) reaction of methyl radical (•CH
3) with •OH, (ii) reaction of •OCH
3 with H
2O, or (iii) reduction of CH
3OOH generated by the integration of •CH
3 and O
2. Methane can also be activated indirectly by any active oxygenated radical species, as illustrated in
Figure 6. This reaction depends on the reaction of the applied system, such as the type of semiconductor and any other additional substance added.
Figure 6. Schematic mechanism of methane activation by semiconductor
[22]. Reproduced with permission from Lin et al. Copyright © 2022 Elsevier.
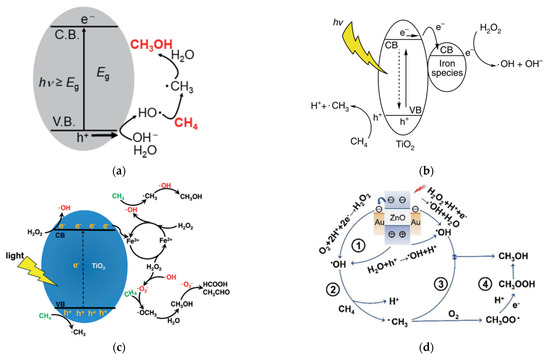
Gondal et al.
[23] proposed a mechanism for methane activation through •OH produced by the photocatalytic process. Several studies have adopted this mechanism, as illustrated in
Figure 7a
[24][25]. Xie et al.
[26] proposed a typical reaction of photocatalytic methanol production from methane with simultaneous production of •OH by reduction of H
2O
2 and •CH
3 by oxidation of methane in the VB by the photogenerated hole. Methanol is obtained through the reaction between •CH
3 and •OH, which probably occurs in iron species as a dopant. Zeng et al.
[27] proposed a mechanism for converting methane to methanol with the addition of iron ions and H
2O
2 species called photocatalysis of the Fenton reaction (PCFR) (
Figure 7b). The schematic mechanism for selective conversion of methane to methanol can also be achieved, as illustrated in
Figure 7c. Zhou
[28] proposed a methane activation mechanism by reducing O
2 in the conduction band and oxidation of H
2O in the valence band. Methanol will be produced by a direct combination of •CH
3 and •OH and reduction of CH
3OOH generated by the integration of •CH
3 and O
2 (
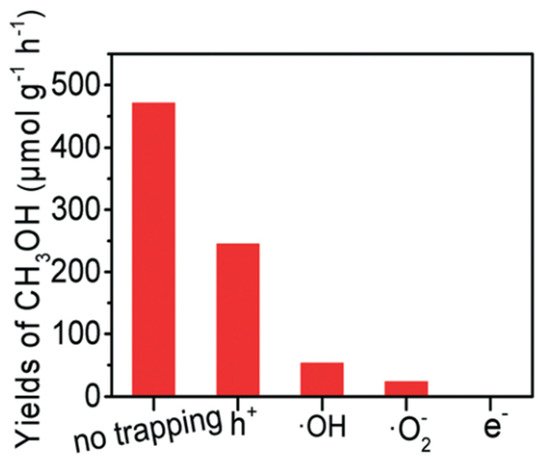
Figure 7d). Moreover, the role of each active species, including e
−, •O
2−, •OH, and h
+ was studied by utilizing K
2Cr
2O
7, para-quinone, salicylic acid and Na
2C
2O
4 as scavengers to trap those active species formed in the PCFR process, respectively (
Figure 8). Based on these findings, e
− played the most dominant role in methanol production, followed by •O
2−, •OH, and h
+.
Figure 7. Several proposed schematic mechanisms for selective conversion of methane to methanol were reported by (
a) Negishi et al.
[25], (
b) Xie et al.
[26], (
c) Zeng et al.
[27], and (
d) Zhou et al.
[28]. Reproduced with permission from Negishi et al., Copyright © 2022 ASM Journals, Xie et al., Copyright © 2022 Springer Nature, Zeng et al., Copyright © 2022 The Royal Society of Chemistry, and Zhou et al. Copyright © 2022 The Royal Society of Chemistry.
Figure 8. Trapping experiment of active species
[23]. Reproduced with permission from Zeng et al. Copyright © 2022 The Royal Society of Chemistry.
Based on several studies, it is found that the hydroxyl radical plays a crucial role in the photocatalytic conversion of methane to methanol. This species can be obtained either by photooxidation of H
2O or photoreduction of H
2O
2. In general, H
2O
2 is added to facilitate the formation of •OH and, thus, enhance photocatalytic efficiency. For example, Noceti et al.
[20] reported that adding H
2O
2 to the reaction carried out at atmospheric pressure, and temperature of 94 °C with irradiation from a mercury lamp (46% visible light) increased methane conversion from 4% to 10%, accompanied by increased CO
2 formation. Zeng et al.
[27] also reported excellent results in reactions using TiO
2 photocatalyst and addition of H
2O
2 under visible light irradiation, with methanol yields up to 850 mol g
−1 h
−1 and a selectivity of 83%.
On the other hand, a study conducted by Gondal et al.
[29] showed the opposite effect, where the addition of H
2O
2 exhibited a lower yield of methanol than the reaction without H
2O
2 addition using WO
3 photocatalyst under visible laser irradiation and ambient conditions. These results are believed to be due to the usage of a visible laser lamp providing higher photon flux density monochromatic light than the conventional lamp so that more •OH was formed. These •OH radicals are also a major source of O
2 that facilitates further oxidation of methanol. These results agree with Villa et al.
[30], which carried out a reaction using WO
3 photocatalyst at atmospheric pressure, the temperature of 55 °C, and under UVC-Vis irradiation. It was found that the formation of ethane accompanied the methanol production due to the higher amount of •OH activated methane to generate more •CH
3, as shown in Equation (11).
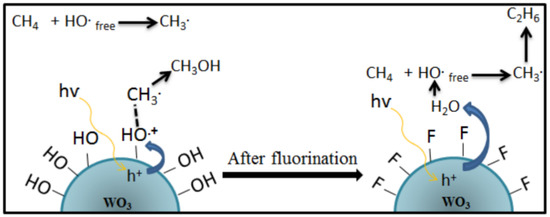
Based on these findings, Villa et al.
[24] proposed a strategy to incorporate fluorine on the WO
3 photocatalyst surface to minimize the interaction between the catalyst and the reactants. The schematic reaction mechanism on the surface of the WO
3 before and after the incorporation of fluorine is shown in
Figure 9. It was found that •OH groups on the catalyst surface are mainly responsible for enhancing the performance of WO
3 in the selective oxidation of methane to methanol. At the same time, an enormous amount of free OH radicals favor the formation of ethane. Therefore, adding a hydroxyl radical generator, i.e., H
2O
2, can increase methane conversion
[31]. However, such an appropriate amount of H
2O
2 is essential for controlling the selectivity of methanol
[21][23][27].
Figure 9. The reaction mechanism of photocatalytic conversion of methane to methanol on the surface of the WO
3 catalyst after fluorination
[24]. Reproduced with permission from Villa et al. Copyright © 2022 Elsevier.
Methanol, the primary desired main product of methane oxidation, is more reactive than methane itself. Thus, methanol can be easily oxidized further when some of the following conditions occur, such as:
1. The formation of superoxide radicals (•O
2−) produced via the reduction of O
2 in CB of semiconductor
[23], shown in Equations (12)–(14).
According to the abovementioned issues, it is essential to control the oxidative power of the system to achieve optimum methanol selectivity. For instance, using a moderate photocatalyst lowers the potential reduction of H
2O
2 [26] and prevents O
2 reduction to form •O
2− by adding an electron scavengers agent
[29]. In addition, the yield of methanol can be enhanced by continuous removal of the methanol after its formation to prevent further oxidation of methanol
[32].