Tabebuia impetiginosa, a plant native to the Amazon rainforest and other parts of Latin America, is traditionally used for treating fever, malaria, bacterial and fungal infections, and skin diseases.
- Tabebuia impetiginosa,
- pharmacological activities, anti-inflammatory
Definition
1. Introduction
Tabebuia impetiginosa, a plant native to the Amazon rainforest and other parts of Latin America, is traditionally used for treating fever, malaria, bacterial and fungal infections, and skin diseases.
1. Introduction
Historically, people have used natural products such as plants, animals, microorganisms, and other biological resources to assuage and cure diseases [1]. Many of the commercial drugs (such as atropine, teniposide, aescin, digoxin, silymarin, and so on) available today were initially developed from plants and other biological resources used in traditional medicines [2,3][2][3]. Therefore, knowledge of the traditional use of natural products plays a large role in drug discovery and development.
Tabebuia impetiginosa (Mart. Ex DC. Mattos) is a plant belonging to the family Bignoniaceae, which is mainly distributed in the Amazon rainforest and other tropical regions of Central and Latin America [4]. It is not only a decorative plant but also has high pharmaceutical value. T. impetiginosa has been used as a traditional medicine to treat various diseases and has antinociceptive, anti-edematogenic, antibiotic, and antidepressant effects [5,6,7][5][6][7]. Moreover, the inner bark of this tree can be made into poultice or concentrated tea to treat various skin inflammatory diseases [8]. Several categories of compounds have been isolated and identified from T. impetiginosa, principally quinones, flavonoids, naphthoquinones, and benzoic acids [9,10,11,12][9][10][11][12]. In recent years, many investigations have demonstrated that extracts or compounds isolated from T. impetiginosa reveal an extensive range of pharmacological activities such as anti-obesity, antifungal, anti-psoriatic, antioxidant, anti-inflammatory, and anti-cancer activities [4,7,13,14,15,16,17,18][4][7][13][14][15][16][17][18]. It is particularly prominent in immunopharmacology. Typically, the mechanism of anti-inflammatory activity of extract from the inner bark of Tabebuia was studied through a molecular biological approach. Nevertheless, the clinical applications of T. impetiginosa have been poorly researched, and there is a void of information on its mechanisms of action.
2. Traditional Uses
T. impetiginosa has been used traditionally to treat cancer [24], obesity [25], depression [26], viral, fungal, and bacterial infections [27], and inflammatory symptoms such as pain [28], arthritis [15], colitis [29], and prostatitis since the Inca civilization. The Callawaya Tribe makes a concentrated tea out of the tree’s inner bark for treating skin inflammatory diseases [8]. Moreover, it can be used as an astringent and diuretic [30]. Caribbean folk healers utilize the bark and leaves ofhas been used traditionally to treat cancer [19], obesity [20], depression [21], viral, fungal, and bacterial infections [22], and inflammatory symptoms such as pain [23], arthritis [15], colitis [24], and prostatitis since the Inca civilization. The Callawaya Tribe makes a concentrated tea out of the tree’s inner bark for treating skin inflammatory diseases [8]. Moreover, it can be used as an astringent and diuretic [25]. Caribbean folk healers utilize the bark and leaves of
T. impetiginosa to cure toothaches, backaches, and sexually transmitted diseases [31]. Latino and Haitian populations were also reported to use this plant for the treatment of infectious disease [32]. Brazilian people have traditionally used this plant for anti-inflammatory, analgesic, and antiophidic purposes against snake venom [33]. Traditional healers in Brazil prescribedto cure toothaches, backaches, and sexually transmitted diseases [26]. Latino and Haitian populations were also reported to use this plant for the treatment of infectious disease [27]. Brazilian people have traditionally used this plant for anti-inflammatory, analgesic, and antiophidic purposes against snake venom [28]. Traditional healers in Brazil prescribed
T. impetiginosa for cancer and tumor prevention or treatment; 69.05% for the treatment of tumors and cancer in general and 30.95% for specific tumors or cancers [34]. Such ethnomedicinal uses offor cancer and tumor prevention or treatment; 69.05% for the treatment of tumors and cancer in general and 30.95% for specific tumors or cancers [29]. Such ethnomedicinal uses of
T. impetiginosaled us to pay attention to it for a full understanding of its immunopharmacological properties for the future development of an effective drug against ethnopharmacologically targeted diseases with this plant.
3. Phytochemistry
Several categories of phytochemicals have been identified in the leaves, bark, and wood of
T. impetiginosa.From
T. impetiginosa bark, 19 glycosides comprised of four iridoid glycosides, two lignan glycosides, two isocoumarin glycosides, three phenylethanoid glycosides, and eight phenolic glycosides were methanol-extracted [35]. Major constituents ofbark, 19 glycosides comprised of four iridoid glycosides, two lignan glycosides, two isocoumarin glycosides, three phenylethanoid glycosides, and eight phenolic glycosides were methanol-extracted [30]. Major constituents of
T. impetiginosaare furanonaphthoquinones, naphthoquinones, anthraquinones (e.g., anthraquinone-2-carboxylic acid (Compound
1in
Figure 2)), quinones, benzoic acid, flavonoids, cyclopentene dialdehydes, coumarins, iridoids, and phenolic glycosides [4,8,36]. The presence of naphthoquinones attracted scientific attention, with lapachol (1)), quinones, benzoic acid, flavonoids, cyclopentene dialdehydes, coumarins, iridoids, and phenolic glycosides [4][8][31]. The presence of naphthoquinones attracted scientific attention, with lapachol (
2) and β-lapachone (
3) especially piquing the interest of professionals in the medical field. Lapachol inhibits proliferation of tumor cells, while β-lapachone exhibits strong toxicity in murine and human cells. Lapachol has been shown to reduce the number of tumors caused by doxorubicin in
Drosophila melanogasterheterozygous for the tumor suppressor gene. Lapachol can also decrease the invasion of HeLa cells, which could represent an interesting scaffold for the development of novel antimetastatic compounds [4].
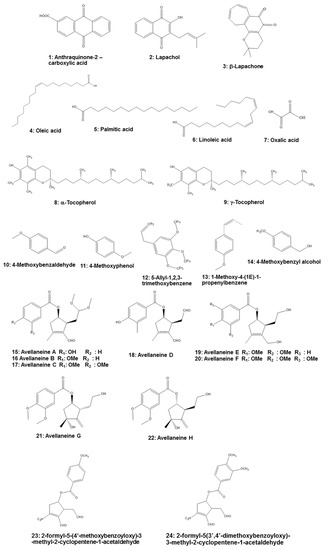
Chemical structures of
-derived components.
Fatty acids, especially oleic acid (
4), palmitic acid (
5), and linoleic acid (
6), are found in the bark of
T. impetiginosa. Free sugars also were identified in the bark, with glucose being the most abundant, followed by fructose and sucrose. Organic acids, especially oxalic acid (
7), are present, as well as the fat-soluble alcohols α-tocopherol (
8) and γ-tocopherol (
9). α-Tocopherol can reduce cardiovascular disease risk and neurodegenerative disorders [4]. In addition,
T. impetiginosahas some volatile constituents that exhibit antioxidant activity. The major volatile constituents in
T. impetiginosainclude 4-methoxybenzaldehyde (
10), 4-methoxyphenol (
11), 5-allyl-1,2,3-trimethoxybenzene (
12), 1-methoxy-4-(1
E)-1-propenylbenzene (
13), and 4-methoxybenzyl alcohol (
14) [37].) [32].
Cyclopentene derivatives are secondary metabolites of plants, and this constituent from
T. impetiginosacontained six known cyclopentenyl esters (avallaneine A–F (
15–20)), two new cyclopentyl esters (avallaneine G (
21) and H (
22)), and two known cyclopentenyl esters. These cyclopentene derivatives may provide a significant anti-inflammatory effect on the lipopolysaccharide (LPS)-mediated inflammatory response by blocking the production of NO and PGE
2; therefore, it is important to determine the molecular mechanism whereby cyclopentenyl esters from
T. impetiginosa inhibit inflammatory responses [16]. Moreover, Koyama et al. [38] isolated two cyclopentene dialdehydes, 2-formyl-5-(4′-methoxybenzoyloxy)-3-methyl-2-cyclopentene-1-acetaldehyde (inhibit inflammatory responses [16]. Moreover, Koyama et al. [33] isolated two cyclopentene dialdehydes, 2-formyl-5-(4′-methoxybenzoyloxy)-3-methyl-2-cyclopentene-1-acetaldehyde (
23) and 2-formyl-5-(3′,4′-dimethoxybenzoyloxy)-3-methyl-2-cyclopentene-1-acetaldehyde (
24), that exert anti-inflammatory activity in human leukocytes. Thus, it is necessary to further investigate their activities.
3. Pharmacological Activities
Previous research has indicated various pharmacological effects of T. impetiginosa and its crude extracts and chemical compounds in a series of in vitro and animal models. It exhibits antibacterial, antioxidant, antifungal, antinociceptive, antidiabetic, anti-edema, anti-inflammatory, and anti-cancer activities at different concentrations or doses. The main pharmacological activities of extracts or compounds isolated from T. impetiginosa reported in in vitro and in vivo studies are briefly summarized in Table 31 and described in detail in the following subsections.
Immunopharmacological effects of
| Pharmacological Activity | Extract/Isolated Compounds | Model | Concentration/Dose | Results | Ref. |
|---|
| Immunomodulatory | Water extract | RAW264.7 (murine macrophage cell), U937 (human promonocytic cell) | 50, 100, 200, and 400 μg/mL | Maintained cluster formation of RAW264.7 cells even after lipopolysaccharide (LPS) treatment. Downregulated the phagocytic uptake of FITC-labeled dextran. Upregulated cell-cell interactions by decreasing migration of cells and enhancing CD-29-mediated cell-cell adhesion and the surface levels of adhesion molecules and costimulatory molecules linked to macrophage stimulation, as seen in upregulation of reaction oxygen species (ROS) release. Suppressed an alteration in the membrane level of macrophages (phagocytic uptake and morphological changes). |
[39] | [34] | ||||||||||||
| Ethanol extract | IL-2-independent T-lymphocyte | 0.25, 0.5, 0.75, 0.9, and 1.0, mg/mL | Inhibited activation and proliferation of IL-2-independent T-lymphocyte | [40] | [35] | |||||||||||||
| Anti-inflammatory | Water extract | LPS-stimulated macrophages, arachidonic acid, or croton oil-induced mouse ear edema models | 0–400 μg/mL, 100–400 mg/kg |
Inhibited the production of NO and PGE | 2 | and suppressed the mRNA levels of COX-2 and iNOS. Curative effect in an in vivo PGE | 2 | -based inflammatory symptoms model induced by arachidonic acid. | [8] | |||||||||
| Ethanol extract | TPA- or arachidonic acid-induced ear edema, hot plate, acetic acid-induced vascular permeability in rats | 100, 200, or 400 mg/kg | Inhibited inflammation of paw edema, ear inflammation, and dye leakage in the vasculature using various animal models including acetic acid-induced vascular permeability, 12- | O | -tetradecanoylphorbol-13-acetate (TPA)-induced ear edema, arachidonic acid-induced mouse ear edema, and carrageenan-induced paw. | [28] | [23] | |||||||||||
| Five novel compounds | Human myeloma THP-1 cells | 25 μM | Showed inhibitory activity on production of the inflammatory cytokines, such as TNF-α and IL-1β. | [41] | [36] | |||||||||||||
| Cyclopentene derivatives | RAW264.7 cells | 12.5, 25, 50 μg/mL | Suppressed the production of NO and PGE | 2 | . | [16] | ||||||||||||
| Anti-cancer | Naphthoquinones | MDA-BB-231, MCF7, and A549 cells | 0–30 μM | Inhibited growth of cancer cell lines and STAT3 phosphorylation activity. | [14] | |||||||||||||
| Water extract | Estrogen receptor (ER) | + | human mammary carcinoma MCF-7 cell line | 0.05, 0.125, 0.25, 0.5, 0.75, 1.5 mg/mL | Exhibited dose-dependent growth inhibition of MCF-7 cells. | [24] | [19] | |||||||||||
| β-lapachone | A549 human lung carcinoma cells | Inhibited growth of A549 cells and telomerase activity; induced apoptosis by reducing the expression of Bcl-2, increasing the expression of Bax, and activating caspase-3 and caspase-9. | [13] | |||||||||||||||
| β-lapachone | HepG2 hepatoma cell line | Inhibited the activity of HepG2 by inducing apoptosis; downregulation of Bcl-2 and Bcl-X | L | , upregulation of Bax expression; induced apoptosis by activating caspase-3 and caspase-9 and degrading poly (ADP-ribose) polymerase protein. | [42] | [37] | ||||||||||||
| Methanol extract | Human tumor cell lines MCF-7, NCI-H460, HeLa, and HepG2; porcine liver primary cells (PLP2). | GI50 values: 110.76 ± 5.33 µg/mL (MCF-7), 76.67 ± 7.09 µg/mL (NCI-H460), 93.18 ± 1.46 µg/mL (HeLa), 83.61 ± 6.61 µg/mL (HepG2), and >400 µg/mL (PLP2). | Showed cytotoxic effects on MCF-7, NCI-H460, HeLa, and HepG2 cells. | [4] | ||||||||||||||
| Antinociceptive | Ethanol extract | Acetic acid-induced writhing response in rats | 100, 200, or 400 mg/kg | Increased the pain threshold in a mouse model when assessed through the hot plate test and inhibited the number of writhes compared to controls in the acetic acid-induced writhing responses mouse model. | [28] | [23] | ||||||||||||
| Osteoarthritis | Ethanol extract | RAW264.7 cells and chondrosarcoma cell line (SW1353); monoiodoacetate (MIA)-induced osteoarthritis in rats | 75, 150, and 300 μg/mL | Showed a chondroprotective effect by preventing cartilage degradation through targeting of NF-κB and AP-1 signaling pathways in macrophage and chondrocyte cells. Downregulated MMP2, MMP9, and MMP13 in a PMA-induced, dose-dependent manner; no effect on the gene expression of COL2A1 and CHSY1. |
[15] | |||||||||||||
| Colitis | Water extract | RAW264.7 cells Dextran sulfate sodium (DSS)-induced colitis in mice |
100, 300, 900, and 2700 μg/mL 2 mg/day, a total of 5 days |
Activated DC to produce immunosuppressive IL10; upregulated anti-inflammatory Th2 and Foxp3 | + | Treg cells in mesenteric lymph node (MLN) and downregulated pro-inflammatory Th1 and Th17 cells. By upregulating type II T-assisted immune response, weight loss and inflammation of colon tissue were downregulated in DSS-induced colitis mice. |
[29] | [24] | ||||||||||
| Antioxidant | Methanol extract | EC50 values: 0.68 ± 0.03 (DPPH scavenging activity), 0.27 ± 0.01 (Reducing power), 0.23 ± 0.04 (β-carotene bleaching inhibition), 0.14 ± 0.01 (thiobarbituric acid Thiobarbituric acid reactive substances (TBARS) inhibition). | Showed the highest antioxidant activity, which may be related to its total phenol content. | [4] | ||||||||||||||
| Methanol, butanol, and water extracts | H | 2 | O | 2 | -induced NIH3T3 cells | 0–2 mg/mL | Regenerated superoxide dismutase (SOD), catalase, and glucose 6-phosphate dehydrogenase activities; enhanced the concentration of glutathione in the cell; protected proteins from oxidative attack of H | 2 | O | 2 | , reduced formation of malondialdehyde in the cell, and protected NIH3T3 cells from H | 2 | O | 2 | -induced oxidative stress. | [43] | [38] | |
| Volatile constituents | 5, 10, 50, 100, and 500 μg/mL | Displayed dose-dependent activity in antioxidant assays | [37] | [32] | ||||||||||||||
| Phenylpropanoid glycosides | Compound 5 had the highest antioxidant activity, with an IC | 50 | of 0.12 µM | Had inhibitory effects on cytochrome CYP3A4 enzyme | [18] | |||||||||||||
| Anti-obesity | n | -butanol extract | Ovariectomized (OVX) mice. 3T3-L1 cells | A total of 16 weeks | Preventing the accumulation of adipocyte in mice, weight loss and fat mass ↓ in ovariectomized mice. | [17] | ||||||||||||
| Ethanol extract | Triton WR-1339-treated Wistar rats | A total of 24,700 kJ/kg energy | Decreased postprandial triglycerides in rats given a fatty meal. | [25] | [20] | |||||||||||||
| Anti-allergic | Five novel compounds | RBL-2H3 cells | 100 μM | Inhibited release of β-hexosaminidase of the allergy marker. | [41] | [36] | ||||||||||||
| Antidepressant | Ethanol extract | Forced swimming test (FST) and tail suspension test (TST) in mice. | 100 mg/kg, p.o. (in the FST) and 10–300 mg/kg, p.o. (in the TST) | Produced antidepressant effects in the tail suspension test and forced swimming test. | [26] | [21] | ||||||||||||
| Antiplatelet | Methanol extract | Rabbit platelets and cultured rat aortic vascular smooth muscle cells (VSMCs) | 10, 50, 100, and 200 μg/mL | Reduced platelet aggregation by inhibiting arachidonic acid release and ERK1/2 MAPK activation. | [30] | [25] |
4. Conclusions
T. impetiginosa has been used as a traditional medicine in Central and South America to treat edema, arthritis, diuretic, and infections. Based on its traditional use, in vivo and in vitro experiments examining its pharmacological potential have been conducted. In vivo experiments were conducted using edema, osteoarthritis, animal paw edema, and writhing (and other) models to screen effects of T. impetiginosa. Moreover, there are numerous studies confirming that extracts or compounds isolated from T. impetiginosa have various pharmacological activities such as anti-obesity, antibacterial, antifungal, antiviral, anti-psoriatic, antioxidant, anti-inflammatory, and anti-cancer activities.
Currently, substantial progress has been made in exploration of the phytochemistry and pharmacological activity of T. impetiginosa. Nonetheless, there are still challenges and gaps in published research papers that should be further explored to establish its clinical application value. Firstly, the extracts and compounds isolated from T. impetiginosa possess multiple pharmacological activities, though most functional mechanisms remain unclear and need to be further explored through in vivo and in vitro experiments. Furthermore, most studies on T. impetiginosa are still in the in vitro and in vivo mouse model stages. Toxicological research can be conducted on other animals such as rabbits in the future to evaluate its safety, which will pave the way for further clinical trials. In addition, further comprehensive experiments are needed to enrich the data and discover other pharmacological uses of T. impetiginosa and to find the exact mechanisms by which its extracts bind to target proteins.
References
- Ngo, L.T.; Okogun, J.I.; Folk, W.R. 21st century natural product research and drug development and traditional medicines. Nat. Prod. Rep. 2013, 30, 584–592.
- Itokawa, H.; Morris-Natschke, S.L.; Akiyama, T.; Lee, K.-H. Plant-derived natural product research aimed at new drug discovery. J. Nat. Med. 2008, 62, 263–280.
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75.
- Pires, T.C.S.P.; Dias, M.I.; Calhelha, R.C.; Carvalho, A.M.; Queiroz, M.-J.R.; Barros, L.; Ferreira, I.C. Bioactive Properties of Tabebuia impetiginosa-Based Phytopreparations and Phytoformulations: A Comparison between Extracts and Dietary Supplements. Molecules 2015, 20, 22863–22871.
- De Miranda, F.G.G.; Vilar, J.C.; Alves, I.A.N.; Cavalcanti, S.C.D.H.; Antoniolli, Â.R. Antinociceptive and antiedematogenic properties and acute toxicity of Tabebuia avellanedae Lor. ex Griseb. inner bark aqueous extract. BMC Pharmacol. 2001, 1, 6.
- Freitas, A.E.; Moretti, M.; Budni, J.; Balen, G.O.; Fernandes, S.C.; Veronezi, P.O.; Heller, M.; Micke, G.A.; Pizzolatti, M.G.; Rodrigues, A.L.S. NMDA Receptors and the l-Arginine-Nitric Oxide-Cyclic Guanosine Monophosphate Pathway Are Implicated in the Antidepressant-Like Action of the Ethanolic Extract fromTabebuia avellanedaein Mice. J. Med. Food 2013, 16, 1030–1038.
- Fernandez, A.; Cock, I.E. Tabebuia impetiginosa (Mart. Ex DC. Mattos) Bark Extracts Inhibit the Growth Gastrointestinal Bacterial Pathogens and Potentiate the Activity of some Conventional Antibiotics. Pharmacogn. Commun. 2020, 10, 75–82.
- Byeon, S.E.; Chung, J.Y.; Lee, Y.G.; Kim, B.H.; Kim, K.H.; Cho, J.Y. In vitro and in vivo anti-inflammatory effects of taheebo, a water extract from the inner bark of Tabebuia avellanedae. J. Ethnopharmacol. 2008, 119, 145–152.
- Sharma, P.K.; Khanna, R.N.; Rohatgi, B.K.; Thomson, R.H. Tecomaquinone-III: A new quinone from Tabebuia pentaphylla. Phytochemistry 1988, 27, 632–633.
- Manners, G.D.; Jurd, L. A new naphthaquinone from Tabebuia guayacan. Phytochemistry 1976, 15, 225–226.
- Blatt, C.T.; Salatino, A.; Salatino, M.L. Flavonoids of Tabebuia caraiba (Bignoniaceae). Biochem. Syst. Ecol. 1996, 24, 89.
- Wagner, H.; Kreher, B.; Lotter, H.; Hamburger, M.O.; Cordell, G.A. Structure Determination of New Isomeric Naphtho[2,3-b] furan-4,9-diones fromTabebuia avellanedae by the selective-INEPT technique. Helvetica Chim. Acta 1989, 72, 659–667.
- Woo, H.; Choi, Y. Growth inhibition of A549 human lung carcinoma cells by β-lapachone through induction of apoptosis and inhibition of telomerase activity. Int. J. Oncol. 2005, 26, 1017–1023.
- Tahara, T.; Watanabe, A.; Yutani, M.; Yamano, Y.; Sagara, M.; Nagai, S.; Saito, K.; Yamashita, M.; Ihara, M.; Iida, A. STAT3 inhibitory activity of naphthoquinones isolated from Tabebuia avellanedae. Bioorganic Med. Chem. 2020, 28, 115347.
- Park, J.G.; Yi, Y.-S.; Hong, Y.H.; Yoo, S.; Han, S.Y.; Kim, E.; Jeong, S.-G.; Aravinthan, A.; Baik, K.S.; Choi, S.Y.; et al. Tabetri™ (Tabebuia avellanedae Ethanol Extract) Ameliorates Osteoarthritis Symptoms Induced by Monoiodoacetate through Its Anti-Inflammatory and Chondroprotective Activities. Mediat. Inflamm. 2017, 2017, 1–14.
- Zhang, L.; Hasegawa, I.; Ohta, T. Anti-inflammatory cyclopentene derivatives from the inner bark of Tabebuia avellanedae. Fitoterapia 2016, 109, 217–223.
- Iwamoto, K.; Fukuda, Y.; Tokikura, C.; Noda, M.; Yamamoto, A.; Yamamoto, M.; Yamashita, M.; Zaima, N.; Iida, A.; Moriyama, T. The anti-obesity effect of Taheebo (Tabebuia avellanedae Lorentz ex Griseb) extract in ovariectomized mice and the identification of a potential anti-obesity compound. Biochem. Biophys. Res. Commun. 2016, 478, 1136–1140.
- Suo, M.; Ohta, T.; Takano, F.; Jin, S. Bioactive Phenylpropanoid Glycosides from Tabebuia avellanedae. Molecules 2013, 18, 7336–7345.
- Telang, N.; Mukherjee, B.; Wong, G.Y. Growth inhibition of estrogen receptor positive human breast cancer cells by Taheebo from the inner bark of Tabebuia avellandae tree. Int. J. Mol. Med. 2009, 24, 253–260.
- Kiage-Mokua, B.N.; Roos, N.; Schrezenmeir, J. Lapacho Tea (Tabebuia impetiginosa) Extract Inhibits Pancreatic Lipase and Delays Postprandial Triglyceride Increase in Rats. Phytotherapy Res. 2012, 26, 1878–1883.
- Freitas, A.E.; Budni, J.; Lobato, K.R.; Binfaré, R.W.; Machado, D.G.; Jacinto, J.; Veronezi, P.O.; Pizzolatti, M.G.; Rodrigues, A.L.S. Antidepressant-like action of the ethanolic extract from Tabebuia avellanedae in mice: Evidence for the involvement of the monoaminergic system. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2010, 34, 335–343.
- Vasconcelos, C.M.; Vasconcelos, T.L.C.; Póvoas, F.T.X.; Santos, R.; Maynart, W.; Almeida, T.J.J.O.C.; Research, P. Antimicrobial, antioxidant and cytotoxic activity of extracts of Tabebuia impetiginosa (Mart ex DC.) Standl. J. Chem. Pharm. Res. 2014, 6, 2673–2681.
- Lee, M.H.; Choi, H.M.; Hahm, D.-H.; Her, E.; Yang, H.-I.; Yoo, M.-C.; Kim, K.S. Analgesic and anti-inflammatory effects in animal models of an ethanolic extract of Taheebo, the inner bark of Tabebuia avellanedae. Mol. Med. Rep. 2012, 6, 791–796.
- Park, H.J.; Lee, S.W.; Kwon, D.-J.; Heo, S.-I.; Park, S.-H.; Kim, S.Y.; Hong, S. Oral administration of taheebo (Tabebuia avellanedae Lorentz ex Griseb.) water extract prevents DSS-induced colitis in mice by up-regulating type II T helper immune responses. BMC Complement. Altern. Med. 2017, 17, 448.
- Son, D.J.; Lim, Y.; Park, Y.H.; Chang, S.K.; Yun, Y.P.; Hong, J.T.; Takeoka, G.R.; Lee, K.G.; Lee, S.E.; Kim, M.R.; et al. Inhibitory effects of Tabebuia impetiginosa inner bark extract on platelet aggregation and vascular smooth muscle cell proliferation through suppressions of arachidonic acid liberation and ERK1/2 MAPK activation. J. Ethnopharmacol. 2006, 108, 148–151.
- Mistrangelo, M.; Cornaglia, S.; Pizzio, M.; Rimonda, R.; Gavello, G.; Conte, I.D.; Mussa, A. Immunostimulation to reduce recurrence after surgery for anal condyloma acuminata: A prospective randomized controlled trial. Color. Dis. 2009, 12, 799–803.
- Camiel, L.D.; Whelan, J. Tropical American Plants in the Treatment of Infectious Diseases. J. Diet. Suppl. 2008, 5, 349–372.
- Malange, K.F.; Dos Santos, G.G.; Parada, C.A.; Kato, N.N.; Toffoli-Kadri, M.C.; Carollo, C.A.; Silva, D.B.; Portugal, L.C.; Alves, F.M.; Rita, P.H.S.; et al. Tabebuia aurea decreases hyperalgesia and neuronal injury induced by snake venom. J. Ethnopharmacol. 2019, 233, 131–140.
- De Melo, J.G.; Santos, A.G.; De Amorim, E.L.C.; Nascimento, S.C.D.; Albuquerque, U.P. Medicinal Plants Used as Antitumor Agents in Brazil: An Ethnobotanical Approach. Evidence-Based Complement. Altern. Med. 2011, 2011, 1–14.
- Warashina, T.; Nagatani, Y.; Noro, T. Constituents from the bark of Tabebuia impetiginosa. Phytochemistry 2004, 65, 2003–2011.
- Jin, Y.; Jeong, K.M.; Lee, J.; Zhao, J.; Choi, S.-Y.; Baek, K.-S. Development and Validation of an Analytical Method Readily Applicable for Quality Control of Tabebuia impetiginosa (Taheebo) Ethanolic Extract. J. AOAC Int. 2018, 101, 695–700.
- Park, B.-S.; Lee, K.-G.; Shibamoto, T.; Lee, S.-E.; Takeoka, G.R. Antioxidant Activity and Characterization of Volatile Constituents of Taheebo (Tabebuia impetiginosaMartius ex DC). J. Agric. Food Chem. 2003, 51, 295–300.
- Koyama, J.; Morita, I.; Tagahara, K.; Hirai, K.-I. Cyclopentene dialdehydes from Tabebuia impetiginosa. Phytochemitry 2000, 53, 869–872.
- Kim, B.H.; Lee, J.; Kim, K.H.; Cho, J.Y. Regulation of macrophage and monocyte immune responses by water extract from the inner bark of Tabebuia avellanedae. JMPR 2010, 4, 431–438.
- Böhler, T.; Nolting, J.; Gurragchaa, P.; Lupescu, A.; Neumayer, H.-H.; Budde, K.; Kamar, N.; Klupp, J. Tabebuia avellanedae extracts inhibit IL-2-independent T-lymphocyte activation and proliferation. Transpl. Immunol. 2008, 18, 319–323.
- Suo, M.; Isao, H.; Kato, H.; Takano, F.; Ohta, T. Anti-inflammatory constituents from Tabebuia avellanedae. Fitoterapia 2012, 83, 1484–1488.
- Woo, H.J.; Park, K.-Y.; Rhu, C.-H.; Lee, W.H.; Choi, B.T.; Kim, G.Y.; Park, Y.-M.; Choi, Y.H. β-Lapachone, a Quinone Isolated from Tabebuia avellanedae, Induces Apoptosis in HepG2 Hepatoma Cell Line Through Induction of Bax and Activation of Caspase. J. Med. Food 2006, 9, 161–168.
- Eun, L.S.; Soo, P.B.; Jong, H.Y. Antioxidative activity of taheebo (Tabebuia impetiginosa Martius ex DC.) extracts on the H2O2-induced NIH3T3 cells. J. Med. Plants Res. 2012, 6, 5258–5265.
