Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ya-Mei Chen and Version 2 by Conner Chen.
The swine coronaviruses replicate in the cellular cytoplasm exerting a wide variety of effects on cells. Some of these effects are particularly pertinent to cell pathology, including endoplasmic reticulum (ER) stress, unfolded protein response (UPR), autophagy, and apoptosis.
- coronaviruses
- ER stress
- UPR
1. Swine Coronaviruses
The family Coronaviridae consists of two subfamilies, Letovirinae and Orthocoronavirinae (Virus Taxonomy: 2020 Release (MSL #36); https://talk.ictvonline.org/taxonomy/ (accessed on 18 January 2022)). Orthocoronavirinae are comprised of four genera: Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus. Six swine coronaviruses (CoVs) have been identified: porcine epidemic diarrhea virus (PEDV), transmissible gastroenteritis virus (TGEV), porcine delta coronavirus (PDCoV), swine acute diarrhea syndrome coronavirus (SADS-CoV), porcine hemagglutinating encephalomyelitis virus (PHEV), and porcine respiratory coronavirus (PRCV) [1]. PEDV, TGEV, SADS-CoV, and PRCV belong to the Alphacoronavirus genus; PHEV belongs to the Betacoronavirus genus; and PDCoV belongs to the Deltacoronavirus genus [2].
Swine CoVs are enveloped, single-stranded, positive-sense RNA viruses, and the viral genome consists of open reading frame (ORF) 1a (ORF1a), ORF1b, HE, S, ORF3, E, M, ORF6, N, and ORF7 [1] (Figure 1). Among these genes, the S, E, M, and N genes encode structural proteins, including spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins, respectively. HE gene, only in PHEV, encodes hemagglutinin esterase. ORF1ab encodes 15–16 nonstructural proteins, and ORF 3, ORF6, and ORF7 encode accessory proteins. PEDV, TGEV, PDCoV, and SADS-CoV are enteropathogenic, leading to vomiting, diarrhea, and dehydration in pigs of all ages, especially in neonatal piglets. These enteropathogenic coronaviruses replicate in absorptive epithelial cells of the intestine, mainly in the jejunum and ileum, which later results in apoptosis, necrosis, and sloughing of epithelial cells [3][4][5][6][3,4,5,6] (Figure 2). PHEV and PRCV cause nervous disorder and respiratory diseases, respectively (Table 1).
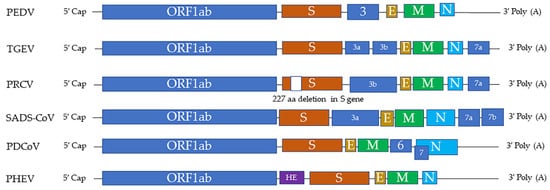
Figure 1. The genome structures of swine coronaviruses. ORF1ab, open reading frame genes 1a and 1b; S, spike; E, envelope; M, membrane; N, nucleocapsid; HE, hemagglutinin esterase; Ns3, Ns3a, Ns3b, Ns6, Ns7, Ns7a, Ns7b, accessory genes.
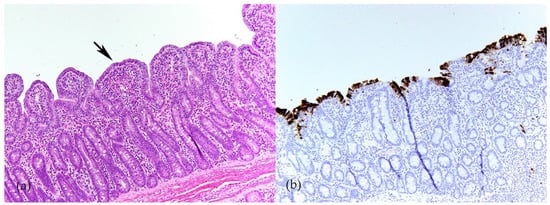
Figure 2. Porcine epidemic diarrhea virus (PEDV) infection in ileum of 4-week-old weaned pig. (a) Severe villous atrophy and villus fusion (arrow). Hematoxylin and eosin (HE). (b) Immunolabeling of PEDV in enterocytes (brown) indicates that PEDV mainly infect mature enterocytes on villi (Y-M Chen and E. Burrough, unpublished data, December 2017).
Table 1.
Diseases caused by natural infections of swine coronaviruses.
| Name of Virus/ Genera |
Major System Affected | Summary and Noteworthy Clinical and Pathological Findings | Ref. |
|---|---|---|---|
| PEDV/ Alphacoronavirus |
Enteric | Morbidity: 100% in piglets, less as pigs age Mortality: 50–100% in piglets ≤ 1 week of age Clinical signs: vomiting, watery diarrhea, anorexia, depression Gross lesions: distended small intestine containing yellow fluid and undigested milk Microscopic lesions: villous atrophy in the jejunum and ileum, necrosis of absorptive enterocytes in jejunum |
[1] |
| TGEV/ Alphacoronavirus |
Enteric | Morbidity: high morbidity in piglets ≤ 2 weeks of age, less as pigs age Mortality: up to 100% in piglets ≤ 2 weeks of age Clinical signs: similar to PEDV Gross lesions: similar to PEDV Microscopic lesions: similar to PEDV |
[1] |
| PDCoV/ Deltacoronavirus |
Enteric | Morbidity: up to 100% in piglets, less with age Mortality: up to 40% in suckling piglets Clinical signs: similar to PEDV Gross lesions: similar to PEDV and TGEV but less extensive Microscopic lesions: similar to PEDV and TGEV but less extensive |
[1] |
| SADS-CoV/ Alphacoronavirus |
Enteric | Morbidity: Up to 90% in piglets ≤ 5 days of age, less with age Mortality: over 35% in piglets ≤ 10 days of age Clinical signs: similar to PEDV Gross lesions: similar to PEDV and TGEV but less extensive Microscopic lesions: similar to PEDV and TGEV but less extensive |
[7][8][7,8] |
| PHEV/ Betacoronavirus |
Nervous | Morbidity: up to 100% in neonatal pigs Mortality: up to 100% in neonatal pigs Clinical signs: sneezing or coughing, nervous disorders, vomiting, wasting Gross lesions: cachexia, stomach dilatation, abdominal distension Microscopic lesions: nonsuppurative encephalomyelitis: perivascular cuffing, gliosis, and neuronal degeneration; most pronounced in the gray matter of the pons Varolii, medulla oblongata, and the dorsal horns of the upper spinal cord; degeneration of the ganglia of the stomach wall and perivascular cuffing |
[1] |
| PRCV/ Alphacoronavirus |
Respiratory | A variant of TGEV with a 227 aa deletion in S gene Morbidity: all ages of pigs can be infected Mortality: minimal (usually subclinical) Clinical signs: coughing, abdominal breathing, dyspnea Gross lesions: mild multifocal consolidation of the lung Microscopic lesions: bronchointerstitial pneumonia, airway epithelial necrosis, type 2 pneumocyte hypertrophy and hyperplasia |
[1] |
Abbreviations: Ref., references.
2. ER Stress
The endoplasmic reticulum (ER) in the cytoplasm of eukaryotic cells is responsible for protein synthesis and folding. ER stress is a condition in which ER homeostasis is disrupted, resulting in the accumulation of unfolded or misfolded proteins in the ER. Various physiological and pathologic factors can induce ER stress, such as gene mutations, hypoxia, nutrient deprivation, cell injury, and pathogen infection [9]. Cells initiate the unfolded protein response (UPR) to restore ER homeostasis [10]. Through a series of signal transduction pathways, the UPR removes aberrant proteins by inhibiting protein translation, increasing protein folding capacity, and promoting ER-associated degradation (ERAD) [11]. Three protein sensors, namely pancreatic ER eIF2α kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring transmembrane kinase/endonuclease 1 (IRE1), play critical roles in the UPR [11].
In the homeostatic ER, PERK, ATF6, and IRE1 are connected to the ER membrane by the ER chaperone glucose-regulated protein 78 (GRP78, also known as BiP) [11]. When the ER is stressed, GRP78 dissociates from the intraluminal domains of sensors into the ER lumen and activates these three proteins. Among the three sensors, PERK is activated firstly. Activated PERK phosphorylates the α subunit of eukaryotic translation initiation factor 2 (eIF2α), followed by the translation of activating transcription factor 4 (ATF4). As a transcription factor, ATF4 regulates genes involved in protein folding and the oxidative stress response. Second, activated ATF6 moves from the ER into the nucleus and stimulates UPR genes, resulting in elevated expression of X-box binding protein 1 (XBP1) and GRP78. As a result, an increased amount of GRP78 is considered the hallmark of ER stress and UPR [12]. Later, activated IRE1 splices XBP1 mRNA to generate a functionally active isoform of XBP1 (XBP1s), which is a transcription factor that regulates most UPR-associated genes [13]. Additionally, ER stress is associated with autophagy (Figure 3), which contributes to removing unnecessary or dysfunctional cellular components. For example, IRE1 is required for autophagy activation [14]. Therefore, the UPR induces a pro-survival adaptation via the PERK, ATF6, and IRE1 pathways.
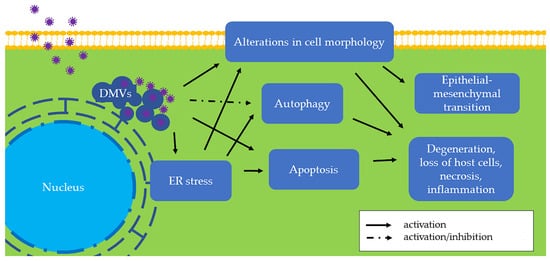
Figure 3. The effects of swine coronaviruses on host cells. Swine coronaviruses replicate in the cytoplasm of host cells and form double-membrane vesicles (DMVs), leading to endoplasmic reticulum (ER) stress, apoptosis, and alterations in cell morphology (Table 2). Swine coronaviruses may either induce or inhibit autophagy. These changes further evolve into degeneration, loss of host cells, necrosis, and inflammation. Furthermore, chronic alteration in cell morphology induces epithelial-mesenchymal transition (EMT). Some of these molecular and subcellular changes can be appreciated in vivo by pathological examination of tissues from infected swine, while others are found only in in vitro settings and remain to be found in clinical specimens (Table 1).
When ER stress is persistently unresolved, cells may undergo apoptosis or chronic ER stress (Figure 3). Apoptosis is a type of programmed cell death, and ER stress-induced apoptosis is responsible for eliminating cells under irremediable ER stress. For example, ATF4 upregulates C/EBP homologous protein (CHOP), which is a proapoptotic transcription factor [15][15]. The relationship between apoptosis and infection of swine coronaviruses is described in the section “Apoptosis in Swine CoV Infection” below. On the other hand, stressed but surviving cells can manage protein synthesis and adapt to chronic ER stress. For example, neoplastic cells under ER stress persistently express an elevated GRP78 level to adapt to a hostile microenvironment [16].
