Photodynamic therapy (PDT), is emerging as a minimally invasive therapeutic modality with precise controllability and high spatiotemporal accuracy in the field of diseases treatment. PDT mainly relies o, has earned significant advancements in the photosensitizers (PSs) to generate oxidative reactive oxygen species (ROS), to play the therapeutic role. Tfield of cancer and other non-cancerous diseases treatment. Thereinto, type I photosensitizers, that undergo hydrogen atom abstraction or electron transfer manner and subsequently produce superoxide radical (O2•−),PDT represents an irreplaceable and meritorious part in contributing to these delightful achievements since its distinctive hydrpoxyl radical (OH•), ia toler hydrogen peroxide (H2O2),ance can perfectly compensate for thetc., is showing more and more prominent advantages high oxygen-dependent type II PDT, particularly in hypoxic tissues, sinc. Regarding the diverse type I PSs-involved PDT usually exhibit distinctive hypoxia tolerance. Regarding the diversephotosensitizers (PSs) that light up type I PSsDT, aggregation-induced emission (AIE)-active type I PSs are currently arousing great research interest owing to their distinguished aggregation-induced emissionAIE and aggregation-induced generation of reactive oxygen species (AIE-ROS) features.
- aggregation-induced emission
- type I photosensitizers
- phototheranostics
1. Introduction
2. Basic Principles of Type I PDT
In general, PSs, light source, and substrates, are recognized as three essential elements in PDT [4]. According to the Jablonski diagram shown in Figure 1, the PSs at ground singlet state (S0) will, firstly, be excited to the unstable excited singlet state (S1) upon light irradiation, and then return to their ground state via radiative or non-radiative decay in the manner of fluorescence emission or heat production. Notably, provided that the energy gap between S1 and T1 (ΔES1–T1) is small enough, the excited singlet state PSs can preferably reach the relatively stable excited triplet-state (T1) by undergoing the ISC process, in which state the PSs can survive long enough to carry out different photochemical reactions (including type I and type II) with surrounding substrates, to yield ROS [6]. Here, type I process will be emphasized.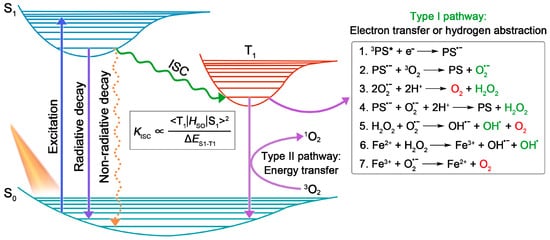
3. Design Strategies of Type I AIE PSs
Encouraged by the distinguished merits of AIE and AIG-ROS of AIE PSs, as well as the great potential that type I PSs hold in practical applications, type I AIE PSs have emerged with abundance in the last five years. As depicted in Figure 1, the ISC process is the foremost step that bears the brunt of following ROS generation, thus, a high ISC rate (kISC) ensuring ample excited triplet-state production is considered as a precondition to boost the photosensitizing properties of PSs. To date, a large number of studies have emerged demonstrating how to promote the ISC process of AIE PSs on the basis of the ISC rate equation (Figure 1), such as a donor (D)–acceptor (A) structured molecular engineering strategy for minimized ΔES1–T1, and spin–orbit coupling (SOC) enhancement strategy for improved SOC, etc. [30]. However, regarding specifically promoting the generation of type I free radical species rather than type II, authoritative and specific strategies are currently still relatively deficient, although several examples of type I AIE PSs have been provided. According to the basic principles of electron transfer in the type I pathway, herein we summarize and classify the currently reported approaches of building type I AIE PSs into two strategies: introducing functional groups with high electron affinity, and enhancing ICT intensity.3.1. Introducing High Electron Affinity Groups
After gaining insight into the mechanism of the type I process, it can easily be observed that before transferring the electron to O2 to yield O2•−, 3PS* first needs to capture one electron from its surroundings. Thus, employing functional groups with high electron affinity will contribute to this step, since they can serve as electronic transfer intermediates. For example, Zhao et al. [48] designed and synthesized two isomeric type I AIE PSs, named α-TPA-PIO and β-TPA-PIO, by employing phosphindole oxide (PIO) as an electron acceptor, and triphenylamine (TPA) as an electron donor. It was proven that owing to the high electron affinity of the PIO core, both of the triplet α-TPA-PIO and β-TPA-PIO could attract one electron from the surrounding substrates and accordingly convert to the intermediate radical anions, α-TPA-PIO•− and β-TPA-PIO•−, respectively, which could be stabilized by several resonance structures. As expected, α-TPA-PIO and β-TPA-PIO were demonstrated to exhibit admirable free radical but low 1O2 generation efficiency, evidently suggesting that the introduction of high electron-affinity PIO indeed favors type I ROS generation by stabilizing an external electron to form radical anion intermediates. Notably, β-TPA-PIO, which possessed stronger electron-accepting ability as indicated by its lower reduction potential, exhibited higher type I ROS generation efficacy than α-TPA-PIO, thus, confirming the superiority of strong electron affinity in dominating the type I pathway. In addition, the higher type I ROS generation efficiency of β-TPA-PIO also gave the credit to the larger SOC value, as well as the multichannel ISC pathways revealed by quantum mechanical calculation. By virtue of the good targeting ability to endoplasmic reticulum (ER) of cells, β-TPA-PIO could efficiently induce ER-stress-mediated apoptosis and autophagy via type I PDT, even in a hypoxic environment. Moreover, distinct in vivo fluorescence imaging of the tumor, and remarkable tumor ablation, could successfully be achieved under light irradiation. Therefore, this work presented a feasible protocol for type I AIE PSs by introducing high electron-affinity groups to capture and stabilize the external electron, which provided the photochemical basis of type I PDT.3.2. Enhancing ICT Intensity
3.2.1. Donor Engineering Based on Anion-π+ AIE Systems
As mentioned above, the type I pathway usually starts at the one-electron transfer step from adjacent substrates to 3PS*. Therefore, it can be speculated that providing electron-rich environments is beneficial for 3PS* to capture the external electron and undergo the type I process upon light irradiation. This assumption has been substantiated by Ding et al. [49]. They successfully modulated the photoreaction of a type II PS from the type II to the type I pathway by encapsulating the PS using electron-rich poly(ethylene glycol)-b-poly(2-(diisopropylamino)ethyl methacrylate (PEG-b-PDPA) as the coating substrate. Motivated by this work, a series of anion-π+ AIE PSs were successively constructed in the pursuit of efficient type I ROS generation, among which anion groups were introduced to offer electron-rich environments [50,51]. In view of the photomechanical basis of the type I process, strong ICT intensity of PSs was also conjectured to be helpful in donating to the type I pathway under the condition of an electronic-rich environment, since enhanced ICT could facilitate the ISC process, thus, promoting ROS generation capacity. In this regard, studies have revealed that anion-π+ PSs fabricated with a strong ICT characteristic would produce type I ROS more efficiently. For instance, through enhancing the ICT strength of the anion-π+ system, Wang et al. [50] successfully obtained robust type I AIE PSs. They firstly synthesized a novel series of anion-π+ structured AIE PSs (TBZPy, MTBZPy, TNZPy, MTNZPy) with enhanced electron-donating ability. TPA and its methoxy-substituted derivative (MTPA), as well as electron-rich heteroatoms (S, N) containing benzo-2,1,3-thiadiozole (BZ)/naphtho[2,3-c][1,2,5]thiadiazole (NZ) moieties groups, served as collaborative AIE-active donors, and the styrylpyridine cations worked as electron acceptors to ensure the ICT intensity; simultaneously, the iodide anion and collaborative donors with strong reducibility were in charge of providing an electron-rich environment to 3PS*. Due to the gradually enhanced electron-donating ability from TBZPy to MTNZPy, MTNZPy possessed the strongest ICT intensity, in the order of TBZPy < MTBZPy < TNZPy < MTNZPy, which was manifested by their absorption spectra in different solvents. It was demonstrated that the generation of 1O2 species gradually decreased, in line with the enhancement of ICT strength, while both total ROS and type I ROS generation efficiency of AIE PSs matched the trend of ICT strength, suggesting the pivotal role of strong ICT strength in facilitating type I ROS species generation. Further experiments revealed that the obtained type I AIE PSs (TNZPy, MTNZPy) could target mitochondria and lysosomes simultaneously, and exhibited low dark toxicity and admirable PDT therapeutic efficiency for HeLa cells in both normoxic and hypoxic conditions, attributing to the highly efficient type I ROS production inside cells. Additionally, their good performances in FLI-guided PDT were subsequently demonstrated in in vivo tumor models. Similarly, the feasibility of this strategy was also confirmed by Zhu and coworkers [51]. In their work, type I ROS generation was significantly boosted by enhancing the electron-donating ability to promote the ICT intensity of electron-rich anion-π+ AIEgens.3.2.2. Acceptor Planarization
In addition to enhancing the electron-donating ability of donors in anion-π+ AIEgens, Wang et al. [52] proposed a parallel strategy of acceptor planarization to enhance the ICT intensity, achieving the transformation from type II PSs to type I PSs. They designed and synthesized three AIE compounds, namely, 2TPAVDPP, TPATPEVDPP and 2TPEVDPP, using a planar core (vinyl-substituted DPP) as the electron acceptor. As a contrast, the previously reported DPP-TPA with thiophene-substituted DPP working as the twisted acceptor core, was also synthesized. The optimized conformation revealed that the dihedral angle between DPP and donor–acceptor linker was around 15° for DPP-TPA, while it decreased to nearly 0° for those three AIE PSs, indicating that the variation of donor–acceptor linker from thienyl (DPP-TPA) to vinyl (2TPAVDPP, TPATPEVDPP and 2TPEVDPP) would bring about better planarity and larger π-conjugation. It was further demonstrated that 2TPAVDPP, TPATPEVDPP and 2TPEVDPP with a planar acceptor exhibited superior type I and inferior type II ROS generation capacity than DPP-TPA, as expected. These results showed that better planarity and larger π-conjugation could effectively promote type I ROS production by enhancing ICT and D−A interaction to facilitate the ISC process, providing an alternative approach for constructing type I AIE PSs.4. Applications of Type I AIE PSs
On the basis of the mechanism described above, type I PSs exhibit relatively low external O2 requirements, owing to the recyclable O2 utilization in the type I ROS generation process. Intrinsically, type I AIE PSs enable the crafty integration of aggregation-induced fluorescence emission and enhanced ROS generation with minimized O2 dependence, presenting significant theranostic potential in different biomedical applications, including, but not limited to cancer ablation, bacterial infection elimination, and harmful algal bloom suppression.3.1. The Anti-Tumor Applications
4.1. The Anti-Tumor Applications
4.1.1. FLI-Guided Type I PDT
ForIn the purpose of redshifted absorption and emission wavelengths, as well as boosted theranostic performance,addition to being assisted by UCNPs, exploring AIE PSs and other organic PSs are generally engineered to contain multiple aromatic rings and/or large conjugated units in their molecular structures, giving rise to their high hydrophobicity. In order to facilitate the in vivo biological applications, hydrophobic AIE PSs are commonly encapsulated within nanovehicles based on amphiphilic biocompatible matriceswith an outstanding two-photon absorption property was another effective method to break through the obstacles encountered by short-wavelength excitation to[64]. fMorm well-dispersed AIE nanoparticles (NPs) in aqueous physiological environments [48]. Addieover, AIE PSs have proved tionally, bright fluorescence, excellent ROS production and enhanced permeability and retention (EPR) effect-driven tumor location can be successfully achieved, simultaneously, after nanofabrication, since the aggregation be promising candidates for developing two-photon excitable PDT agents, as the two-photon absorption cross section (δ2PA) of AIE PSs wcould obvithin the intraparticle limited room is capable of effectively astrictously be raised by simply increasing their active intramolecular motions, thus, blocking nonradiative thermal dissipation and saving the excited state energy for the fluorescence and ISC pathway [28,31]. In addloading amount in the NPs, exhibiting a unique aggregation-enhanced nonlinear optition to pcassively targetedl effect tumor[65]. Genrichment by the EPR effect, actively targeting transport of AIE PSs favored by specific recognition will be able to further enhance PDT efficacy. From those, Lou et al. [49] develerally, the wavelength in two-photon excitation is twice as long as that of one-photoped an amphiphilic polymeric matrix with conjugated targeting peptides to co-assemble with a type I AIE PS of TTB to fabricate tumor-specific targeting TTB NPs for amplifying type I bsorption, thus, making the NIR light-excitable photodynamic cancer treatment. In addition, Duotheranostics feasible. In this regard, Tang et al. [50][66] pconstrut forward an innovative protocol for efficiently targeted delivery of type I AIE PSs to tumor tissues by taking full advantage of the hypoxia condition in solid tumors and selective hypoxia tropism of some bacteria. For this approach, a novel bacteria-based AIE hybrid system was built, enabling the powerful delivery of tycted amphiphilic lipids-enveloping AIE NPs by encapsulating a tactfully designed two-photon excitable type I AIE PS of TBP-2 into the hypoxic tumor microenvironments for hypoxia-tolerant PDT of orthotopic colon cancer.
Consi(TPE-PTB). With strong D–A interaction andering that the eeffective killing range of ROS is typically confined to the immediate vicinityπ-conjugation strength, TPE-PTB-formed NPs resulted in a high δ2PA of PSs560 on a subcellular scale, an appropriate organelle-targeting location of PSs is, therefore, highly desired for implementing final PDT outcomes. Different subcellular organelles play their own unique roles in maintaining the normal physiological function of cells. It has been acknowledged that the organelles, including cell membrane, mitochondria, lysosomes, ER, and nucleus, are all valid sites for performing PDT [51].GM under 800 nm two-photon laser irradiation. Moreover, TPE-PTB NPs exhibited a far-red fluorescence emission with a high quantum yield of 23%. These advantageous superiorities enabled TPE-PTB NPs Tto date, diverse subcellular organelle-targeted type I AIE PSs-based anti-tumor systems have been exploited in succession [52]. For image deep-seated tumors and vessels winstance, Feng et al. [53] dh a high spatial revelsoped a class of cationic AIE PSs possessing a specific tumor cell mitochondrial targeting feature to facilitate bothlution on a mouse melanoma model. Notably, type I and type II PDT. In addition, Tang et al. [54]ROS species of OH• prcoposed a useful molecular design guideline for constructing efficient AIE PSs and tailoring their organelle specificity.
Amuld be effectively generated by AIE NPs under 800 nm laser illuminationg. the various subcellular organelles, of particular importance is the cell nucleus as it dominates the cellular gene expression, metabolism and proliferation [55].Further mechanistic explanation showed that TPE-PTB could take one electron from an environmental hydroxyl anion to Mforeover, the DNA and RNA parts of the nuclei are very sensitive to type I ROS due to its extremely high m anionic PS, for a subsequent series of photochemical reactivity [56]. Ion view of this, Wang et al. [56] to eventually genexplored, forate OH•. tThe first time, a nucleus-targeting PDT strategy based on type I AIE PSs, by making full use of theranostic agents and nanocarrier systems (Figure 2a). Twlive–dead staining experiment showed that TPE-PTB NPs plus NIR laser irradiation could cause mo AIE PSs, namred TFMN and TTFMN, with typical D–A structures and sufficient molecular rotors, were firstly designed and synthesized. Compared with TFMN, TTFMN was equipped with additional TPE moiety in structure, which endowed it with much better AIE peculiarity. Various ROS indicators were employed to distinguish the ROS species produced by TFMN and TTFMN, through fluorescence spectroscopy and ESR measurements, which was discriminated to t than 90% cell death rate as analyzed by flow cytometry, while no obvious cell death was found in other control groups. More importantly, TPE-PTB NPs performed well for in vivo FLI-guided type I ROS of OH•. Mtwo-phoretover, the TTFMN showed stronger ESR signal intensity than TFMN (Figure 2b), n PDT, with significant indhibicating its better generation capacity of OH•tion of tumor growth. In addition, whichTPE-PTB was attributed to its superior AIE tendency and smaller ΔES1–T1. Wible to be effectively cleared from th the help of a lysosomal acid-activated nuclear localization signal peptide (TAT)-modified amphiphilic polymer, the resultant TTFMN-loaded NPs (TTFMN-NPs) exhibited nucleus-anchoring delivery ability, visualized by the intrinsic fluorescence property of TTFMN (Figure 2c). Furmouse body after completing the treatment, guaranteeing favorable in vivo biosecurity. A pother in vivo investigations uncovered that TTFMN-NPs with good biosecurity and long blood circulation time could specifically accumulate at tumor nt NIR-excited type I PDT nanoplatform based on AIEgens was successfully constructed in this work.
4.1.2. The Cooperation of Type I PDT and PTT
Despites (Figure 2d). Upon w thite light irradiation, TTFMN-NPsow O2 idependuced high-efficiency tumoricidal resultsence of the type I process enduing it with a 75.1% tumor growhypoxia tolerance, O2 is sthill inhibition rate (Figure 2e,f)requisite during type I PDT. This woerk offered a new perspective in the constructionefore, the efficiency of type I PS-based and nucleus-targeted nanotheranostic systems.
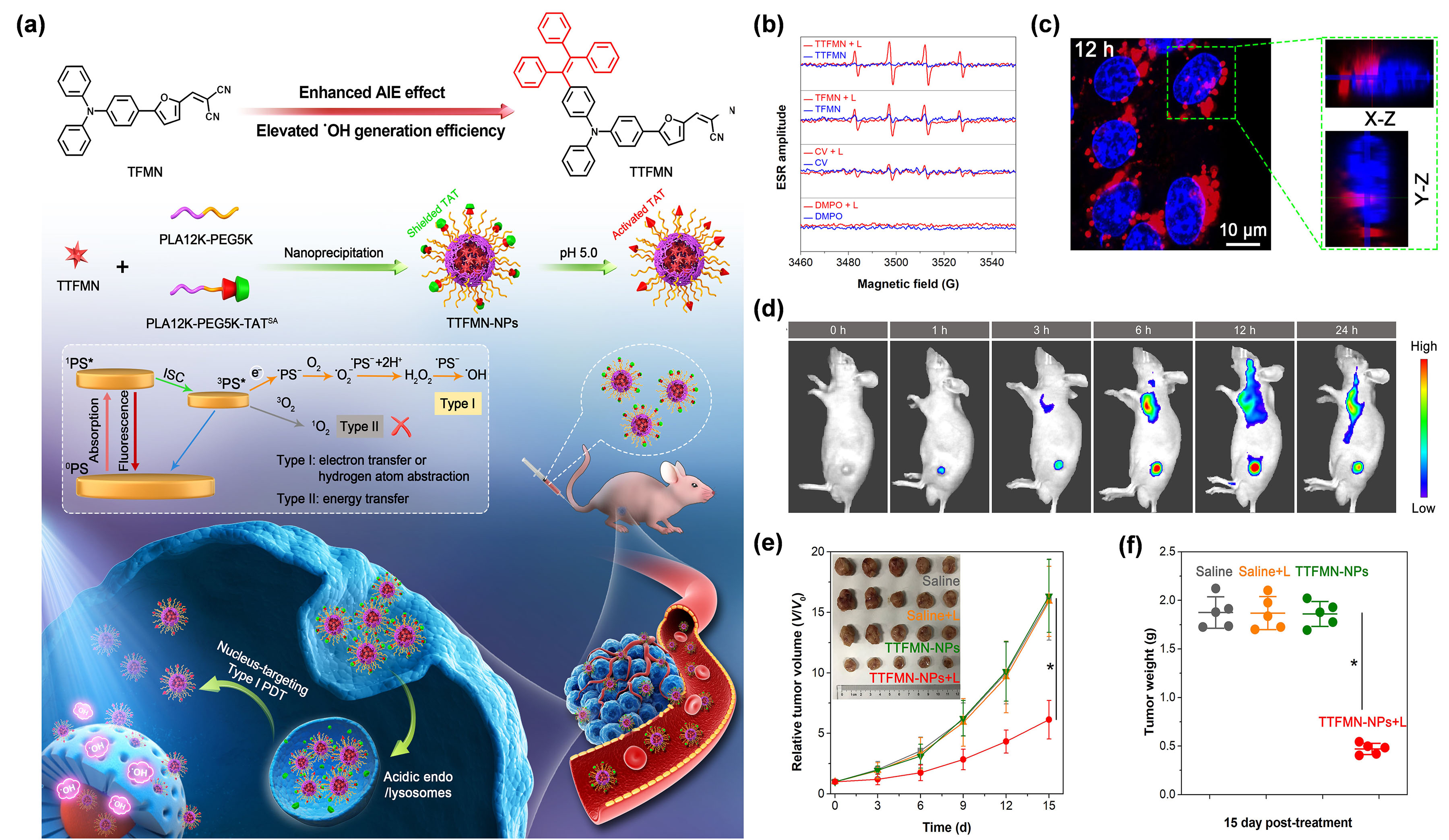
Figure 2. (a)DT can be further improved by appropriately increasing the O2 Illusconcentration of molecular design principle, nanotheranostics fabrication, and its application in nucleus-targeted type I photodynamic cancer treatment. (b) ESR analysis fthe lesions. Recent reports have demonstrated that the coor OH• genperation of type I TTFMN and TFMN after white light irradiation (200 mW/cm2PDT and photothermal therapy (PTT). (c) CLSM images wof nuclear targeting delivery of TTFMN-NPs (2 µg/mL TTFMN) after incubation with 4T1 cells for 12 h. The blue color represents the fluorescence of Hoechst 33342 for locating cell nucleuld be a superb strategy to conquer their respective shortcomings and the red color representsboost therapeutic outcomes [31,32]. tThe fluorescence of TTFMN-NPs. (d)is is because PTT can Timgene-dependent in vivo FLI of tumor-bearing mice after injection with TTFMN-NPs. (e) The grrate localized heat, not only for cancer ablation, but alsowth curves of tumors in different treatmentto increase the O2 grosups (npply in = 5, *p < 0.001). (f) Tthe tumor wtissueights of mice after treatments for 15 days (n = 5, * p < 0.001). Reprs by virtue of raising ted with permission from [56], che blood flow, thus, promopyrtight 2021, Wiley-VCH.
At ng typresent, most AIE PSs can only be effectively excited by short wavelength of UV or visible lights I PDT efficiency, which conversely, further improves the treatment outcome of PTT [37]. However, the shallcow penetration depth nstruction of the excited light presented a major scientifictype I PDT-PTT combinational system based on a single organic molecule is a challenge for AIE PSs to treat deep-seated tumors. Based on this, the combinnging task, since the energy dissipation of rare earth doped upconversion NPs (UCNPs) would provide an effective solution to this problem, since UCNPs can serve as a near-infrared (NIR) light transducer to harness and convert the NIR laser to UV-visible light, enabling the construction of robust NIR laser excitable nanotheranostic systems [57]. Enchannels are generally competitive. Under this circumstance, AIE molecules have a remarkable capability which can tailor the equilibrium of energy dissipation by regulating the intramolecouraged by the synergistic effect of combining UCNPs and AIE PSs toward cancer therapylar motions. In 2022, Wang et al. [5867] creatively designed and developed a triple-jump photodynamic nanotheranostic agent, termed MUM NPs, by integrating aported a multifunctional type I AIE PS of MeOTTI into the multifunctional nanoplatform built by UCNPs and manganese dioxide (MnO2), namely, DCTBT, applying to a second near-infrared (NIR-II) FLI-guided type I PDT, simultaneous with high-efficiency PTT functions, for pancrenhanced theranostic outputs in PDTatic cancer, the king of cancer [68,69,70]. (Figure 3a).A Spconjugatecifically, MeOTTI was engineered to afford the type I ROS capacity verified by the ESR test (Figure 3b).d small molecule (CTBT) was modified with diphenylamine moieties on Wiboth the aid of UCNPs whose emission spectrum matched wellof the two ends, to endow DCTBT with the absorption spectrum of MeOTTI, the resulting Förster resonance energy transfer (FRET) effect between UCNPs and MeOTTI not only achieved the excitation light extension from UV-visible to NIR region, but also significantly elesufficient intramolecular motions for enhanced PTT efficiency, stronger electron D–A interaction for longer absorption and emission wavelengths, and smaller ΔES1–T1 for improvated the ROS generation effabiciency (Figure 3c)lity. Afttractively, the introduction of the MnO2 componer employing different wasROS aimed at depleting the intracellularly upregulated glutathione (GSH), thus, significantly facilitating the production of highly oxidative type I ROS in cells. Meanwhile, the yielded Mindicators, DCTBT NPs fabricated by using DSPE-PEG2000 as the encapsulation matrix were demon2+ was tralso able to catalyze the intracellular H2ted to have distinguished O2•− tand mo genederate OH•, as well as for magnetic resonance imaging (MRI). Therefore, the triple-jump type I Rgeneration efficiencies. Moreover, DCTBT NPs exhibited comparable OS2•− generation ability tof MUM NPs could be smoothly achieved inside the tumor cells after NIR laser irradiation. This splendid triple-jump rose bengal (RB), which was much stronger than that of CTBT NPs. In vitro photodynamic theranostic protocol was confirmed by a series of cell and animal thermal experiments (Figure 3d,e).
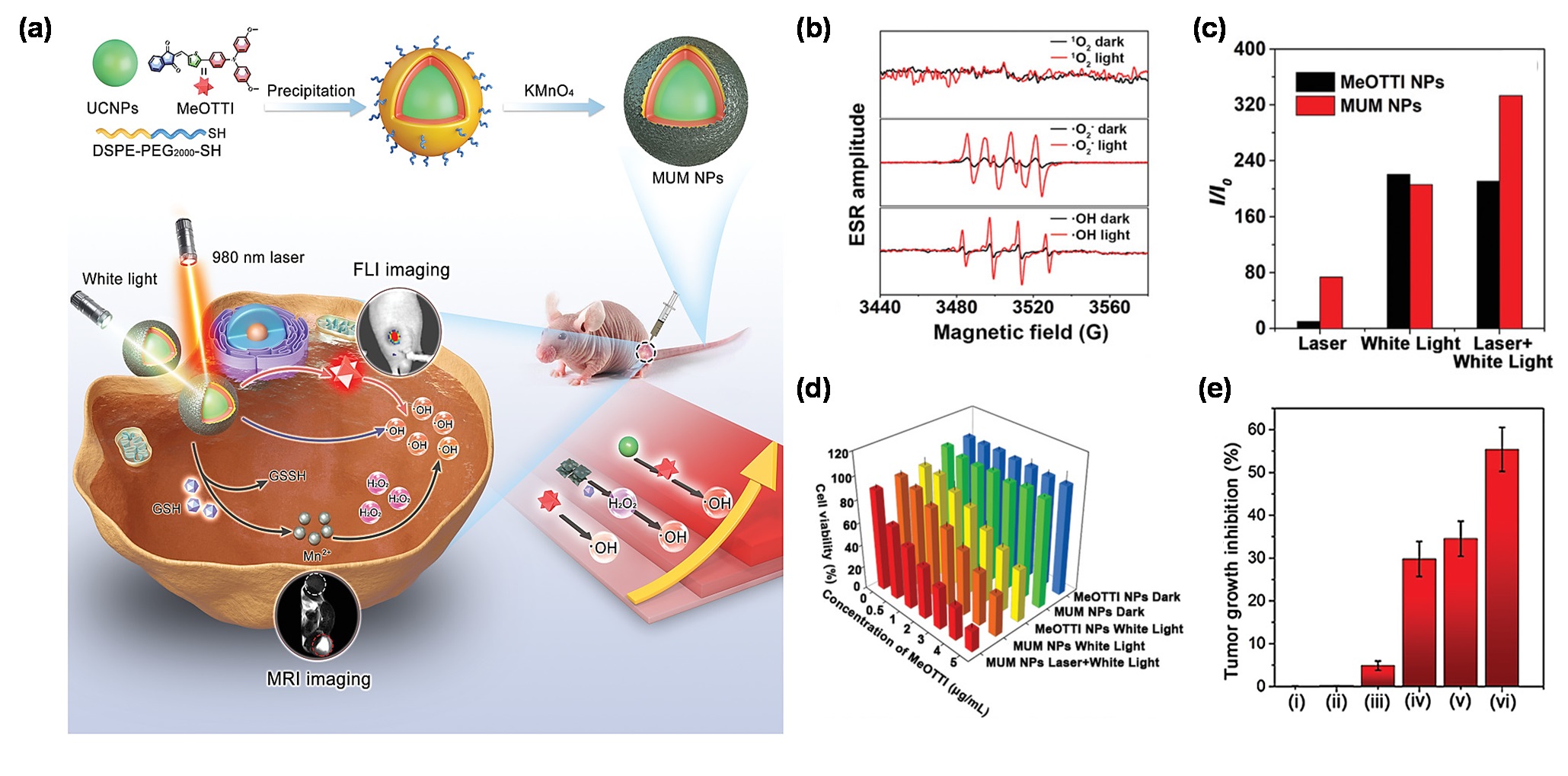
Figure 3. (a) Cshowemical structure of MeOTTI and schematic illustd that DCTBT NPs achieved concentration of triple-jump-dependent photodynamic theranostic protocol. (b) ROS genethermal performation type of MeOTTI determined by ESR test. (c) ROS productnce, and the hion ghefficiency of MeOTTI NPs and MUM NPs at the same MeOTTI concentrationst temperature could reach up to 62.8℃ under the808 nm laser irradiation of different light sources. (d) Cfor 5 min as thell viability of 4T1 cells treated with different conditions. (e)concentration rose to 300 Tuμg/mor inhibition ratios of mice after different treatments, namely: (i) PBS, (ii) MUM NPs, (iii) 980 nm laser and white light, (iv) MeOTTI NPs and white light, (v) MUM NPs and white light, (vi) MUM NPs, 980 nm laser and white light (nL, which implied the excellent photothermal performance of DCTBT NPs. With the aid of the GE11 peptide, a specific ligand for = 5, * p < 0.001). Reprinted with permission from [58], dermal growth factopyright 2021, Wiley-VCH.
In addir reception to being assisted by UCNPs, exploring AIE PSs with an outstanding two-photon absorption property was another effective method to break through the obstacles encountered by short-wavelength excitation [59]. Mr (EGFR), target NPs, whose surface was decorated with DSPE-PEG2000-GE11, were prepared to facilitate the intracellular intake oreover,f AIE PSs have proved to be promising candidates for developing two-photon excitable PDT agents, as the two-photon absorption cross section (δ2PA)lip-DCTBT NPs (the DCTBT-loaded liposomes). Considering the remarkable NIR-II emission property, in vivo NIR-II of AFLIE PSs could obviously be raised by simply increasing their loading amount in the NPs, exhibiting a unique aggregation-enhanced nonlinear optical effect [60]. Gen was performed for the real time observation of NPs distribution and preciserally, the wavelength in two-photon excitation is twice as long as that of one-photon absorption, thus, making the NIR light-excitable photodynamic theranostics feasible. In this regard, Tang et al. [61]detection of tumors. As expected, the target NPs showed more intensive fluorescence signal in constructed amphiphilic lipids-enveloping AIE NPs by encapsulating a tactfully designed two-photon excitable type I AIE PS (TPE-PTB) (Figure 4a). Wast with non-target NPs (without DSPE-PEG2000-GE11), whitch strong D–A interaction and effective π-conjugation strength, TPE-PTB-formed NPs resulted in a high δ2PA was beneficial for enhanced tumor targeting ability, by virtue of 560the GM under 800 nm two-photon laser irradiation (Figure 4b)EGFR ligand. MoreNover, TPE-PTB NPs exhibited a far-red ftably, fluorescence emission with a high quantum yield of 23%. These advantageous superiorities enabled TPE-PTB NPs to image deep-seated tumors and vessels with a high spatial resolution on a mouse melanoma model. Notably, type I ROS species of OH• could be effecsignals were still observed at 48 h post-injection, indicating the enormous potential of target NPs for long-term NIR-II FLI in the real-time tracing of tumors. After treatment, the tively argenerated by AIE NPs under 800 nm laser illumination (Figure 4c). Fuet NPs group provided the most significant tumor growther mechanistic explanation showed that TPE-PTB could take one electron from an environmental hydroxyl anion to form anionic PS, for a subsequent series of photochemical reactions, to eventually generate OH• (Figure 4d). As inhibition on both subcutaneous and orthotopic PANC-1 tumor-bearing mice modelshown in Figure 4e, a live–dead staining experiment showed that TPE-PTB NPs plus NIR laser irradiation could cause more than 90% cell death rate as analyzed by flow cytometry, while no obvious cell death was found in other control groups. More importantly, TPE-PTB NPs performed well for in vivo FLI-guided type I two-photon PDT, with demonstrating that target NPs possessed extraordinary anti-tumor efficacy through synergetic type I PDT-PTT pathways. Furthermore, in all groups, no significant inhibition of tumor growth (Figure 4f).change of body weight of Inmice addition, TPE-PTB was able to be effectively cleared from the mouse body after completing the treatment, guaranteeing favorable in vivo biosecurity. A potent NIR-excited type I PDT nanoplatform based on AIEgens was successfully constructed in this work.
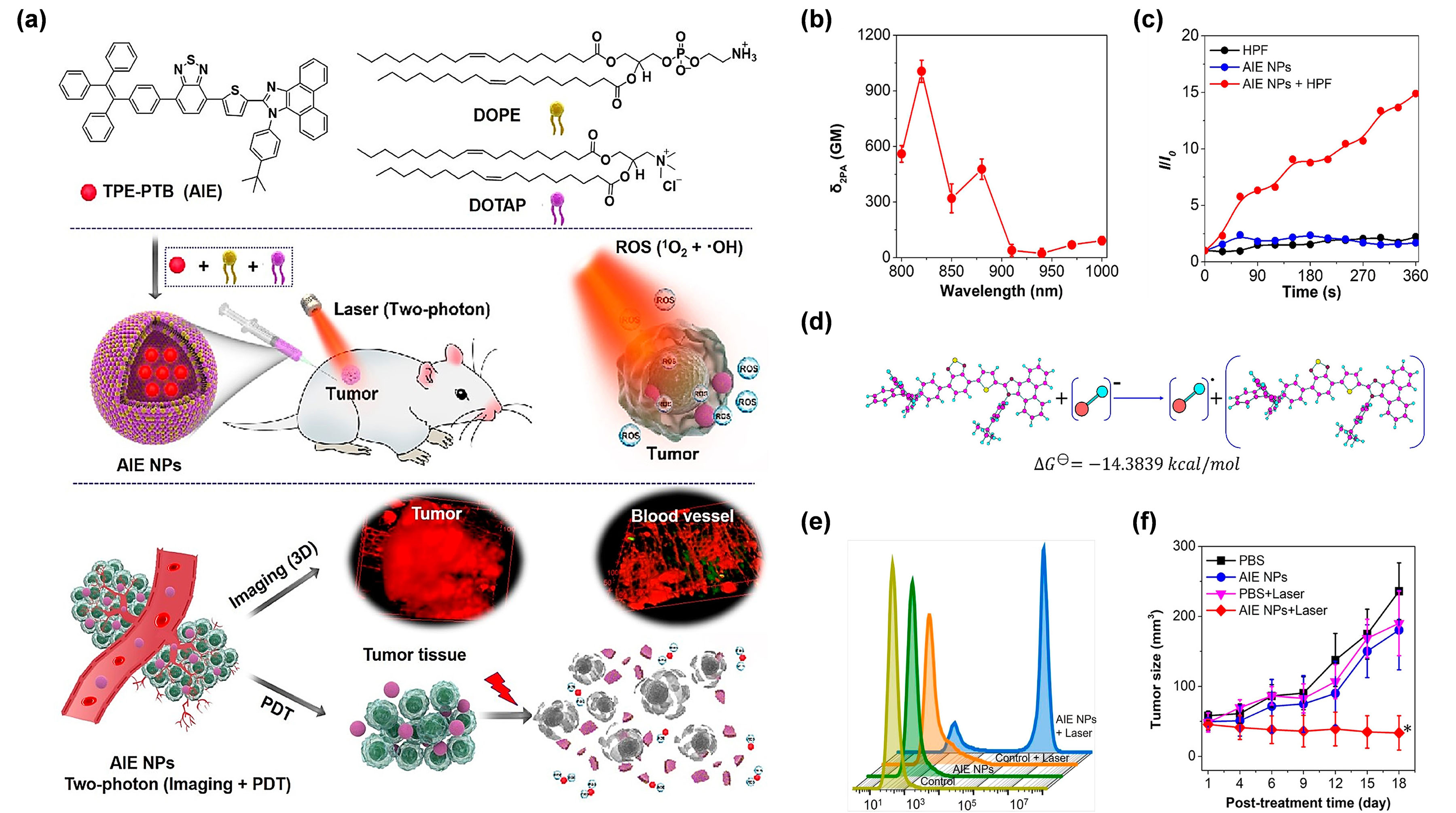
Figure 4. (a) Cwas discovered, as well as no noticeable changes of the complete blood panel test and serum biochemistry in the target NPs treated groups compared with themical PBS structure of TPE-PTB and illustration of two-photon-excited FLI-guided PDT applications. (b) δ2PA treated groups, which proved the negligible systemic toxicity of the TPElip-PTB NPs under different excitation wavelengths. (c)DCTBT NPs. This study not only provided a OH• paroduction ability of TPE-PTB NPs indicated by HPF. (d) Mechanisadigm for exploring advanced m and calculation of OH• getifuneraction analysis. (e) Flow cal tytompetry of A375 cells stained by PI after treatment with different conditions. (f) I AIE PS for the application of TumorNIR-II growth curves of mice in different treatments. (*FLI-guided type-I PDT-PTT p < 0.05). Rsyneprinted with permission from [61], cgistic therapy, but also brought favopyright 2020, American Chemical Societyable insights into pancreatic cancer treatment.
34.2. The Antimicrobial Applications
Infectious diseases caused by pathogenic microbes including bacteria, fungi, and viruses, pose serious threats to humans, since they usually cause severe diseases such as foodborne illness, tuberculosis, sepsis, meningitis, and pneumonia, for which the situation continues to worsen, along with the appearance of antibiotics-resistant microbes [6271,6372]. In view of this rigorous challenge, PDT has stood out as a promising candidate for antimicrobial applications including the inactivation of multidrug resistant (MDR) microbe species; ROS could provide an aggressive attack on microbes without the need for complete entrance of PSs into the microbial interior, which can potentially avoid the generation of microbial resistance [6473]. In this respect, type I PDT has been widely employed due to the longer half-life of O2•−, as well as the strong oxidizing property of OH•. Possessing the advantage of AIE and AIG-ROS, periodical achievements in the FLI-guided PDT of pathogenic microbes have been attained, based on AIE-active type I PSs [6574,6675,767].
For example, Wang et al. [6877] reprepared a functional nanofibrous membrane (TTVB@NM) by doping a type I AIE PSs TTVB in an electroactive polymer (PVDF-HFP) matrix using the electrospinning technique, and achieved the photodynamic elimination of pathogenic droplets and aerosols under sunlight (Figure 5a). Due to the inherent positive charge, TTVB was able to effectively stain several kinds of bacteria and fungi (Figure 5b). Under sunlight irradiation, TTVB possessed outstanding type I ROS generation efficiency (Figure 5c–e), owing to its typical D–A structure and electron-rich heteroatoms (S and N). After doping into the PVDF-HFP, the obtained nanofibrous membrane (TTVB@NM) was demonstrated to exhibit similar photophysical performances as TTVB, as well as favorable washability and photostability, indicating great potential for effective antimicrobial effect. The antimicrobial activity of TTVB@NM was subsequently verified by the significantly decreased survival rates of four kinds of pathogenic droplets (Gram-positive bacteria S. S. aureusaureus, Gram-negative bacteria E. coli, fungi C. albicans, and M13 bacteriophage) after 1 h under sunlight irradiation (Figure 5f). Further evaluation of the antimicrobial effect of TTVB@NM against pathogenic aerosols was carried out by placing the pathogenic aerosols-loaded TTVB@NM outdoors (Figure 5g). The results revealed that TTVB@NM could effectively inactivate pathogenic aerosols containing bacteria (inhibition rate: > 99%), fungi (~88%), and viruses (>99%) within only 10 min under sunlight irradiation (Figure 5h,i). The author also stated that TTVB was measured to show moderate photothermal conversion performance, which could play an adjuvant role for microbe inhibition.
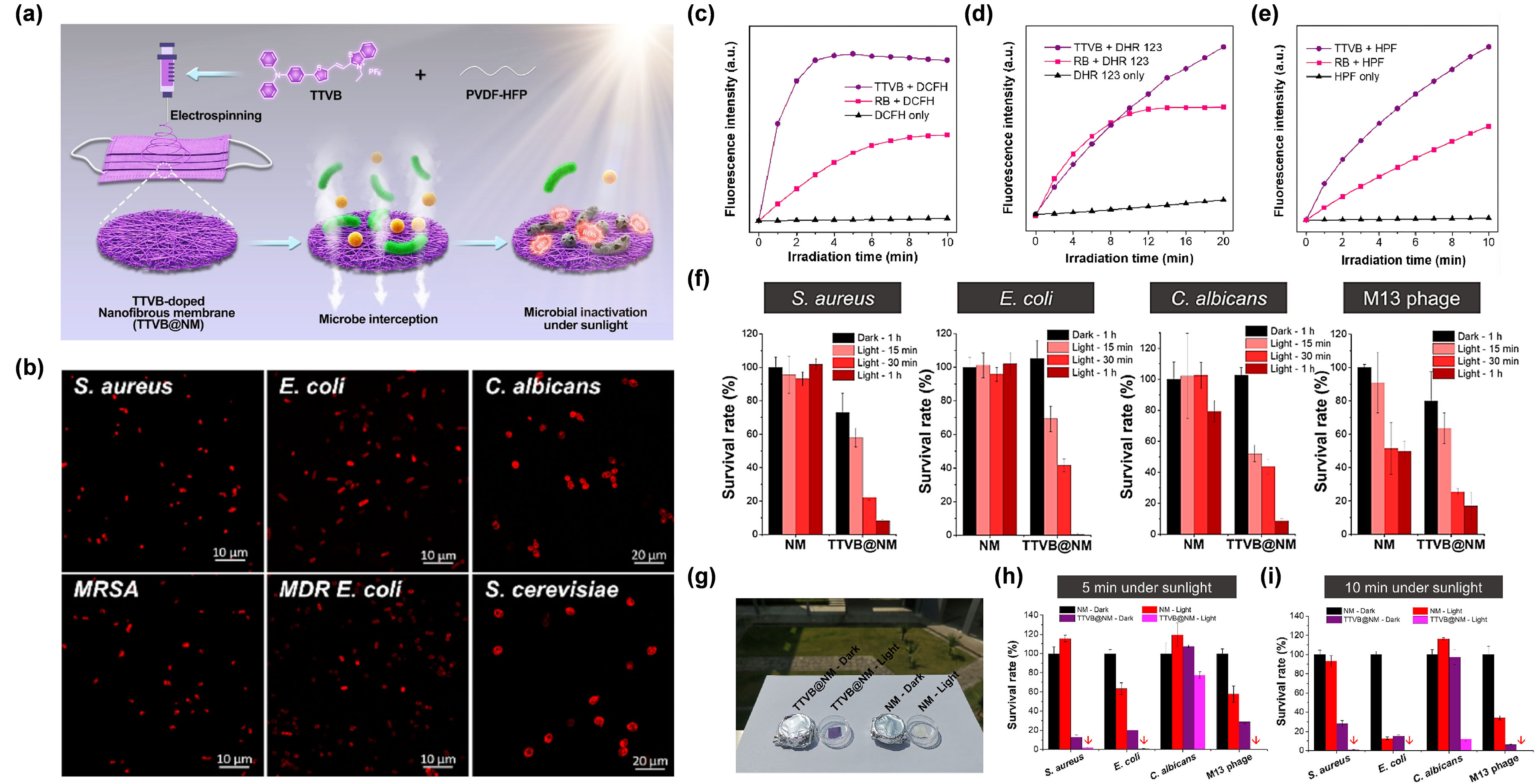
Figure 5. (a) Diagram of the preparation of TTVB@NM for antimicrobial applications. (b) CLSM imaging of bacteria and fungi co-incubated with TTVB. Relative fluorescence intensity of (c) DCFH for total ROS detection, (d) DHR123 for O2•− detection, and (e) HPF for OH• detection of TTVB and RB under light irradiation (34 mW/cm2). (f) Microbial survival rate treated with NM or TTVB@NM in dark or under sunlight irradiation. (g) Antimicrobial experiment against pathogenic aerosols in dark or under sunlight irradiation. Survival rate of microbes under sunlight irradiation for (h) 5 min and (i) 10 min. Reprinted with permission from [68], copyright 2021, Elsevier.
34.3. The Inhibition of Harmful Algal Bloom
Harmful algal bloom (HAB) has become a global environmental problem, causing serious impact on aquatic ecology and economy [6978]. The rapid growth of algae aggravates O2 depletion and the release of harmful toxins, consequently threatening the survival of aquatic animals, resulting in widespread freshwater and marine area pollution [709]. Although many physical and chemical methods have been developed to inhibit HAB, their inherent drawbacks, such as low suppression rate, limited application area, and the possibility of secondary and persistent pollution, have hindered their widespread application [7180]. In recent years, ROS-generating algaecides have aroused extensive interest owing to their effective, eco-friendly and cost-efficient properties [7281]. Therefore, exploring PSs which show excellent elimination effect of algae upon light irradiation without causing toxicity to other aquatic organisms, will be a promising strategy. Of particular interest are the type I AIE PSs, which can exhibit excellent ROS generation ability under low O2 concentration, suitable for the relatively low O2 environment of algal bloom.
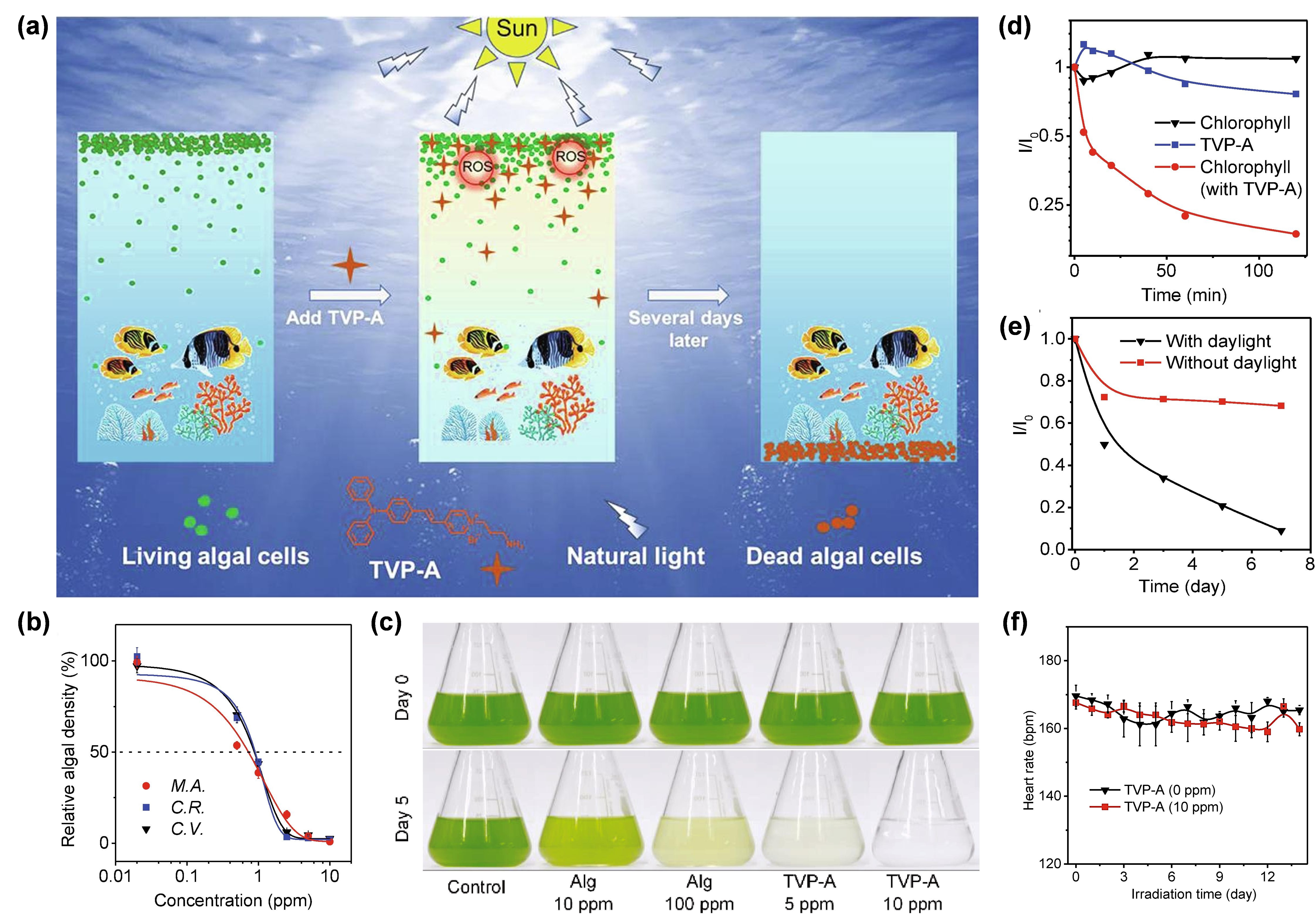
Under this circumstance, Luo et al. [7382] developed a water-soluble type I AIE PS with self-degrading ability, termed TVP-A, which could selectively eliminate HAB upon exposure to natural light (Figure 6a). TVP-A was constructed with a typical D–A structure with a primary amino group modified onto the terminal pyridinium, endowing the molecule with good water solubility. Moreover, the positively charged property also endowed TVP-A with a specific algae-targeting feature, on account of the negatively charged cell membrane of the algae. Upon white light irradiation, TVP-A exhibited superb ROS generation ability through both type I and type II mechanisms, particularly ·OH•. In this work, they co-incubated one cyanobacteria (M. aeruginosa) and two freshwater green algae (C. vulgaris, and C. reinhardtii) with TVP-A at different concentrations, respectively, under 16 h light (50 μEinstein/m2/s1)/8 h dark cycles to explore the effective concentrations in controlling the HAB. It was found that the 50% effective concentration (EC50) value of TVP-A was less than 1 ppm for these three kinds of algae (Figure 6b). As showIn in Figure 6c, in contrast with the commercial algaecide (Alg), which still had a large amount of algae residue when the concentration was as high as 100 ppm, TVP-A exhibited ultra-efficient control of HAB, with effective inhibition of the algae bloom C. reinhardtii at 5 ppm and a clear color of water after five natural daily cycles at 10 ppm. The fluorescence change of chlorophyll in C. reinhardtii was measured to prove the irreversible damage of chloroplast due to the photodynamic process (Figure 6d). After 2 h of illumination, the fluorescence intensity of chlorophyll decreased to less than 20%, indicating that TVP-A could rapidly cause irreversible damage to these important organelles of algal cells, at especially low concentration, upon illumination. Collectively, TVP-A could be employed as a powerful agent for inhibiting HAB by destroying the chloroplast of algal cells. In addition, the strong self-degradation ability of TVP-A (Figure 6e) suggested that it was an eco-friendly agent with little environmental residue left under sufficient natural light irradiation, avoiding secondary pollution to the environment. Meanwhile, the daily heart rates of fish in the groups with or without TVP-A showed no significant difference (Figure 6f), generally indicating the good biocompatibility of TVP-A within the working concentration. This strategy afforded a favorable insight into developing novel type I ROS-generating algaecides for green HAB governance.
5. Conclusions and Perspectives
Figure 6. (a)Profiting from the Scinhematic illustration of selectively removing HAB by TVP-A upon natural light irradiation. (b) Rrent advantages of AIE and AIG-ROS of AIE PSs, as well as the predominant role that type I PDT exhibited in breaking through the bottleneck of conventional type II PDT, after five years of development, AIE-active type I PS is emerging as a rising star that holds great potential in illuminating the future of PDT. Based on the recent significant advancements in novel AIE-ative cell densityctive type I PSs, a systemic summary, involving the photophysical and photochemical mechanisms of three algae after incubating with TVP-A at different concentrations after 96 h under the simulated daily cycles. M.A.:e type I pathway, molecular design strategies, as well as practical bioapplications of type I AIE PSs, was provided in this review. In addition to a high ISC rate ensuring sufficient excited triplet-state production to undergo subsequent electron-transfer or energy-transfer pathway, approaches that could preferentially trigger the type I electron-transfer, rather than the type II energy-transfer process, are particularly needed in constructing type I PSs. Currently, according to the M. aeruginosa;cascade C.R.:reactions C. reinhardtii;of C.V.:the C. vulgaris.type (c)I Ppathotos ofway, introducing functional groups with a high electron affinity C. reinhardtiito capture and (1.6stabilize × 107the external elecells/mL) in the presence of Alg (10 ppm and 100 ppm) or TVP-A (5 ppm and 10 ppm) under the simulated daily cycles on day 0 and day 5. (d) Ttron, as well as enhancing the ICT intensity of PSs through donor engineering or acceptor planarization, represent two strategies to promote type I ROS generation. Guided by these strategies, a number of type I AIE PSs have been constructed and successfully applied in the type I ROS-mediated eradication of tumors and pathogen-caused infectious diseases, as well as the cinhibition of hange of the relativermful algae bloom. Additionally, due to the excellent features of bright fluorescence intensity (I/I0)at aggregated state, rotator or vibrator-rich structure, and easy tailorability, FLI-guided type I PDT or ieven C. reinhardtiiFLI-guided (1.6type × 107I PDT-PTT synergisticells/mL) in the p theranostic platforms were also witnessed by ingeniously adjusting the excited-state energy dissipation pathways of type I AIE PSs.
Promising as it is, theresence or absen still exist challenges or perhaps opportunities to be considered, regarding the further advance of TVP-A (5 ppm) under different times of simulated natural light illumination. (e)type I AIE PSs. Primarily, although the design strategies of promoting ISC for developing highly efficient AIE PSs have already been proposed, the authoritative approach that specifically activates the following type I pathway is, to date, still far from satisfactory. In this Vregariation in relative absorbance of TVP-A at 462 nm with or without different natural light for evaluating the degradability of TVP-A. (f)d, the successful examples guided by those two above-mentioned approaches are still limited, and their universal applicability remains to be validated by more attempts, despite their success in designing some type I AIE PSs. Hence, an in-depth understanding of the photochemical mechanisms of the type I pathway, the accordingly effectual design principles, as well as more ingenious trails, are urgently called for. Then, in order to avoid misjudgment about type I ROS generation capacity and to accelerate the progressive advancement of type I AIE PSs, a standard Tmethe change of averaod capable of precisely assessing the quantum yield of type I ROS, particularly O2•− and OH•, is highly neede heart rates of fish with or without TVP-A durd. This is because current detection methods can only provide qualitative data instead of quantitative, and it is difficult to make direct comparison between the newly synthesized PSs and the previously reported ones, when only depending on qualitative data. Lastly, notwithstanding the 14 days of cultivation time under the simulated daily cycles. Reprinted with permission from [73], at some other bioapplications of type I AIE PSs, besides tumor treatment, have emerged, for example, the inhibition of harmful algae bloom, continuing efforts are still required for exploring a broader scope of application of type I AIE PSs, such as anti-biofilm application. We expect this review can provide valuable insights into the underlying photophysical and photochemical mechanism of the type I pathway, and inspire researchers to develop authoritative design strategies and rationales for the novel copynstright 2021, Elsevieruction of ideal type I AIE PSs in the future.
