Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 3 by Sirius Huang.
Stretching is one of the popular elements in physiotherapy and rehabilitation. When correctly guided, it can help minimize or slow down the disabling effects of chronic health conditions. Most likely, the benefits are associated with reducing inflammation.
- stretching
- inflammation
1. Inflammatory Lesion and Tissue Morphology
The inflammatory infiltrate formed by the infiltration or accumulation of inflammatory cells is the result of tissue reactions to harmful stimuli. The infiltrate is formed by the action of neutrophils, lymphocytes, plasmacytes, eosinophils, macrophages, and mast cells and is accompanied by the vasodilation and accumulation of exudate, resulting in tissue swelling [1].
Corey et al. (2012) showed that stretching reduces inflammatory infiltration around subcutaneous lesions, improves gait, and reduces pain sensitivity. In their model, inflammation was induced by injecting carrageenan (a polysaccharide that, when injected subcutaneously, causes severe swelling) into the subcutaneous connective tissues of the lower back of studied animals (mice). The stretching model used was similar to methods used in physiotherapy or yoga (active stretching) [2], and the effect of the exercises performed was a significant change in macrophage marker expression (CD68) around the tissue analyzed. The carrageenan/stretch group had reduced macrophage marker expression in the connective tissue of the lumbar region compared to the carrageenan/no-treatment group and the carrageenan/sham group. Ultrasound measurements additionally showed a reduced thickness of the examined tissues, understood as the size of the inflammatory infiltrate in the stretch group [2].
In another study using the carrageenan inflammation model, Berrueta et al. (2016) demonstrated that active and passive stretching (under anesthesia) activates local anti-inflammatory processes in mice [3]. Ultrasound results showed that stretching reduced the thickness of the inflammatory lesion and its cross-sectional areas, as well as reduced the number of neutrophil granulocytes and the total number of cells in the inflamed area. Both passive and active stretching produced similar results, and these effects were similar to those observed after treatment with resolvin D2 (Rvd2), a specialized pro-resolving mediator (SPM) with anti-inflammatory properties [3][4]. SPMs are a wide group of cell signaling agents produced from polyunsaturated fatty acids (PUFA). They take part in the resolution and silencing of the inflammatory response by inhibiting the migration and/or infiltration of inflammatory cells and the release of proinflammatory mediators [5][6]. Researchers also conducted ex vivo studies to investigate the local effects of tissue stretching independent of the vascular, lymphatic, and neuromuscular systems. They showed that tissue stretching 48 h after carrageenan injection was associated with a significant reduction in neutrophil migration in the connective tissue in mice [3]. Similar results were obtained by Wang et al. (2022) where, in a posttraumatic knee contracture model, the implementation of static progressive stretching (30 min) resulted in a reduction in the number of inflammatory cells [7].
Looking for the basis of the analgesic action of MFR, Meltzer et al. used the RMS-induced inflammation model in human fibroblast cultures [8]. They observed elongated lamellopodia, cellular decentralization, larger intercellular distances, and reduced cell–cell contact area, which resulted in induced inflammatory responses. Reductions in the fibroblast structure were noticed following MFR therapy (3 h after RMS). The apoptosis rate in the RMS group was elevated, and the implementation of MFR reduced this. The application of both techniques did not alter the proliferation rate of fibroblasts [8].
In 2018, Langevin et al. used domestic swine to study the effect of stretching on inflammation, as their back structure is more similar to humans than were the rodents used in previous studies [2][3]. The animals of the study groups underwent a unilateral fascia injury in the dorsal trunk, then were subjected to movement restriction (prevented from full hip extension and pelvic lateral flexion in the transverse plane during gait) and then stretching. The stretching model differed from those used in previous studies [2][3] because the focus was on stretching the hip and lower back [9]. The fascia thickness increased overall from week 8 to week 12, despite the animals returning to normal gait speed. However, this effect may be linked to non-inflammatory pathologies and physiological processes such as tissue growth, muscle contraction, and mucosal physiology [10]. By week 12, there were no significant differences in fascia thickness between the groups. This shows that reduced fascia mobility in response to an injury along with movement restriction is a plastic phenomenon that worsens over time and persists even when movement is restored. Four weeks of daily 10 min passive stretching of the hip and lower back tissues after the restriction was removed was not superior to simply removing the restriction [9]. Despite the lack of difference between the stretching and non-stretched groups, the study suggests that an important issue in controlling inflammation through stretching may be the stretching method itself, with the method used not exerting sufficient tension on the thoracolumbar fascia [9].
Studies using domestic swine were also conducted by Vergara et al. (2020), this time using stretching techniques similar to those used by Berrueta et al. and Corey et al. [2][3]. A carrageenan-induced inflammation model was used and the pigs were stretched by holding their legs twice a day for 5 min over 48 h [11]. The results obtained showed reduced inflammatory lesions and lesion mass in the stretching (S) group compared to the non-stretched (NS) group. The S group’s lesions had 71% fewer granulocytes and 49% fewer macrophages compared to the NS group. However, the observed differences were not statistically significant (probably due to the small size of the study groups, n = 4 for each group) [11].
The results obtained in the aforementioned studies confirm that stretching affects local inflammation and accelerates the resolution of inflammation [2][3]. They also indicate a significant role of rehabilitation and stretching in reducing inflammatory infiltration, which is likely underpinned by both systemic and local mechanisms [2][3]. Furthermore, the mode of stretching seems to be significant [9][11]. Stimulation of connective tissue may be an important therapeutic goal, and stretching may serve as a viable method of treatment [3][8].
2. Proinflammatory Genes and Cytokine Expression
Most proinflammatory genes are not expressed under physiological conditions but are rather controlled by phosphorylation and dephosphorylation of many transcription factors, and can be triggered by stress factors that activate intracellular signaling pathways such as mitogen-activated protein kinase (MAPK) cascades, the NF-κB, and the JAK–STAT signaling pathway [12][13][14]. Dysregulation of these genes is associated with inflammation and the progression of diseases such as cancer, diabetes, and autoimmune diseases [14][15]. The cytokines secreted as a result of inflammatory response also activate the aforementioned cascades [12].
2.1. Patient Studies
Stretching exercises, as well as exercises with a prominent stretching component (e.g., yoga, Tai Chi), can reduce levels of circulating proinflammatory cytokines [16][17][18][19]. A study by Sarvottam et al. (2013), based on hourly whole-body stretching exercises (yoga) performed for 10 days, showed a reduction in IL-6 levels as well as increased levels of adiponectin—a potential endogenous anti-atherogenic factor produced by mature fat cells [18][20]. Overweight and obese patients often exhibit low-grade inflammation, which can result in chronic inflammatory disease [21]. These patients also often have elevated circulating IL-6, a proinflammatory interleukin that is a risk indicator for cardiovascular disease (CVD) [22]. According to Sarvottam et al. (2013), even a short-term change in lifestyle can lead to lower blood pressure and weight loss, and can exert anti-inflammatory and anti-atherogenic effects. Despite the study being conducted on a group of only 51 men, the results obtained indicate the relevance of stretching exercises in reducing the risk of CVD [18]. Similar results have been obtained by trainers of TCC, which is shown to be a useful behavioral intervention resulting in lower circulating levels of IL-6 [16].
2.2. Animal Studies
The study mentioned earlin the previous section er in Section 2.1 was based on a carrageenan-induced inflammation model [3] in which stretching resulted not only in morphological improvements, but also in higher concentrations of resolvin D1 (RvD1), a signaling molecule with anti-inflammatory properties [23][24][25]. The concentration of LTB4, an eicosanoid produced during inflammation [26], was not significantly altered by stretching. However, the studies showed a two times higher ratio of RvD1 to LTB4 following stretching, compared to controls, thus demonstrating the additional anti-inflammatory potential of stretching [3].
Vergara et al. conducted a study on pigs (n = 4) to analyze the production of SPMs in carrageenan-induced inflammation and found no changes in the expression of any of the investigated proinflammatory genes, with differences in protein expression not statistically significant, most likely due to the small group size [11]. However, some trends were observed. In the serum, both lipoxin A4 and RvD1 were higher in the stretching group, with the proinflammatory mediator prostaglandin D2 (PGD2) exhibiting an almost twofold decrease. Within the lesion, the stretching did not alter RvD1 or LXA4 levels; however, the ratio of serum LXA4 or RvD1 to PGD2 showed a nearly twofold increase following stretching compared with the control group [11]. Lipoxins are specialized pro-resolving mediators (SPMs), and their synthesis increases in response to increased concentrations of arachidonic acid metabolites [4]. The increased ratio between LXA4 and PGD2 could, therefore, suggest the initiation of resolution of the inflammation.
2.3. Cell Culture Studies
Mechanically Induced Inflammation
Meltzer and Standley (2007) used RMS in their study to model IOMT and investigate the response of fibroblasts [27]. OMT is a set of techniques used by osteopathic physicians, during which elements of stretching are combined with the application of appropriate pressure to muscles and joints [28], and is often prescribed for the management of many health conditions [29]. RMS in the fibroblast culture resulted in decreased cell proliferation, decreased secretion of interleukin-1 receptor antagonist (IL-1ra) with anti-inflammatory properties, and increased secretion of proinflammatory factors IL-1α, IL-1β, IL-2, IL-3, IL-6, and IL-16. In contrast, IOMT treatment alone resulted in a decrease only in the secretion of the proinflammatory IL-3 [27]. IOMT following stimulation of the RMS cells did not lead to an induction of interleukin secretion that could be observed after RMS alone, but resulted in a decrease in proinflammatory IL-6 secretion and an increase in cell proliferation. Thus, the inflammatory response of fibroblasts is dependent on the stretching model used, with IOMT appearing to reverse the proinflammatory effects caused by RMS stimulation by regulating cytokine secretion [27].
Eagan et al. (2007) investigated the cellular mechanisms behind the positive clinical outcomes of manual medicine treatments (MMT) and showed that regulation of the inflammatory response was influenced by the stretching model used, with equibiaxially strained cells having increased fractalkine (CX3CL1) secretion [30]. A soluble fraction of fractalkine serves as a chemoattractant for T cells, monocytes, and NK cells [26]. The aforementioned cytokine regulates apoptosis and can promote the death of damaged neural cells [31], but when released from apoptotic cells, it induces both antiapoptotic and mitogenic effects on neighboring vascular smooth muscle cells, and promotes proper wound healing and regeneration by inhibiting fibrotic responses to cell death [32][33]. MMT was also associated with reduced secretion of the pulmonary and activation-regulated chemokine/CCL18, as well as the macrophage-derived chemoattractant/chemokine MDC and, compared to the model of heterobiaxial strain, equibiaxially stretched cells showed reduced proliferation and reduced secretions of MDC and IL-6 [30].
Meltzer et al. (2010) observed an increased apoptosis rate in RMS-induced inflammation, which was most likely based on the observed upregulation of apoptosis-associated signaling protein kinase 2 (DAPK-2). After implementing MFR as a treatment for inflammation, a downregulation of this protein was observed, as well as an increase in serine 133-phosphorylated cyclic adenosine monophosphate (cAMP) response element-binding protein (CREBS133) in the RMS groups [8]. Phosphorylation of CREB at site S133 activates this protein, leading to an altered expression of many genes [34]. It has been proven that overexpression of CREB protects against tunicamycin-induced apoptosis [35]. Upregulation of phosphorylated CREB also increased in the MFR and RMS+MFR vs. RMS groups, which may indicate the implementation of processes aimed at limiting apoptosis, the rate of which is faster following MFR. A similar effect may arise from the upregulation of phosphorylated focal adhesion kinase (FAK), an enzyme suppressing apoptosis [36], observed both in RMS vs. control and following MFR in groups with no inflammation and the RMS-induced inflammation group [8]. The lack of change in the proliferation rate in this study was probably masked by DAPK-2-associated apoptosis [8]. In terms of the secretion of cytokines and growth-promoting mediators, Meltzer et al. (2010) observed no changes indicative of modulation of the inflammation but, as they noted, their results do not exclude the existence of changes in the expression of receptors of the studied mediators, as well as in the expression of intracellular effectors or expression/secretion in non-measured mediators [8].
Another study was undertaken by Anloague et al. 2020, analyzing the effect of mechanical stimulation of human dermal fibroblasts on inflammatory processes, wherein primary human dermal fibroblasts were subjected to an 8-hour-long CSDS or CSDS combined with ALDS. Anloague et al. confirmed that cyclical mechanical strain increases levels of IL-6 and, adding long-duration stretching intended to mimic therapeutic soft-tissue stimulation, results in a reduced IL-6 levels [37]. Expanding the cytokine profile also allowed the team to prove that long-duration stretching (CLDS+ALDS) results in lowered levels of IL-8, one of the most potent chemotactic factors [37]. Similar results were obtained by Nazet et al. (2020), where advanced stretching led to a reduction in the inflammatory effects of TNF-α, IL-6, and IL-1β, but the implementation of short-term high-frequency cyclic tension or static isotropic tension was associated with proinflammatory effects [38].
IL-1-Induced Inflammation
More than any other cytokine family, the IL-1 family is primarily associated with innate immunity [39] and IL-1β is often used to induce inflammation in studies on rodents [40][41][42][43][44].
In a study on rabbit articular cartilage, Xu et al. (2000) demonstrated that CTS acts as a potent antagonist of IL-1β [40]. High levels of IL-1 in the joint synovium of patients with osteoarthritis are associated with cartilage destruction [45][46]. IL-1 increases leucocyte recruitment and increases the activity of matrix metalloproteinases (MMPs), leading to joint destruction through both degradation and decreased synthesis of matrix components [47]. Due to the increased production of reactive nitrogen species, IL-1 is also associated with elevated deoxyribonucleic acid (DNA) damage [48]. In contrast, stimulation (CTS) of chondrocytes cultured in the presence of IL-1β leads to the suppression of the expression of proinflammatory genes such as inducible nitric oxide synthase (iNOS) and COX-2 and, consequently, to a decrease in the synthesis of NO and prostaglandin E2 (PGE2). The anti-inflammatory and regenerative effects observed following CTS are similar to those obtained with drugs that reduce cartilage degradation [40].
Further studies on the mechanisms responsible for the anti-inflammatory properties of CTS were conducted by Madhavan et al. in 2006. They cultured CTS chondrocytes in the presence of IL-1β for various periods, followed by a period of rest. The researchers showed that 90% of the expression of IL-1β-induced proinflammatory genes—iNOS, COX-2, matrix metalloproteinases 9 (MMP-9), and matrix metalloproteinases 13 (MMP-13)—was able to be blocked by continuous CTS [49]. Eight hours of CTS was able to reverse the changes produced by 16 h of exposure to IL-1β, but was unable to reduce iNOS expression after 28 h and 40 h of exposure. The data suggest that continuous CTS inhibits IL-1β-induced proinflammatory gene expression at the transcriptional level, and that the signals generated by CTS are sustained after cessation, with the persistence depending on the duration of exposure [49].
Research on the effects of biomechanical signals in joint inflammation was also conducted by Dossumbekova et al. (2007), who, using a rat chondrocyte culture, demonstrated that CTS inhibits the IL-1β-induced activation of proinflammatory genes by the nuclear factor kappa-light-chain-enhancer of activated B-cell (NF-κB) cascades [50]. The analysis by Dossumbekova et al. (2007) shows that CTS rapidly inhibited the IL-1β-induced nuclear translocation of NF-κB, but not its phosphorylation at serine 536 and serine 276. Scientists also showed that the stretching method they used repressed gene transcription of IκBα and IκBβ (associated with the NF-κB pathway); however, it inhibited their cytoplasmic protein degradation. The reduction in degradation of IκB was caused by downregulation of IκB kinase activity. A rapid nuclear translocation of IκBα, presumably to prevent the binding of NF-κB to DNA, was also observed [50]. The results indicate that the NF-κB signaling cascade is indirectly affected by CTS at multiple points (sites), resulting in the attenuation of IL-1β-induced proinflammatory gene expression [50] (Figure 1).
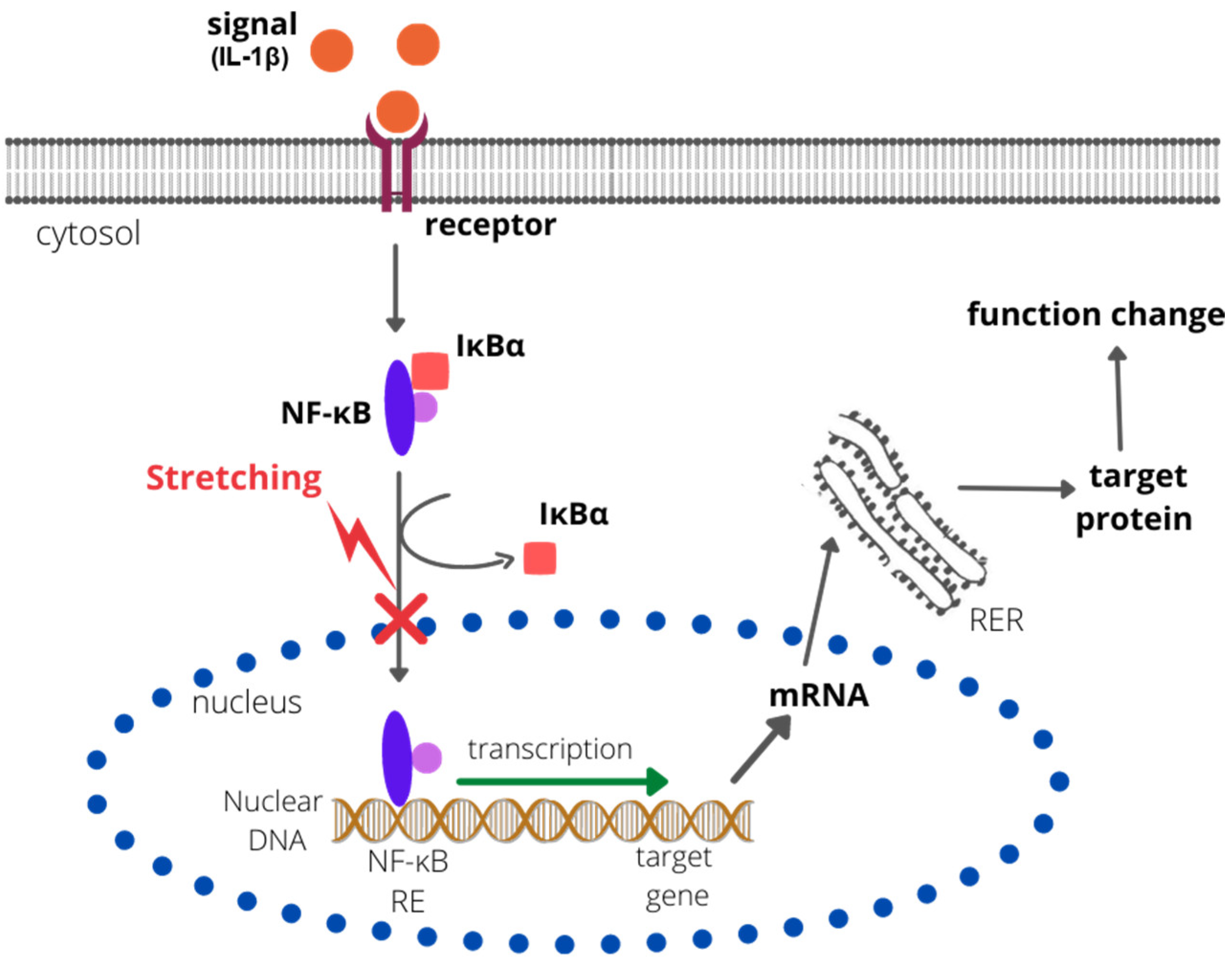
Figure 1. The effect of CTS on the NF-κB pathway. CTS inhibits the degradation of IκBα and IκBβ and rapidly inhibits the IL-1β-induced nuclear translocation of NF-κB. Therefore, the NF-κB response element (NF-κB RE) is not bound by the NF-κB complex, the transcription is not activated, and no target protein is synthesized. RER—rough endoplastic reticulum; IκBα/β—nuclear factor of kappa light polypeptide gene enhancer in B-cell inhibitors alpha/beta).
Similar results were obtained by Branski et al., 2007, using rabbit vocal cord fibroblasts cultured in the presence of IL-1β following different magnitudes of CTS [51]. The results obtained confirm earlier reports on the increase in expression of iNOS, COX-2, and MMP-1 in vocal cord fibroblasts at the mRNA and protein level under the influence of IL-1β. CTS nullified the IL-1β-induced activation of the mentioned genes in a magnitude-dependent manner [51].
Mechanical signals of low magnitudes applied in dynamic mechanical stimulation show a strong anti-inflammatory potential. Their application leads to the attenuation of proinflammatory gene induction by IL-1β and TNF-α [52][53]. A study on the time-dependent effects of dynamic tensile forces (DTF) on fibrochondrocytes harvested from rat knees by Ferretti et al. (2006) showed, similarly to Madhavan et al. (2006), inhibition of IL-1β-dependent induction of iNOS [53]. The observed effect depended on the magnitude used and was present for up to 20 h after the end of stimulation. The mRNA expression of IL-1β decreased successively after the application at magnitudes ranging from 5% to 20%, which translated into NO accumulation as well as iNOS synthesis in IL-1β-induced inflammation. The results obtained by Ferretti et al. were also dependent on the frequency of the signals used—the greatest decreases in iNOS mRNA expression were observed at the lowest frequency applied—0.025 Hz. At other applied frequencies, the decrease in iNOS expression was not as spectacular. DTF also strongly inhibited the mRNA expression of TNF-α and MMP-13 and their proteins. An increase in the expression of these molecules is observed after injury and, therefore, the introduction of DTF blocks the expression of inflammatory mediators and protects inflamed joints from a loss of function. The results reported by Ferretti et al. in 2006 indicate that mechanical signals act as strong anti-inflammatory signals and this response is magnitude- and frequency-dependent and continues even after DTF cessation. The use of mechanical forces of appropriate intensity may, therefore, be recommended in the rehabilitation of meniscal cartilage [53].
The aforementioned studies confirm that CTS and DTF reduce cartilage degradation, producing effects similar to those observed following pharmacotherapy. IL-1β-induced inflammation under stretching may be reduced by changes in the expression of iNOS and COX-2, MMP-9 and MMP-13, MMP-1, and TNF-α, causing a decrease in NO and PGE2 synthesis, and affecting the NF-κB signaling cascade [49][50][51][53]. The presence of IL-1β in the joint synovium of patients with RA or osteoarthritis (OA) plays a key role in cartilage destruction [41][45][46]. Therefore, CTS may be particularly important in alleviating and controlling arthritic diseases of different etiologies. Further research is required to more precisely establish the molecular consequences of tissue stimulation by stretching.
References
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435.
- Corey, S.M.; Vizzard, M.A.; Bouffard, N.A.; Badger, G.J.; Langevin, H.M. Stretching of the Back Improves Gait, Mechanical Sensitivity and Connective Tissue Inflammation in a Rodent Model. PLoS ONE 2012, 7, e29831.
- Berrueta, L.; Muskaj, I.; Olenich, S.; Butler, T.; Badger, G.J.; Colas, R.A.; Spite, M.; Serhan, C.N.; Langevin, H.M. Stretching Impacts Inflammation Resolution in Connective Tissue. J. Cell. Physiol. 2016, 231, 1621–1627.
- Chiurchiù, V.; Leuti, A.; Dalli, J.; Jacobsson, A.; Battistini, L.; MaCcarrone, M.; Serhan, C.N. Proresolving Lipid Mediators Resolvin D1, Resolvin D2, and Maresin 1 Are Critical in Modulating T Cell Responses. Sci. Transl. Med. 2016, 8, 353ra111.
- Qu, Q.; Xuan, W.; Fan, G.H. Roles of Resolvins in the Resolution of Acute Inflammation. Cell Biol. Int. 2015, 39, 3–22.
- Basil, M.C.; Levy, B.D. Specialized Pro-Resolving Mediators: Endogenous Regulators of Infection and Inflammation. Nat. Rev. Immunol. 2016, 16, 51–67.
- Wang, L.; Cui, J.B.; Xie, H.M.; Zuo, X.Q.; He, J.L.; Jia, Z.S.; Zhang, L.N. Effects of Different Static Progressive Stretching Durations on Range of Motion, Myofibroblasts, and Collagen in a Posttraumatic Knee Contracture Rat Model. Phys. Ther. 2022, 102, pzab300.
- Meltzer, K.R.; Cao, T.V.; Schad, J.F.; King, H.; Stoll, S.T.; Standley, P.R. In Vitro Modeling of Repetitive Motion Injury and Myofascial Release. J. Bodyw. Mov. Ther. 2010, 14, 162–171.
- Langevin, H.M.; Bishop, J.; Maple, R.; Badger, G.J.; Fox, J.R. Effect of Stretching on Thoracolumbar Fascia Injury and Movement Restriction in a Porcine Model. Am. J. Phys. Med. Rehabil. 2018, 97, 187–191.
- Gusev, E.; Zhuravleva, Y. Inflammation: A New Look at an Old Problem. Int. J. Mol. Sci. 2022, 23, 4596.
- Vergara, D.M.; Berrueta, L.; Carmody, C.; An, X.; Wayne, P.M.; Zavacki, A.M.; Langevin, H.M. Establishment of a Novel Porcine Model to Study the Impact of Active Stretching on a Local Carrageenan-Induced Inflammation. Am. J. Phys. Med. Rehabil. 2020, 99, 1012–1019.
- Dinarello, C.A. Proinflammatory Cytokines. Chest 2000, 118, 503–508.
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218.
- Plotnikov, A.; Zehorai, E.; Procaccia, S.; Seger, R. The MAPK Cascades: Signaling Components, Nuclear Roles and Mechanisms of Nuclear Translocation. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 1619–1633.
- Rao, S.K.; Pavicevic, Z.; Du, Z.; Kim, J.G.; Fan, M.; Jiao, Y.; Rosebush, M.; Samant, S.; Gu, W.; Pfeffer, L.M.; et al. Pro-Inflammatory Genes as Biomarkers and Therapeutic Targets in Oral Squamous Cell Carcinoma. J. Biol. Chem. 2010, 285, 32512–32521.
- Irwin, M.R.; Olmstead, R. Mitigating Cellular Inflammation in Older Adults: A Randomized Controlled Trial of Tai Chi Chih. Am. J. Geriatr. Psychiatry 2012, 20, 764–772.
- Irwin, M.R.; Olmstead, R.; Carrillo, C.; Sadeghi, N.; Breen, E.C.; Witarama, T.; Yokomizo, M.; Lavretsky, H.; Carroll, J.E.; Motivala, S.J.; et al. Cognitive Behavioral Therapy vs. Tai Chi for Late Life Insomnia and Inflammatory Risk: A Randomized Controlled Comparative Efficacy Trial. Sleep 2014, 37, 1543–1552.
- Sarvottam, K.; Magan, D.; Yadav, R.K.; Mehta, N.; Mahapatra, S.C. Adiponectin, Interleukin-6, and Cardiovascular Disease Risk Factors Are Modified by a Short-Term Yoga-Based Lifestyle Intervention in Overweight and Obese Men. J. Altern. Complement. Med. 2013, 19, 397–402.
- Morgan, N.; Irwin, M.R.; Chung, M.; Wang, C. The Effects of Mind-Body Therapies on the Immune System: Meta-Analysis. PLoS ONE 2014, 9, e100903.
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321.
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory Cytokines IL-6 and TNF-α and the Development of Inflammation in Obese Subjects. Eur. J. Med. Res. 2010, 15, 120–122.
- Yudkin, J.S.; Kumari, M.; Humphries, S.E.; Mohamed-Ali, V. Inflammation, Obesity, Stress and Coronary Heart Disease: Is Interleukin-6 the Link? Atherosclerosis 2000, 148, 209–214.
- Benabdoun, H.A.; Kulbay, M.; Rondon, E.P.; Vallières, F.; Shi, Q.; Fernandes, J.; Fahmi, H.; Benderdour, M. In Vitro and in Vivo Assessment of the Proresolutive and Antiresorptive Actions of Resolvin D1: Relevance to Arthritis. Arthritis Res. Ther. 2019, 21, 72.
- Cao, D.; Pi, J.; Shan, Y.; Tang, Y.; Zhou, P. Anti-Inflammatory Effect of Resolvin D1 on LPS-Treated MG-63 Cells. Exp. Ther. Med. 2018, 16, 4283–4288.
- Markworth, J.F.; Brown, L.A.; Lim, E.; Floyd, C.; Larouche, J.; Castor-Macias, J.A.; Sugg, K.B.; Sarver, D.C.; Macpherson, P.C.D.; Davis, C.; et al. Resolvin D1 Supports Skeletal Myofiber Regeneration via Actions on Myeloid and Muscle Stem Cells. JCI Insight 2020, 5, e137713.
- Li, P.; Oh, D.Y.; Bandyopadhyay, G.; Lagakos, W.S.; Talukdar, S.; Osborn, O.; Johnson, A.; Chung, H.; Mayoral, R.; Maris, M.; et al. LTB4 Promotes Insulin Resistance in Obese Mice by Acting on Macrophages, Hepatocytes and Myocytes. Nat. Med. 2015, 21, 239–247.
- Meltzer, K.R.; Standley, P.R. Modeled Repetitive Motion Strain and Indirect Osteopathic Manipulative Techniques in Regulation of Human Fibroblast Proliferation and Interleukin Secretion. J. Am. Osteopath. Assoc. 2007, 107, 527–536.
- Campbell, S.M.; Winkelmann, R.R.; Walkowski, S. Osteopathic Manipulative Treatment: Novel Application to Dermatological Disease. J. Clin. Aesthet. Dermatol. 2012, 5, 24–32.
- Steel, A.; Sundberg, T.; Reid, R.; Ward, L.; Bishop, F.L.; Leach, M.; Cramer, H.; Wardle, J.; Adams, J. Osteopathic Manipulative Treatment: A Systematic Review and Critical Appraisal of Comparative Effectiveness and Health Economics Research. Musculoskelet. Sci. Pract. 2017, 27, 165–175.
- Eagan, T.S.; Meltzer, K.R.; Standley, P.R. Importance of Strain Direction in Regulating Human Fibroblast Proliferation and Cytokine Secretion: A Useful in Vitro Model for Soft Tissue Injury and Manual Medicine Treatments. J. Manip. Physiol. Ther. 2007, 30, 584–592.
- Sokolowski, J.D.; Chabanon-Hicks, C.N.; Han, C.Z.; Heffron, D.S.; Mandell, J.W. Fractalkine Is a “Find-Me” Signal Released by Neurons Undergoing Ethanol-Induced Apoptosis. Front. Cell. Neurosci. 2014, 8, 360.
- Engel, D.R.; Krause, T.A.; Snelgrove, S.L.; Thiebes, S.; Hickey, M.J.; Boor, P.; Kitching, A.R.; Kurts, C. CX 3 CR1 Reduces Kidney Fibrosis by Inhibiting Local Proliferation of Profibrotic Macrophages. J. Immunol. 2015, 194, 1628–1638.
- White, G.E.; Tan, T.C.C.; John, A.E.; Whatling, C.; McPheat, W.L.; Greaves, D.R. Fractalkine Has Anti-Apoptotic and Proliferative Effects on Human Vascular Smooth Muscle Cells via Epidermal Growth Factor Receptor Signalling. Cardiovasc. Res. 2010, 85, 825–835.
- Parker, D.; Ferreri, K.; Nakajima, T.; LaMorte, V.J.; Evans, R.; Koerber, S.C.; Hoeger, C.; Montminy, M.R. Phosphorylation of CREB at Ser-133 Induces Complex Formation with CREB-Binding Protein via a Direct Mechanism. Mol. Cell. Biol. 1996, 16, 694–703.
- Balogh, A.; Németh, M.; Koloszár, I.; Markó, L.; Przybyl, L.; Jinno, K.; Szigeti, C.; Heffer, M.; Gebhardt, M.; Szeberényi, J.; et al. Overexpression of CREB Protein Protects from Tunicamycin-Induced Apoptosis in Various Rat Cell Types. Apoptosis 2014, 19, 1080–1098.
- Kurenova, E.; Xu, L.-H.; Yang, X.; Baldwin, A.S.; Craven, R.J.; Hanks, S.K.; Liu, Z.; Cance, W.G. Focal Adhesion Kinase Suppresses Apoptosis by Binding to the Death Domain of Receptor-Interacting Protein. Mol. Cell. Biol. 2004, 24, 4361–4371.
- Anloague, A.; Mahoney, A.; Ogunbekun, O.; Hiland, T.A.; Thompson, W.R.; Larsen, B.; Loghmani, M.T.; Hum, J.M.; Lowery, J.W. Mechanical Stimulation of Human Dermal Fibroblasts Regulates Pro-Inflammatory Cytokines: Potential Insight into Soft Tissue Manual Therapies. BMC Res. Notes 2020, 13, 400.
- Nazet, U.; Grässel, S.; Jantsch, J.; Proff, P.; Schröder, A.; Kirschneck, C. Early OA Stage Like Response Occurs after Dynamic Stretching of Human Synovial Fibroblasts. Int. J. Mol. Sci. 2020, 21, 3874.
- Dinarello, C.A. Overview of the IL-1 Family in Innate Inflammation and Acquired Immunity. Immunol. Rev. 2018, 281, 8–27.
- Xu, Z.; Buckley, M.J.; Evans, C.H.; Agarwal, S. Cyclic Tensile Strain Acts as an Antagonist of IL-1β Actions in Chondrocytes. J. Immunol. 2000, 165, 453–460.
- Fei, J.; Liang, B.; Jiang, C.; Ni, H.; Wang, L. Luteolin Inhibits IL-1β-Induced Inflammation in Rat Chondrocytes and Attenuates Osteoarthritis Progression in a Rat Model. Biomed. Pharmacother. 2019, 109, 1586–1592.
- Pan, T.; Wu, D.; Cai, N.; Chen, R.; Shi, X.; Li, B.; Pan, J. Alpha-Mangostin Protects Rat Articular Chondrocytes against IL-1β-Induced Inflammation and Slows the Progression of Osteoarthritis in a Rat Model. Int. Immunopharmacol. 2017, 52, 34–43.
- Tu, C.; Ma, Y.; Song, M.; Yan, J.; Xiao, Y.; Wu, H. Liquiritigenin Inhibits IL-1β-Induced Inflammation and Cartilage Matrix Degradation in Rat Chondrocytes. Eur. J. Pharmacol. 2019, 858, 172445.
- Yang, X.M.; Downey, J.M.; Cohen, M.V.; Housley, N.A.; Alvarez, D.F.; Audia, J.P. The Highly Selective Caspase-1 Inhibitor VX-765 Provides Additive Protection Against Myocardial Infarction in Rat Hearts When Combined with a Platelet Inhibitor. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 574–578.
- Griffin, T.M.; Guilak, F. The Role of Mechanical Loading in the Onset and Progression of Osteoarthritis. Exerc. Sport Sci. Rev. 2005, 33, 195–200.
- Kurz, B.; Lemke, A.K.; Fay, J.; Pufe, T.; Grodzinsky, A.J.; Schünke, M. Pathomechanisms of Cartilage Destruction by Mechanical Injury. Ann. Anat. 2005, 187, 473–485.
- Giacomelli, R.; Ruscitti, P.; Alvaro, S.; Ciccia, F.; Liakouli, V.; Di Benedetto, P.; Guggino, G.; Berardicurti, O.; Carubbi, F.; Triolo, G.; et al. IL-1β at the Crossroad between Rheumatoid Arthritis and Type 2 Diabetes: May We Kill Two Birds with One Stone? Expert Rev. Clin. Immunol. 2016, 12, 849–855.
- Davies, C.M.; Guilak, F.; Weinberg, J.B.; Fermor, B. Reactive Nitrogen and Oxygen Species in Interleukin-1-Mediated DNA Damage Associated with Osteoarthritis. Osteoarthr. Cartil. 2008, 16, 624–630.
- Madhavan, S.; Anghelina, M.; Rath-Deschner, B.; Wypasek, E.; John, A.; Deschner, J.; Piesco, N.; Agarwal, S. Biomechanical Signals Exert Sustained Attenuation of Proinflammatory Gene Induction in Articular Chondrocytes. Osteoarthr. Cartil. 2006, 14, 1023–1032.
- Dossumbekova, A.; Anghelina, M.; Madhavan, S.; He, L.; Quan, N.; Knobloch, T.; Agarwal, S. Biomechanical Signals Inhibit IKK Activity to Attenuate NF-ΚB Transcription Activity in Inflamed Chondrocytes. Arthritis Rheum. 2007, 56, 3284–3296.
- Branski, R.C.; Perera, P.; Verdolini, K.; Rosen, C.A.; Hebda, P.A.; Agarwal, S. Dynamic Biomechanical Strain Inhibits IL-1β-Induced Inflammation in Vocal Fold Fibroblasts. J. Voice 2007, 21, 651–660.
- Chowdhury, T.T.; Bader, D.L.; Lee, D.A. Dynamic Compression Inhibits the Synthesis of Nitric Oxide and PGE2 by IL-1β-Stimulated Chondrocytes Cultured in Agarose Constructs. Biochem. Biophys. Res. Commun. 2001, 285, 1168–1174.
- Ferretti, M.; Madhavan, S.; Deschner, J.; Rath-Deschner, B.; Wypasek, E.; Agarwal, S. Dynamic Biophysical Strain Modulates Proinflammatory Gene Induction in Meniscal Fibrochondrocytes. Am. J. Physiol. Cell Physiol. 2006, 290, C1610–C1615.
More
