Stimuli-responsive supramolecular gels comprising synthetic short peptides as building blocks have been explored for various biological and material applications. Though a wide range of stimuli has been tested depending on the structure of the peptides, light as a stimulus has attracted extensive attention due to its non-invasive, non-contaminant, and remotely controllable nature, precise spatial and temporal resolution, and wavelength tunability. The integration of molecular photo-switch and low-molecular-weight synthetic peptides may thus provide access to supramolecular self-assembled systems, notably supramolecular gels, which may be used to create dynamic, light-responsive “smart” materials with a variety of structures and functions.
- peptide
- stimuli responsive
- gel
- trans-cis isomerization
- azobenzene
- arylazopyrozoles
- spiropyran
1. Introduction
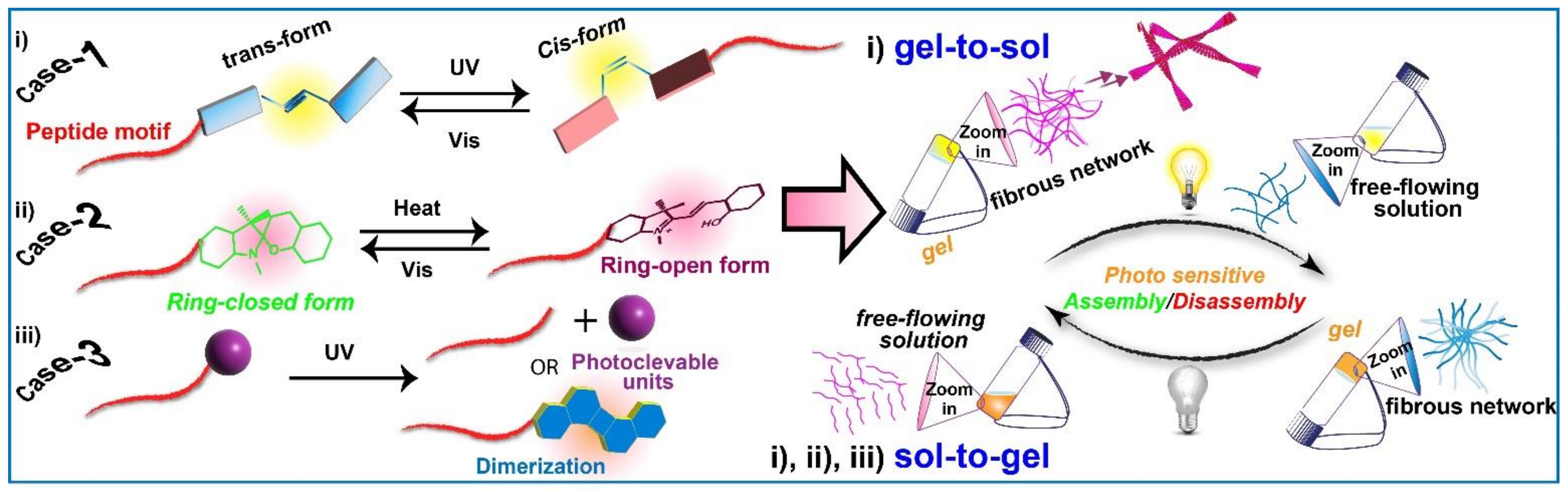
2. Light-Responsive Molecular Switches
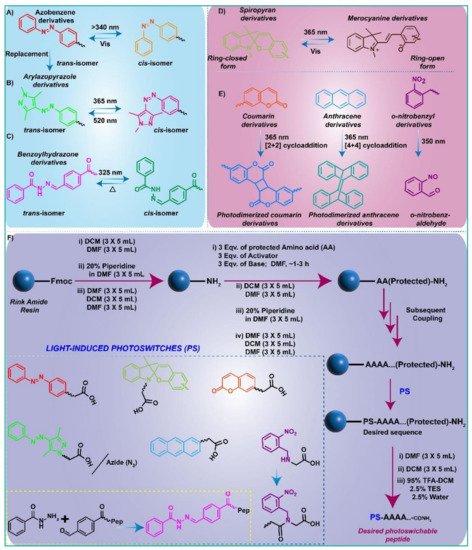
2.1. Azobenzene Conjugated Peptide Derivatives and Light-Assisted Self-Assembly/Disassembly Phenomenon
2.2. Arylazopyrazoles Conjugated Peptide Derivatives with Light-Sensitive Gelation Characteristics
2.3. Spiropyran Conjugated Peptide Derivatives and Light-Induced Gelation Behaviour
2.4. Other Photo-Responsive Peptide Derivatives and Light-Induced Gel-Sol Transition or Vice-Versa
References
- Das, S.; Das, D. Rational Design of Peptide-based Smart Hydrogels for Therapeutic Applications. Front. Chem. 2021, 9, 770102.
- Okesola, B.O.; Wu, Y.; Derkus, B.; Gani, S.; Wu, D.; Knani, D.; Smith, D.K.; Adams, D.J.; Mata, A. Supramolecular Self-Assembly to Control Structural and Biological Properties of Multicomponent Hydrogels. Chem. Mater. 2019, 31, 7883–7897.
- Dasgupta, A.; Mondal, J.H.; Das, D. Peptide hydrogels. RSC Adv. 2013, 3, 9117–9149.
- Busseron, E.; Ruff, Y.; Moulin, E.; Giuseppone, N. Supramolecular self-assemblies as functional nanomaterials. Nanoscale 2013, 5, 7098–7140.
- Makam, P.; Gazit, E. Minimalistic peptide supramolecular co-assembly: Expanding the conformational space for nanotechnology. Chem. Soc. Rev. 2018, 47, 3406–3420.
- Ahmed, S.; Mondal, J.H.; Behera, N.; Das, D. Self-Assembly of Peptide-Amphiphile Forming Helical Nanofibers and in Situ Template Synthesis of Uniform Mesoporous Single Wall Silica Nanotubes. Langmuir 2013, 29, 14274–14283.
- Singha, N.; Gupta, P.; Pramanik, B.; Ahmed, S.; Dasgupta, A.; Ukil, A.; Das, D. Hydrogelation of a Naphthalene Diimide Appended Peptide Amphiphile and Its Application in Cell Imaging and Intracellular pH Sensing. Biomacromolecules 2017, 18, 3630–3641.
- Seow, W.Y.; Hauser, C.A.E. Short to ultrashort peptide hydrogels for biomedical uses. Mater. Today 2014, 17, 381–388.
- Tsutsumi, H.; Tanaka, K.; Chia, J.Y.; Mihara, H. Short self-assembling peptides with a urea bond: A new type of supramolecular peptide hydrogel materials. Pept. Sci. 2021, 113, e24214.
- Falcone, N.; Shao, T.; Andoy, N.M.O.; Rashid, R.; Sullan, R.M.A.; Sun, X.; Kraatz, H.-B. Multi-component peptide hydrogels—A systematic study incorporating biomolecules for the exploration of diverse, tuneable biomaterials. Biomater. Sci. 2020, 8, 5601–5614.
- Ligorio, C.; Hoyland, J.A.; Saiani, A. Self-assembling peptide hydrogels as functional tools to tackle intervertebral disc degeneration. Gels 2022, 8, 211.
- Pramanik, B.; Singha, N.; Das, D. Sol-, Gel-, and Paper-Based Detection of Picric Acid at Femtogram Level by a Short Peptide Gelator. ACS Appl. Polym. Mater. 2019, 1, 833–843.
- Singha, N.; Srivastava, A.; Pramanik, B.; Ahmed, S.; Dowari, P.; Chowdhuri, S.; Das, B.K.; Debnath, A.; Das, D. Unusual confinement properties of a water insoluble small peptide hydrogel. Chem. Sci. 2019, 10, 5920–5928.
- Pramanik, B.; Ahmed, S.; Singha, N.; Das, B.K.; Dowari, P.; Das, D. Unorthodox Combination of Cation−π and Charge-Transfer Interactions within a Donor–Acceptor Pair. Langmuir 2019, 35, 478–488.
- Liu, C.; Zhang, Q.; Zhu, S.; Liu, H.; Chen, J. Preparation and applications of peptide-based injectable hydrogels. RSC Adv. 2019, 9, 28299–28311.
- Xie, X.; Gao, B.; Ma, Z.; Liu, J.; Zhang, J.; Liang, J.; Chen, Z.; Wu, L.; Li, W. Host-Guest Interaction Driven Peptide Assembly into Photoresponsive Two-Dimensional Nanosheets with Switchable Antibacterial Activity. CCS Chem. 2021, 3, 1949–1962.
- Panja, S.; Adams, D.J. Stimuli responsive dynamic transformations in supramolecular gels. Chem. Soc. Rev. 2021, 50, 5165–5200.
- Draper, E.R.; Adams, D.J. Photoresponsive gelators. Chem. Commun. 2016, 52, 8196–8206.
- Fatás, P.; Bachl, J.; Oehm, S.; Jiménez, A.I.; Cativiela, C.; Díaz Díaz, D. Multistimuli-Responsive Supramolecular Organogels Formed by Low-Molecular-Weight Peptides Bearing Side-Chain Azobenzene Moieties. Chem. Eur. J. 2013, 19, 8861–8874.
- Diaferia, C.; Balasco, N.; Sibillano, T.; Ghosh, M.; Adler-Abramovich, L.; Giannini, C.; Vitagliano, L.; Morelli, G.; Accardo, A. Amyloid-Like Fibrillary Morphology Originated by Tyrosine-Containing Aromatic Hexapeptides. Chem. Eur. J. 2018, 24, 6804–6817.
- Diaferia, C.; Rosa, E.; Balasco, N.; Sibillano, T.; Morelli, G.; Giannini, C.; Vitagliano, L.; Accardo, A. The Introduction of a Cysteine Residue Modulates the Mechanical Properties of Aromatic-Based Solid Aggregates and Self-Supporting Hydrogels. Chem. Eur. J. 2021, 27, 14886–14898.
- Draper, E.R.; McDonald, T.O.; Adams, D.J. Photodimerisation of a coumarin-dipeptide gelator. Chem. Commun. 2015, 51, 12827–12830.
- Fichman, G.; Gazit, E. Self-assembly of short peptides to form hydrogels: Design of building blocks, physical properties and technological applications. Acta Biomat. 2014, 10, 1671–1682.
- Jones, C.D.; Steed, J.W. Gels with sense: Supramolecular materials that respond to heat, light and sound. Chem. Soc. Rev. 2016, 45, 6546–6596.
- Li, Z.; Zhou, Y.; Li, T.; Zhang, J.; Tian, H. Stimuli-responsive hydrogels: Fabrication and biomedical applications. View 2022, 3, 20200112.
- Ahmed, S.; Pramanik, B.; Sankar, K.N.A.; Srivastava, A.; Singha, N.; Dowari, P.; Srivastava, A.; Mohanta, K.; Debnath, A.; Das, D. Solvent Assisted Tuning of Morphology of a Peptide-Perylenediimide Conjugate: Helical Fibers to Nano-Rings and their Differential Semiconductivity. Sci. Rep. 2017, 7, 9485.
- Ahmed, S.; Amba Sankar, K.N.; Pramanik, B.; Mohanta, K.; Das, D. Solvent Directed Morphogenesis and Electrical Properties of a Peptide–Perylenediimide Conjugate. Langmuir 2018, 34, 8355–8364.
- Wang, J.; Tao, K.; Zhou, P.; Pambou, E.; Li, Z.; Xu, H.; Rogers, S.; King, S.; Lu, J.R. Tuning self-assembled morphology of the Aβ(16–22) peptide by substitution of phenylalanine residues. Colloids Surf. B Biointerfaces 2016, 147, 116–123.
- Zhao, Y.; Li, X.; Zhang, L.; Wang, D.; Wang, W.; Wang, L.; Chen, C. Tuning the self-assembled nanostructures of ultra-short bola peptides via side chain variations of the hydrophobic amino acids. J. Mol. Liq. 2020, 315, 113765.
- Li, L.; Sun, R.; Zheng, R. Tunable morphology and functionality of multicomponent self-assembly: A review. Mater. Des. 2021, 197, 109209.
- Pashuck, E.T.; Cui, H.; Stupp, S.I. Tuning Supramolecular Rigidity of Peptide Fibers through Molecular Structure. J. Am. Chem. Soc. 2010, 132, 6041–6046.
- Edwards-Gayle, C.J.C.; Hamley, I.W. Self-assembly of bioactive peptides, peptide conjugates, and peptide mimetic materials. Org. Biomol. Chem. 2017, 15, 5867–5876.
- Jonker, A.M.; Löwik, D.W.P.M.; van Hest, J.C.M. Peptide- and Protein-Based Hydrogels. Chem. Mater. 2012, 24, 759–773.
- Habibi, N.; Kamaly, N.; Memic, A.; Shafiee, H. Self-assembled peptide-based nanostructures: Smart nanomaterials toward targeted drug delivery. Nano Today 2016, 11, 41–60.
- Ashwanikumar, N.; Kumar, N.A.; Nair, S.A.; Kumar, G.S.V. Phenylalanine-containing self-assembling peptide nanofibrous hydrogel for the controlled release of 5-fluorouracil and leucovorin. RSC Adv. 2014, 4, 29157–29164.
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48.
- He, R.; Finan, B.; Mayer, J.P.; DiMarchi, R.D. Peptide Conjugates with Small Molecules Designed to Enhance Efficacy and Safety. Molecules 2019, 24, 1855.
- Pethő, L.; Kasza, G.; Lajkó, E.; Láng, O.; Kőhidai, L.; Iván, B.; Mező, G. Amphiphilic drug–peptide–polymer conjugates based on poly(ethylene glycol) and hyperbranched polyglycerol for epidermal growth factor receptor targeting: The effect of conjugate aggregation on in vitro activity. Soft Matter 2020, 16, 5759–5769.
- Jeong, W.-J.; Bu, J.; Kubiatowicz, L.J.; Chen, S.S.; Kim, Y.; Hong, S. Peptide–nanoparticle conjugates: A next generation of diagnostic and therapeutic platforms? Nano Converg. 2018, 5, 38.
- Wei, G.; Wang, Y.; Huang, X.; Hou, H.; Zhou, S. Peptide-Based Nanocarriers for Cancer Therapy. Small Methods 2018, 2, 1700358.
- Zheng, Y.; Mao, K.; Chen, S.; Zhu, H. Chirality Effects in Peptide Assembly Structures. Front. Bioeng. Biotechnol. 2021, 9, 703004.
- Garifullin, R.; Guler, M.O. Supramolecular chirality in self-assembled peptide amphiphile nanostructures. Chem. Commun. 2015, 51, 12470–12473.
- Hu, K.; Jiang, Y.; Xiong, W.; Li, H.; Zhang, P.-Y.; Yin, F.; Zhang, Q.; Geng, H.; Jiang, F.; Li, Z.; et al. Tuning peptide self-assembly by an in-tether chiral center. Sci. Adv. 2018, 4, eaar5907.
- Dowari, P.; Pramanik, B.; Das, D. pH and secondary structure instructed aggregation to a thixotropic hydrogel by a peptide amphiphile. Bull. Mater. Sci. 2020, 43, 70.
- Dowari, P.; Saha, S.; Pramanik, B.; Ahmed, S.; Singha, N.; Ukil, A.; Das, D. Multiple Cross-Linking of a Small Peptide to Form a Size Tunable Biopolymer with Efficient Cell Adhesion and Proliferation Property. Biomacromolecules 2018, 19, 3994–4002.
- Apostolopoulos, V.; Bojarska, J.; Chai, T.-T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules 2021, 26, 430.
- Hirst, A.R.; Huang, B.; Castelletto, V.; Hamley, I.W.; Smith, D.K. Self-Organisation in the Assembly of Gels from Mixtures of Different Dendritic Peptide Building Blocks. Chem. Eur. J. 2007, 13, 2180–2188.
- Rosa, E.; Diaferia, C.; Gianolio, E.; Sibillano, T.; Gallo, E.; Smaldone, G.; Stornaiuolo, M.; Giannini, C.; Morelli, G.; Accardo, A. Multicomponent Hydrogel Matrices of Fmoc-FF and Cationic Peptides for Application in Tissue Engineering. Macromol. Biosci. 2022, 22, 2200128.
- Jain, R.; Roy, S. Designing a bioactive scaffold from coassembled collagen–laminin short peptide hydrogels for controlling cell behaviour. RSC Adv. 2019, 9, 38745–38759.
- Giraud, T.; Bouguet-Bonnet, S.; Stébé, M.-J.; Richaudeau, L.; Pickaert, G.; Averlant-Petit, M.-C.; Stefan, L. Co-assembly and multicomponent hydrogel formation upon mixing nucleobase-containing peptides. Nanoscale 2021, 13, 10566–10578.
- Jain, R.; Pal, V.K.; Roy, S. Triggering Supramolecular Hydrogelation Using a Protein–Peptide Coassembly Approach. Biomacromolecules 2020, 21, 4180–4193.
- Tang, W.; Yang, J.; Zhao, Z.; Lian, Z.; Liang, G. Intracellular coassembly boosts the anti-inflammation capacity of dexamethasone. Nanoscale 2017, 9, 17717–17721.
- Radvar, E.; Azevedo, H.S. Supramolecular Nanofibrous Peptide/Polymer Hydrogels for the Multiplexing of Bioactive Signals. ACS Biomater. Sci. Eng. 2019, 5, 4646–4656.
- Wang, Q.; Hou, X.; Gao, J.; Ren, C.; Guo, Q.; Fan, H.; Liu, J.; Zhang, W.; Liu, J. A coassembled peptide hydrogel boosts the radiosensitization of cisplatin. Chem. Commun. 2020, 56, 13017–13020.
- Ji, W.; Tang, Y.; Makam, P.; Yao, Y.; Jiao, R.; Cai, K.; Wei, G.; Gazit, E. Expanding the Structural Diversity and Functional Scope of Diphenylalanine-Based Peptide Architectures by Hierarchical Coassembly. J. Am. Chem. Soc. 2021, 143, 17633–17645.
- Halperin-Sternfeld, M.; Ghosh, M.; Sevostianov, R.; Grigoriants, I.; Adler-Abramovich, L. Molecular co-assembly as a strategy for synergistic improvement of the mechanical properties of hydrogels. Chem. Commun. 2017, 53, 9586–9589.
- Okesola, B.O.; Mata, A. Multicomponent self-assembly as a tool to harness new properties from peptides and proteins in material design. Chem. Soc. Rev. 2018, 47, 3721–3736.
- Diaferia, C.; Morelli, G.; Accardo, A. Fmoc-diphenylalanine as a suitable building block for the preparation of hybrid materials and their potential applications. J. Mater. Chem. B 2019, 7, 5142–5155.
- Diaferia, C.; Ghosh, M.; Sibillano, T.; Gallo, E.; Stornaiuolo, M.; Giannini, C.; Morelli, G.; Adler-Abramovich, L.; Accardo, A. Fmoc-FF and hexapeptide-based multicomponent hydrogels as scaffold materials. Soft Matter 2019, 15, 487–496.
- Raymond, D.M.; Nilsson, B.L. Multicomponent peptide assemblies. Chem. Soc. Rev. 2018, 47, 3659–3720.
- Jorgensen, M.D.; Chmielewski, J. Co-assembled Coiled-Coil Peptide Nanotubes with Enhanced Stability and Metal-Dependent Cargo Loading. ACS Omega 2022, 7, 20945–20951.
- Carrick, L.M.; Aggeli, A.; Boden, N.; Fisher, J.; Ingham, E.; Waigh, T.A. Effect of ionic strength on the self-assembly, morphology and gelation of pH responsive β-sheet tape-forming peptides. Tetrahedron 2007, 63, 7457–7467.
- Lee, S.; Trinh, T.H.T.; Yoo, M.; Shin, J.; Lee, H.; Kim, J.; Hwang, E.; Lim, Y.-b.; Ryou, C. Self-Assembling Peptides and Their Application in the Treatment of Diseases. Int. J. Mol. Sci. 2019, 20, 5850.
- Lopez-Silva, T.L.; Leach, D.G.; Li, I.C.; Wang, X.; Hartgerink, J.D. Self-Assembling Multidomain Peptides: Design and Characterization of Neutral Peptide-Based Materials with pH and Ionic Strength Independent Self-Assembly. ACS Biomater. Sci. Eng. 2019, 5, 977–985.
- Tan, W.; Zhang, Q.; Quiñones-Frías, M.C.; Hsu, A.Y.; Zhang, Y.; Rodal, A.; Hong, P.; Luo, H.R.; Xu, B. Enzyme-Responsive Peptide Thioesters for Targeting Golgi Apparatus. J. Am. Chem. Soc. 2022, 144, 6709–6713.
- Liu, S.; Zhang, Q.; Shy, A.N.; Yi, M.; He, H.; Lu, S.; Xu, B. Enzymatically Forming Intranuclear Peptide Assemblies for Selectively Killing Human Induced Pluripotent Stem Cells. J. Am. Chem. Soc. 2021, 143, 15852–15862.
- Li, J.; Xu, B. 19—Enzyme-mediated self-assembly. In Self-Assembling Biomaterials; Azevedo, H.S., da Silva, R.M.P., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 399–417.
- Zhou, J.; Xu, B. Enzyme-Instructed Self-Assembly: A Multistep Process for Potential Cancer Therapy. Bioconjug. Chem. 2015, 26, 987–999.
- Xie, Y.; Huang, R.; Qi, W.; Wang, Y.; Su, R.; He, Z. Enzyme–substrate interactions promote the self-assembly of amino acid derivatives into supramolecular hydrogels. J. Mater. Chem. B 2016, 4, 844–851.
- Huang, R.; Wang, Y.; Qi, W.; Su, R.; He, Z. Temperature-induced reversible self-assembly of diphenylalanine peptide and the structural transition from organogel to crystalline nanowires. Nanoscale Res. Lett. 2014, 9, 653.
- Kopeček, J.; Yang, J. Peptide-directed self-assembly of hydrogels. Acta Biomater. 2009, 5, 805–816.
- Dehsorkhi, A.; Castelletto, V.; Hamley, I.W. Self-assembling amphiphilic peptides. J. Pept. Sci. 2014, 20, 453–467.
- Pugliese, R.; Gelain, F. Programmable stiffness and stress–relaxation of cross-linked self-assembling peptide hydrogels. J. Appl. Polym. Sci. 2022, 139, 51759.
- Das, B.K.; Pramanik, B.; Chowdhuri, S.; Scherman, O.A.; Das, D. Light-triggered syneresis of a water insoluble peptide-hydrogel effectively removes small molecule waste contaminants. Chem. Commun. 2020, 56, 3393–3396.
- Mondal, J.H.; Ahmed, S.; Ghosh, T.; Das, D. Reversible deformation–formation of a multistimuli responsive vesicle by a supramolecular peptide amphiphile. Soft Matter 2015, 11, 4912–4920.
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and Applications of Photoresponsive Hydrogels. Adv. Mater. 2019, 31, 1807333.
- Jia, S.; Fong, W.-K.; Graham, B.; Boyd, B.J. Photoswitchable Molecules in Long-Wavelength Light-Responsive Drug Delivery: From Molecular Design to Applications. Chem. Mater. 2018, 30, 2873–2887.
- Volarić, J.; Szymanski, W.; Simeth, N.A.; Feringa, B.L. Molecular photoswitches in aqueous environments. Chem. Soc. Rev. 2021, 50, 12377–12449.
- Yao, X.; Li, T.; Wang, J.; Ma, X.; Tian, H. Recent Progress in Photoswitchable Supramolecular Self-Assembling Systems. Adv. Opt. Mater. 2016, 4, 1322–1349.
- Wu, D.; Xie, X.; Kadi, A.A.; Zhang, Y. Photosensitive peptide hydrogels as smart materials for applications. Chin. Chem. Lett. 2018, 29, 1098–1104.
- Garifullin, R.; Guler, M.O. Electroactive peptide-based supramolecular polymers. Mater. Today Bio 2021, 10, 100099.
- Devika, V.; Sreelekshmi, P.J.; Rajeev, N.; Aiswarya Lakshmi, S.; Chandran, A.; Gouthami, G.B.; Sadanandan, S. Recent Advances in Peptides-Based Stimuli-Responsive Materials for Biomedical and Therapeutic Applications: A Review. Mol. Pharm. 2022, 19, 1999–2021.
- Jervis, P.J.; Hilliou, L.; Pereira, R.B.; Pereira, D.M.; Martins, J.A.; Ferreira, P.M.T. Evaluation of a Model Photo-Caged Dehydropeptide as a Stimuli-Responsive Supramolecular Hydrogel. Nanomaterials 2021, 11, 704.
- Smith, D.J.; Brat, G.A.; Medina, S.H.; Tong, D.; Huang, Y.; Grahammer, J.; Furtmüller, G.J.; Oh, B.C.; Nagy-Smith, K.J.; Walczak, P.; et al. A multiphase transitioning peptide hydrogel for suturing ultrasmall vessels. Nat. Nanotechnol. 2016, 11, 95–102.
- Xing, P.; Chen, H.; Xiang, H.; Zhao, Y. Selective Coassembly of Aromatic Amino Acids to Fabricate Hydrogels with Light Irradiation-Induced Emission for Fluorescent Imprint. Adv. Mater. 2018, 30, 1705633.
- Navarro-Barreda, D.; Angulo-Pachón, C.A.; Galindo, F.; Miravet, J.F. Photoreversible formation of nanotubes in water from an amphiphilic azobenzene derivative. Cheml. Commun. 2021, 57, 11545–11548.
- Karcher, J.; Kirchner, S.; Leistner, A.-L.; Hald, C.; Geng, P.; Bantle, T.; Gödtel, P.; Pfeifer, J.; Pianowski, Z.L. Selective release of a potent anticancer agent from a supramolecular hydrogel using green light. RSC Adv. 2021, 11, 8546–8551.
- Li, L.; Chen, J.; Wang, Z.; Xie, L.; Feng, C.; He, G.; Hu, H.; Sun, R.; Zhu, H. A supramolecular gel made from an azobenzene-based phenylalanine derivative: Synthesis, self-assembly, and dye adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127289.
- Larik, F.A.; Fillbrook, L.L.; Nurttila, S.S.; Martin, A.D.; Kuchel, R.P.; Al Taief, K.; Bhadbhade, M.; Beves, J.E.; Thordarson, P. Ultra-Low Molecular Weight Photoswitchable Hydrogelators. Angew. Chem. Int. Ed. 2021, 60, 6764–6770.
- Chu, C.-W.; Stricker, L.; Kirse, T.M.; Hayduk, M.; Ravoo, B.J. Light-Responsive Arylazopyrazole Gelators: From Organic to Aqueous Media and from Supramolecular to Dynamic Covalent Chemistry. Chem. Eur. J. 2019, 25, 6131–6140.
- Sallee, A.; Ghebreyessus, K. Photoresponsive Zn2+–specific metallohydrogels coassembled from imidazole containing phenylalanine and arylazopyrazole derivatives. Dalton Trans. 2020, 49, 10441–10451.
- Nakamura, K.; Tanaka, W.; Sada, K.; Kubota, R.; Aoyama, T.; Urayama, K.; Hamachi, I. Phototriggered Spatially Controlled Out-of-Equilibrium Patterns of Peptide Nanofibers in a Self-Sorting Double Network Hydrogel. J. Am. Chem. Soc. 2021, 143, 19532–19541.
- Weyandt, E.; ter Huurne, G.M.; Vantomme, G.; Markvoort, A.J.; Palmans, A.R.A.; Meijer, E.W. Photodynamic Control of the Chain Length in Supramolecular Polymers: Switching an Intercalator into a Chain Capper. J. Am. Chem. Soc. 2020, 142, 6295–6303.
- Behanna, H.A.; Rajangam, K.; Stupp, S.I. Modulation of Fluorescence through Coassembly of Molecules in Organic Nanostructures. J. Am. Chem. Soc. 2007, 129, 321–327.
- Nakayama, K.; Heise, I.; Görner, H.; Gärtner, W. Peptide Release upon Photoconversion of 2-Nitrobenzyl Compounds into Nitroso Derivatives. Photochem. Photobiol. 2011, 87, 1031–1035.
- Peters, F.B.; Brock, A.; Wang, J.; Schultz, P.G. Photocleavage of the Polypeptide Backbone by 2-Nitrophenylalanine. Chem. Biol. 2009, 16, 148–152.
- Tatsu, Y.; Nishigaki, T.; Darszon, A.; Yumoto, N. A caged sperm-activating peptide that has a photocleavable protecting group on the backbone amide. FEBS Lett. 2002, 525, 20–24.
- Grunwald, C.; Schulze, K.; Reichel, A.; Weiss, V.U.; Blaas, D.; Piehler, J.; Wiesmüller, K.-H.; Tampé, R. In situ assembly of macromolecular complexes triggered by light. Proc. Natl. Acad. Sci. USA 2010, 107, 6146–6151.
- Mason, M.L.; Lalisse, R.F.; Finnegan, T.J.; Hadad, C.M.; Modarelli, D.A.; Parquette, J.R. pH-Controlled Chiral Packing and Self-Assembly of a Coumarin Tetrapeptide. Langmuir 2019, 35, 12460–12468.
- Wang, C.; Fu, L.; Hu, Z.; Zhong, Y. A mini-review on peptide-based self-assemblies and their biological applications. Nanotechnology 2021, 33, 062004.
- Zhong, Y.; Zhan, J.; Xu, G.; Chen, Y.; Qin, Q.; Liao, X.; Ma, S.; Yang, Z.; Cai, Y. Enzyme-Instructed Self-Assembly Enabled Monomer–Excimer Transition to Construct Higher Ordered Luminescent Supramolecular Assembly for Activity-based Bioimaging. Angew. Chem. Int. Ed. 2021, 60, 8121–8129.
- Chivers, P.R.A.; Dookie, R.S.; Gough, J.E.; Webb, S.J. Photo-dissociation of self-assembled (anthracene-2-carbonyl)amino acid hydrogels. Chem. Commun. 2020, 56, 13792–13795.
- Mondal, S.; Chakraborty, P.; Das, S.; Bairi, P.; Nandi, A.K. A Comparative Account of the Kinetics of Light-Induced E–Z Isomerization of an Anthracene-Based Organogelator in Sol, Gel, Xerogel, and Powder States: Fiber to Crystal Transformation. Langmuir 2016, 32, 5373–5382.
- Truong, V.X.; Li, F.; Forsythe, J.S. Versatile Bioorthogonal Hydrogel Platform by Catalyst-Free Visible Light Initiated Photodimerization of Anthracene. ACS Macro Lett. 2017, 6, 657–662.
- Nishitani, N.; Hirose, T.; Matsuda, K. Self-assembly of photochromic diarylethene–peptide conjugates stabilized by β-sheet formation at the liquid/graphite interface. Chem. Commun. 2019, 55, 5099–5102.
- Cheng, H.-B.; Zhang, S.; Bai, E.; Cao, X.; Wang, J.; Qi, J.; Liu, J.; Zhao, J.; Zhang, L.; Yoon, J. Future-Oriented Advanced Diarylethene Photoswitches: From Molecular Design to Spontaneous Assembly Systems. Adv. Mater. 2022, 34, 2108289.
- de Loos, M.; van Esch, J.; Kellogg, R.M.; Feringa, B.L. Chiral Recognition in Bis-Urea-Based Aggregates and Organogels through Cooperative Interactions. Angew. Chem. Int. Ed. 2001, 40, 613–616.
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825.
- Doran, T.M.; Ryan, D.M.; Nilsson, B.L. Reversible photocontrol of self-assembled peptide hydrogel viscoelasticity. Polym. Chem. 2014, 5, 241–248.
- Pianowski, Z.L.; Karcher, J.; Schneider, K. Photoresponsive self-healing supramolecular hydrogels for light-induced release of DNA and doxorubicin. Chem. Commun. 2016, 52, 3143–3146.
- Samai, S.; Sapsanis, C.; Patil, S.P.; Ezzeddine, A.; Moosa, B.A.; Omran, H.; Emwas, A.-H.; Salama, K.N.; Khashab, N.M. A light responsive two-component supramolecular hydrogel: A sensitive platform for the fabrication of humidity sensors. Soft Matter 2016, 12, 2842–2845.
- Sahoo, J.K.; Nalluri, S.K.M.; Javid, N.; Webb, H.; Ulijn, R.V. Biocatalytic amide condensation and gelation controlled by light. Chem. Commun. 2014, 50, 5462–5464.
- Huang, Y.; Qiu, Z.; Xu, Y.; Shi, J.; Lin, H.; Zhang, Y. Supramolecular hydrogels based on short peptides linked with conformational switch. Org. Biomol. Chem. 2011, 9, 2149–2155.
- Matsuzawa, Y.; Tamaoki, N. Photoisomerization of Azobenzene Units Controls the Reversible Dispersion and Reorganization of Fibrous Self-Assembled Systems. J. Phys. Chem. B 2010, 114, 1586–1590.
- Stricker, L.; Fritz, E.-C.; Peterlechner, M.; Doltsinis, N.L.; Ravoo, B.J. Arylazopyrazoles as Light-Responsive Molecular Switches in Cyclodextrin-Based Supramolecular Systems. J. Am. Chem. Soc. 2016, 138, 4547–4554.
- Browne, W.R.; Feringa, B.L. Making molecular machines work. Nat. Nanotechnol. 2006, 1, 25–35.
- Wegner, H.A. Molecular Switches. Second Edition. Edited by Ben L. Feringa and Wesley R. Browne. Angew. Chem. Int. Ed. 2012, 51, 2281.
- Nalluri, S.K.M.; Voskuhl, J.; Bultema, J.B.; Boekema, E.J.; Ravoo, B.J. Light-Responsive Capture and Release of DNA in a Ternary Supramolecular Complex. Angew. Chem. Int. Ed. 2011, 50, 9747–9751.
- Moratz, J.; Samanta, A.; Voskuhl, J.; Mohan Nalluri, S.K.; Ravoo, B.J. Light-Triggered Capture and Release of DNA and Proteins by Host–Guest Binding and Electrostatic Interaction. Chem. Eur. J. 2015, 21, 3271–3277.
- Roling, O.; Stricker, L.; Voskuhl, J.; Lamping, S.; Ravoo, B.J. Supramolecular surface adhesion mediated by azobenzene polymer brushes. Chem. Commun. 2016, 52, 1964–1966.
- Knie, C.; Utecht, M.; Zhao, F.; Kulla, H.; Kovalenko, S.; Brouwer, A.M.; Saalfrank, P.; Hecht, S.; Bléger, D. ortho-Fluoroazobenzenes: Visible Light Switches with Very Long-Lived Z Isomers. Chem. Eur. J. 2014, 20, 16492–16501.
- Weston, C.E.; Richardson, R.D.; Haycock, P.R.; White, A.J.P.; Fuchter, M.J. Arylazopyrazoles: Azoheteroarene Photoswitches Offering Quantitative Isomerization and Long Thermal Half-Lives. J. Am. Chem. Soc. 2014, 136, 11878–11881.
- Stricker, L.; Böckmann, M.; Kirse, T.M.; Doltsinis, N.L.; Ravoo, B.J. Arylazopyrazole Photoswitches in Aqueous Solution: Substituent Effects, Photophysical Properties, and Host–Guest Chemistry. Chem. Eur. J. 2018, 24, 8639–8647.
- Kortekaas, L.; Browne, W.R. The evolution of spiropyran: Fundamentals and progress of an extraordinarily versatile photochrome. Chem. Soc. Rev. 2019, 48, 3406–3424.
- Qiu, Z.; Yu, H.; Li, J.; Wang, Y.; Zhang, Y. Spiropyran-linked dipeptide forms supramolecular hydrogel with dual responses to light and to ligand–receptor interaction. Chem. Commun. 2009, 23, 3342–3344.
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2014, 43, 148–184.
- Liu, M.; Creemer, C.N.; Reardon, T.J.; Parquette, J.R. Light-driven dissipative self-assembly of a peptide hydrogel. Chem. Commun. 2021, 57, 13776–13779.
- Moldenhauer, D.; Gröhn, F. Water-Soluble Spiropyrans with Inverse Photochromism and Their Photoresponsive Electrostatic Self-Assembly. Chem. Eur. J. 2017, 23, 3966–3978.
- Parthenopoulos, D.A.; Rentzepis, P.M. Three-Dimensional Optical Storage Memory. Science 1989, 245, 843–845.
- Rosario, R.; Gust, D.; Hayes, M.; Jahnke, F.; Springer, J.; Garcia, A.A. Photon-Modulated Wettability Changes on Spiropyran-Coated Surfaces. Langmuir 2002, 18, 8062–8069.
- Raymo, F.M.; Giordani, S. Signal Processing at the Molecular Level. J. Am. Chem. Soc. 2001, 123, 4651–4652.
- Minkin, V.I. Photo-, Thermo-, Solvato-, and Electrochromic Spiroheterocyclic Compounds. Chem. Rev. 2004, 104, 2751–2776.
- Wojtyk, J.T.C.; Wasey, A.; Xiao, N.-N.; Kazmaier, P.M.; Hoz, S.; Yu, C.; Lemieux, R.P.; Buncel, E. Elucidating the Mechanisms of Acidochromic Spiropyran-Merocyanine Interconversion. J. Phys. Chem. A 2007, 111, 2511–2516.
- Wagner, K.; Byrne, R.; Zanoni, M.; Gambhir, S.; Dennany, L.; Breukers, R.; Higgins, M.; Wagner, P.; Diamond, D.; Wallace, G.G.; et al. A Multiswitchable Poly(terthiophene) Bearing a Spiropyran Functionality: Understanding Photo- and Electrochemical Control. J. Am. Chem. Soc. 2011, 133, 5453–5462.
- Chen, L.; Wu, J.; Schmuck, C.; Tian, H. A switchable peptide sensor for real-time lysosomal tracking. Chem. Commun. 2014, 50, 6443–6446.
- Keyvan Rad, J.; Balzade, Z.; Mahdavian, A.R. Spiropyran-based advanced photoswitchable materials: A fascinating pathway to the future stimuli-responsive devices. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100487.
- Yang, Y.; Li, Y.; Chen, Y.; Wang, Z.; He, Z.; He, J.; Zhao, H. Dynamic Anticounterfeiting Through Novel Photochromic Spiropyran-Based Composites. ACS Appl. Mater. Interfaces 2022, 14, 21330–21339.
- Zhu, C.N.; Li, C.Y.; Wang, H.; Hong, W.; Huang, F.; Zheng, Q.; Wu, Z.L. Reconstructable Gradient Structures and Reprogrammable 3D Deformations of Hydrogels with Coumarin Units as the Photolabile Crosslinks. Adv. Mater. 2021, 33, 2008057.
- Kim, S.H.; Sun, Y.; Kaplan, J.A.; Grinstaff, M.W.; Parquette, J.R. Photo-crosslinking of a self-assembled coumarin-dipeptide hydrogel. New J. Chem. 2015, 39, 3225–3228.
- Liu, Q.; Wang, H.; Li, G.; Liu, M.; Ding, J.; Huang, X.; Gao, W.; Huayue, W. A photocleavable low molecular weight hydrogel for light-triggered drug delivery. Chin. Chem. Lett. 2019, 30, 485–488.
- Zheng, Z.; Hu, J.; Wang, H.; Huang, J.; Yu, Y.; Zhang, Q.; Cheng, Y. Dynamic Softening or Stiffening a Supramolecular Hydrogel by Ultraviolet or Near-Infrared Light. ACS Appl. Mater. Interfaces 2017, 9, 24511–24517.
