1. Introduction
Supramolecular self-assembly, governed by multiple non-covalent interactions, has been explored as a powerful and elegant strategy for the hierarchical bottom-up synthesis of soft materials across length scales
[1][2][3][4][5][1,2,3,4,5]. Though the individual supramolecular interactions are weak, the resultant interaction is strong enough to make soft materials with different nanostructures, functions, and elegant properties when they work in tandem. An extreme case of higher order self-assembly is the formation of supramolecular gels, basically, semi-solid materials composed of three-dimensional (3D) networked structures with a large amount of entrapped solvents (water in the case of hydrogels and other solvents for organogels)
[6][7][8][9][10][11][12][13][6,7,8,9,10,11,12,13]. Due to the reversible nature of the supramolecular interactions, such as hydrogen bonding, π−π stacking, hydrophobic interactions, van der Waals interactions, charge-transfer interactions, etc., the resultant gels are highly sensitive to different external stimuli and thus making those gels highly dynamic in nature
[14][15][16][14,15,16]. Over the past couple of decades, a plethora of supramolecular gels with structural sophistication and functional variations, particularly aromatic peptides because of their built π-interactions environment, have been reported
[17][18][19][20][21][17,18,19,20,21].
In light of this, peptides, because of their unique properties, are proven to be an excellent class of building blocks for devising supramolecular gels
[22][23][24][25][22,23,24,25]. They offer a wide range of structural diversity, self-assembling propensities, and morphological variations due to large possible combinations of amino acids which form peptide sequences
[26][27][28][29][30][31][32][26,27,28,29,30,31,32]. In addition, the design rules for the self-assembly of peptides are well documented. Moreover, peptides offer bioactive functionalities, biocompatibility
[33][34][33,34], and biodegradability
[35]. In addition to this, they can possess unique specific functions like cell targeting and environmental responsiveness owing to their bio-active nature
[36]. Chemically, the side chains, free amino (–NH
2), and carboxyl (–COOH) terminus further open up ample opportunities to integrate drugs
[37][38][37,38], carriers
[39][40][39,40], and other functional molecules of interest. Due to the chiral nature of the amino acids (except Glycine (Gly)), often, molecular chirality gets transferred to the supramolecular level causing nano-structures with specific chirality
[41][42][43][41,42,43]. Additionally, peptides are synthetically accessible due to the well-established straightforward, efficient procedure of Solid Phase Peptide Synthesis (SPPS) that makes them a promising candidate for assembling, programming, and recognizing with utmost efficacy and minimum toxicity
[44][45][44,45]. Additionally, peptides are well-known for their smaller size (length ranging from 10 to 15 amino acids), even smaller than antibodies, and they are less immunogenic and highly stable in physiological conditions, making them a reliable candidate for conjugation with various kinds of nano-carriers for biological application
[46]. Finally, peptides are well-known for their co-assembling and co-aggregating propensity with a wide range of molecular entities such as other peptide sequences, proteins, polymers, drug molecules, inorganic and other molecules
[47][48][49][50][51][52][53][54][55][47,48,49,50,51,52,53,54,55]. Co-assembly can occur at the molecular level in mainly four different ways, viz. (a) cooperative co-assembly, (b) self-sorting (or orthogonal co-assembly), (c) random co-assembly, and (d) destructive co-assembly
[50]. These newly generated multicomponent co-assembled systems give access to tailored features, enhanced mechanical and architectural scope, desired morphology, improved bioavailability, and functional complexity with emergent behavior
[48][49][50][51][52][53][54][55][56][57][58][59][48,49,50,51,52,53,54,55,56,57,58,59]. In fact, in recent years, a considerable amount of effort have been dedicated in the direction of designing peptide-based multicomponent systems decorated with desired structures, properties, and functions with multitasking abilityies via co-assembly, which is difficult for a monocomponent peptide assembly to realize
[48][57][58][59][60][61][48,57,58,59,60,61].
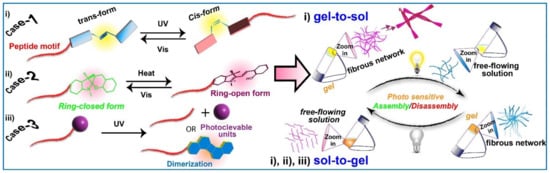
One potentially helpful feature of supramolecular gels is their switchable behavior in different physical states in response to various external stimuli. Although a plethora of incentives, for example, ionic strength
[62][63][64][62,63,64], pH
[44][64][44,64], enzyme
[65][66][67][68][69][65,66,67,68,69], temperature
[14][70][71][72][14,70,71,72], mechanical stress
[73], light
[74][75][74,75], etc., have been reported extensively to show the switching ability, among them, light has received extensive attention because of its non-invasive nature and more importantly, light permits to target a specific area of gel remotely by using photo masks with a high level of spatiotemporal resolution causing patterned gel surfaces and rapid phase transitions reversibly
[17][18][76][77][78][17,18,76,77,78]. On top of that, the system is free from waste generation/chemical contaminants hence closed systems can be stimulated without introducing any foreign chemicals, and finally, the light can be conveniently switched on and off with specific wavelengths and tunable intensities to modulate and program supramolecular gelation
[18][77][79][18,77,79]. Considering the utmost advantages of peptides and light, in recent years, a variety of photo-responsive moieties has been incorporated into the peptides to design photo-responsive gelators which can display switchable, smart, and emergent features
[15][80][81][82][83][84][15,80,81,82,83,84].
This short review features the recent advancement toward developing low molecular weight supramolecular light-responsive peptide gels. Although a massive number of light-responsive peptide assemblies have been documented in recent years, considering the scope of this
research, the researchersshort review, we have only included the special cases where ‘gels’ are involved, as shown in
Scheme 1.
Scheme 1.
Schematic representation of light-responsive supramolecular gels.
2. Light-Responsive Molecular Switches
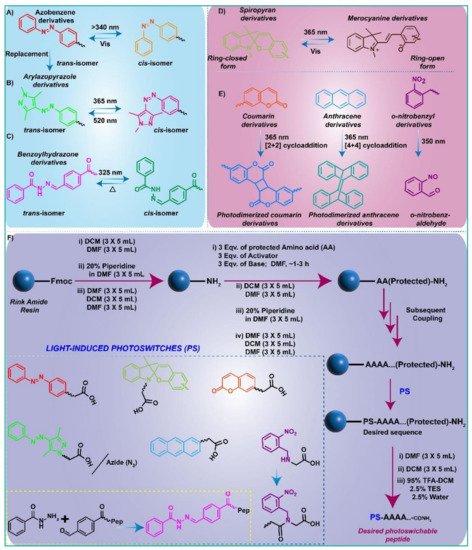
In view of light-responsive supramolecular gels, azobenzene
[55][85][86][87][88][55,85,86,87,88], arylazopyrozole
[89][90][91][89,90,91], benzoylhydrozone
[92][93][92,93], stilbene
[60][94][60,94], etc., are more frequently used photoisomerizable molecules that switch between
trans- and
cis-isomeric forms under the illumination of light (
Figure 1). Spiropyran
[24][78][80][24,78,80] is another critical photo-sensitive unit for light-induced ring-opening and closing behavior. The 2-nitrobenzyl (NB) group
[95][96][97][98][95,96,97,98], coumarin
[99][100][101][99,100,101], anthracene
[102][103][104][102,103,104], and diarylethene
[105][106][105,106] units are also used to create light-responsive gels (
Figure 1). The molecular structure and light-induced structural changes of the most well-studied and explored photo-switches in recent years are shown in
Figure 1. Additionally, in view of chemical approaches, a schematic illustration is depicted to synthesize the photo-switchable peptide monomers (
Figure 1F).
Figure 1. Molecular structure of: (A) Azobenzene; (B) Arylazopyrazoles; (C) Benzoylhydrazone derivatives and light-induced reversible trans- and cis- isomerization; (D) Chemical structure and light-assisted reversible equilibrium between Spiropyran and Merocyanine derivatives; (F) Schematic illustration for synthesis of photo-responsive peptide conjugates using SPPS.
2.1. Azobenzene Conjugated Peptide Derivatives and Light-Assisted Self-Assembly/Disassembly Phenomenon
Azobenzene core is the most common photo-responsive moiety incorporated in peptide sequences to design low molecular weight peptide gelators to develop numerous functional soft materials
[78][107][108][109][110][111][112][113][78,107,108,109,110,111,112,113]. Under UV-light irradiation, the azobenzene core undergoes
trans- (
E-) to
cis- (
Z-) isomerization, while the reverse
cis to
trans isomerization process is carried out by visible light or thermally in a dark environment (
Figure 1A)
[78][107][108][109][110][111][112][113][78,107,108,109,110,111,112,113]. The
trans-isomer is a thermodynamically favored state. The photoisomerization leads to the change in molecular planarity, which in turn affects π−π stacking interaction amongst the azobenzene moieties causing alteration of the molecular packing of azobenzene-incorporated peptides, which ultimately results in the formation or disruption of gels
[109][113][114][109,113,114]. Hence, it is fascinating to incorporate azobenzene into short peptide sequences to create light-responsive peptide hydrogels with variable properties and functions due to light-induced changes in the steric profile of the installed azobenzene.
2.2. Arylazopyrazoles Conjugated Peptide Derivatives with Light-Sensitive Gelation Characteristics
Although a lot of progress has been made with azobenzene as a light-responsive molecular switch, certain disadvantages restrict their application
[115][132]. For example, the UV-light used to trigger
E→
Z isomerization is harmful and can be vastly distributed in biological tissue or nanomaterials
[115][116][117][132,133,134]. Additionally, most azobenzene derivatives exhibited low thermodynamic stability of the
Z-form in comparison with other molecular photo-switches
[115][132]. Consequently, incomplete photoisomerization behavior is noticed owing to the overlapping absorbances of both
E- and
Z-isomers. The photostationary state (PSS) for classic azobenzene derivatives is about 80% for the
E→
Z and 70% for the
Z→
E isomerization
[115][118][132,135]. Because of this drawback, in highly multivalent systems, a substantial fraction of the remaining
E-isomer can still dictate the material properties, causing partial switching
[115][118][119][120][132,135,136,137].
For the last few decades, researchers have been trying to develop azobenzene derivatives that can undergo visible light-induced isomerization to aim either to move the π→π* transition to a longer wavelength or to acquire a splitting n→π* transition of the
E- and
Z-isomer that typically fuse in 400–500 nm wavelength window
[115][121][132,138]. Therefore, to solve the issue, the pyrazole hetero cycle was introduced
[115][122][132,139]. The replacement of one benzene ring in azobenzene with a pyrazole ring resulted in arylazopyrazoles (AAP,
Figure 1B), as an alternative and a new light-responsive molecular switch. Introduced by Fuchter et al. they have received enormous attention to the peptide chemist and pharmacist because of their ease and scalable synthesis, good water solubility, and superior photophysical properties
[122][123][139,140]. As expected, APP displayed a noteworthy red shift of the n→π* transition band of the
Z-isomer, enabling almost quantitative isomerization by UV(
E→Z) or green light (
Z→
E) irradiation
[115][132]. Additionally, AAP showed half-life times up to 1000 days, which can be attributed to the decreased steric repulsion within the
Z-form
[115][132].
2.3. Spiropyran Conjugated Peptide Derivatives and Light-Induced Gelation Behaviour
In light of the molecular photo-switches, spiropyrans have received extraordinary attention from photo chemists and peptide chemists because of their outstanding photophysical properties
[124][141]. Depending on the nature of illuminating light, two distinct structural thermodynamically stable isomers exist with the gigantic difference in properties: (i) colored planar merocyanine (MC) form, a charged hydrophilic ring-open form, and (ii) colorless non-planar spiropyran (SP) form, a non-charged hydrophobic ring-closed form which ultimately make spiropyran a unique photo-switch (
Figure 1D)
[124][125][126][119,141,142]. Because of the planar structure, SP shows a high propensity to form aggregate-like structures through intermolecular π−π stacking (
Figure 1D)
[125][127][128][119,121,143]. It is well-documented in literature that a range of stimuli such as temperature, solvents, redox potential, acids, bases, metal ions, mechanical forces, etc., can stimulate spiropyran’s reversible isomerization
[129][130][131][132][133][134][144,145,146,147,148,149]. Based on the properties mentioned above, increasing effort has been made to create spiropyran appended novel materials over the decades
[135][136][137][150,151,152].
2.4. Other Photo-Responsive Peptide Derivatives and Light-Induced Gel-Sol Transition or Vice-Versa
Coumarins are well-known for their photodimerization tenacity when irradiated with light of wavelength greater than 280 nm (
Figure 1E)
[22][138][22,155]. The photo-induced nature of the coumarins has inspired scientists to prepare stimuli-responsive LMWGs
[22][139][22,122]. As anticipated, the solubility of the light-induced dimerized coumarin decreases as the coumarin monomer becomes double in size. As a result, hydrophobicity of the system increases, which disrupt the gel network and, eventually, decreases in rheological parameters observed
[139][140][141][122,123,156].
Benzoylhydrazone is another interesting moiety that also exhibits reversible
E-
Z isomerization on photoirradiation, but this moiety is less explored in the context of peptide-based gelators (
Figure 1C)
[92].