The molecular structure of pillar[n]arene could serve different roles in the fabrication of attractive carbon nanotube-based materials. Firstly, pillar[n]arene has the ability to provide the structural basis for enlarging the cylindrical pillar-like architecture by forming one-dimensional, rigid, tubular, oligomeric/polymeric structures with aromatic moieties as the linker, or forming spatially “closed”, channel-like, flexible structures by perfunctionalizing with peptides and with intramolecular hydrogen bonding. Interestingly, such pillar[n]arene-based carbon nanotube-resembling structures were used as porous materials for the adsorption and separation of gas and toxic pollutants, as well as for artificial water channels and membranes. In addition to the art of organic synthesis, self-assembly based on pillar[n]arene, such as self-assembled amphiphilic molecules, is also used to promote and control the dispersion behavior of carbon nanotubes in solution. Furthermore, functionalized pillar[n]arene derivatives integrated carbon nanotubes to prepare advanced hybrid materials through supramolecular interactions, which could also incorporate various compositions such as Ag and Au nanoparticles for catalysis and sensing.
1. Mimicking the Structure and Characteristics of Carbon Nanotubes by Functionalized Pillar[n]arene
1.1. Preparing Linear Pillar[n]arene-Based Oligomer/Polymer via Rigid Aromatic Bridges
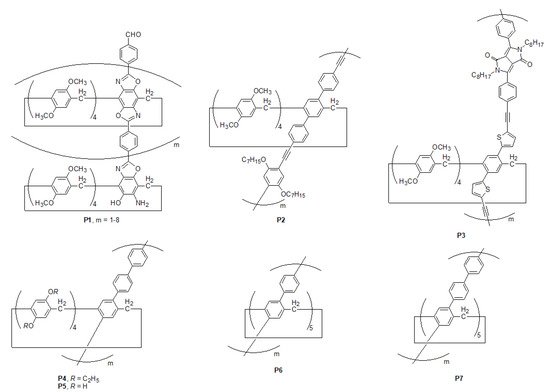
Due to the possession of rigid pillar-like molecular structures and an electron-rich cavity
[1][48], the skeleton of functionalized pillar[n]arene was employed as previous pieces for the construction of linear oligomeric and polymeric architectures, such as
P1–
P7 (
Scheme 1), by introducing rigid aromatic bridging subunits to mimic carbon nanotubes
[2][3][4][5][6][42,49,50,51,52]. Several classic organic syntheses such as heterocyclization and Pd-catalyzed coupling reactions
[7][8][9][10][53,54,55,56] have been thoroughly employed. Particularly, those carbon nanotube-resembling linear pillar[n]arene-based porous materials have been used in diverse applications such as gas absorption
[11][12][57,58], as well as the absorption and separation of toxic pollutants in water
[13][59]. For example,
P2 exhibits a recognition towards solvent molecules such as dichloromethane
[4][50], whereas
P3 has the ability to capture toxic pollutants such as adiponitrile
[5][51]. In addition,
P5 can selectively absorb CO
2 rather than N
2 or methane
[6][52], whereas
P6 and
P7 were used for separating propane gas from the simulated gas mixture of methane and propane
[2][42].
Scheme 1. Chemical structures of pillar[5]arene-based polymers P1–P7.
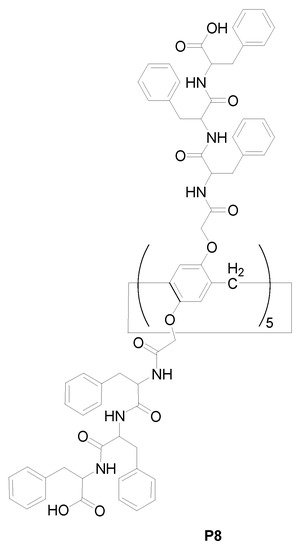
1.2. Preparing Peptide-Appended Pillar[n]arene Processing Intramolecular Hydrogen Bonds
Except for introducing rigid aromatic moieties as the bridging linker for the construction of pillar[n]arene-based polymeric architectures, diverse designs and synthetic strategies were carried out for mimicking carbon nanotubes; for example
[14][60], the peptide-appended pillar[n]arene
P8 (
Scheme 2) was produced to introduce intramolecular interactions such as hydrogen bonding
[15][61] to form “closed”, tubular, molecular architectures
[16][62], as well as to resemble the performance of carbon nanotubes as artificial water channels
[17][18][63,64] and permeable membranes
[19][20][21][65,66,67]. It was revealed that the average single-channel osmotic water permeability
[19][65] and the ion rejection of
P8 were greatly analogous to those of carbon nanotubes
[21][67]. Furthermore, the pore density
[22][68] of
P8-based channel arrays was much higher than that of carbon nanotube-based ones
[19][65]. It was also found that the flexible conformation of the peptide-appended pillar[n]arene was available for water permeability
[20][23][24][66,69,70].
Scheme 2. Chemical structures of pillar [5]arene derivative
P8 [19][65].
2. Dispersion of Carbon Nanotube by Using Functionalized Pillar[n]arene
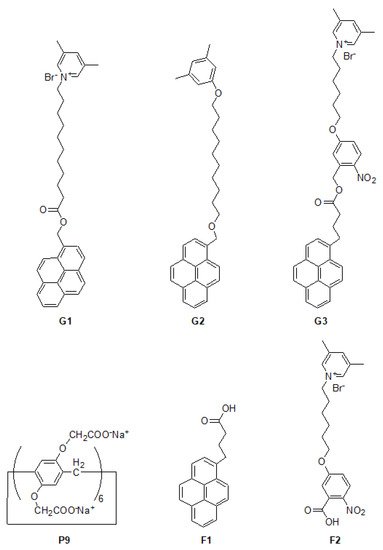
Water-soluble pillar[n]arene could include hydrophobic guest molecules
[25][26][71,72], producing pillar[n]arene-based self-assembled amphiphiles (PSAs)
[27][28][73,74] and resembling the performance of general surfactant-dispersing carbon nanotubes
[29][75]. For example
[30][76], water-soluble carboxylate-perfunctionalized
[31][77] pillar[6]arene
P9 (
Scheme 3) has the ability to recognize the hydrophobic pyrene
[32][78] derivative
G1 (
Scheme 3) in the stoichiometry of 1/1, and aggregate into vesicular architectures
[33][79] in an aqueous solution as confirmed by transmission electron microscopy (TEM)
[34][80]. Since the water solubility of
P9 changes with the change of pH value, the morphology of
P9 ⸧
G1-based self-assemblies could be also controlled. In addition
[30][76],
P9 could include another pyrene derivative
G2 (
Scheme 3) with the association constant (
Ka) of (8.04 ± 0.68) 10
4 M
−1. Additionally, the obtained amphiphilic inclusion could further disperse multi-walled carbon nanotubes well by sonication in aqueous solutions
as confirmed by TEM and scanning electron microscopy (SEM). The π–π stacking interactions
[35][81] between pyrene subunits and carbon nanotubes played a significant role in this process as confirmed by fluorescence spectroscopy
[36][82].
Scheme 3. Chemical structures of pillar[6]arene derivative P9, guests such as pyrene derivatives
G1–G3, as well as functional molecules
F1 and
F2 [30][37][76,83].
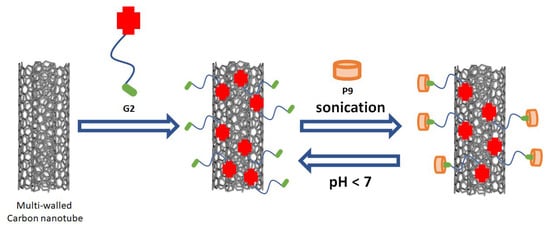
Figure 1. Illustration of dispersing multi-walled carbon nanotube by using amphiphilic host–guest inclusion
P9 ⸧
G2 in aqueous solution
[30][76].
Similarly
[37][83], another pyrene derivative
G3 (
Scheme 3 and Table 1) which is responsive to UV irradiation and degrades into
F1 and
F2 (
Scheme 3) was further employed as the hydrophobic guest to be included by
P9. Thus, the amphiphilic inclusion
P9 ⸧
G3 was obtained and exhibited the critical aggregation concentration (CAC)
[38][84] of 1.0 × 10
6 mol L
−1, which has the capacity of dispersing multi-walled carbon nanotubes in aqueous solutions as confirmed by TEM. Interestingly, the dispersion of carbon nanotubes could be controlled upon UV irradiation according to the formation/deformation of
G3.
3. Decoration of Carbon Nanotube by Using Functional Pillar[n]arene
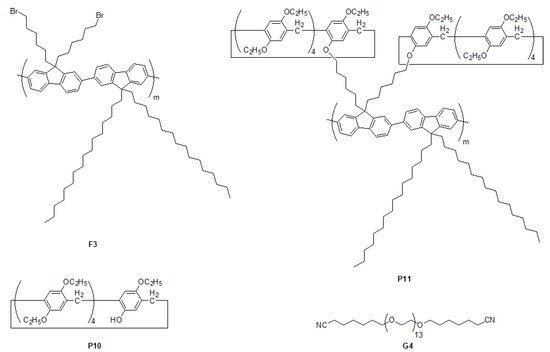
The mono-hydroxyl functionalized pillar[5]arene
P10 (
Scheme 4) coupled with the alkyl bromide-modified polyfluorene
F3 (
Scheme 4) via a classic nucleophilic substitution reaction led to the formation of pillar[5]arene-functionalized polymer
P11 (
Scheme 4 and Table 1)
[39][85]. Due to the possession of the pillar[5]arene cavity on the structural skeleton,
P11 could form a complex with a neutral poly(ethylene glycol) (PEG) derivative with two ending subunits—alkylnitrile
G4 (
Scheme 4) via donor–acceptor interactions. Particularly, this obtained host–guest inclusion
P11 ⸧
G4 not only has the capacity for properly exfoliating and dispersing single-walled carbon nanotubes in organic solvents (600 μg mL
−1)as confirmed by
1H NMR, Raman, UV–Vis–near-infrared (NIR) spectra, and thermogravimetric analysis (TGA), but also affords the preparation of pillar[5]arene-based polymer-carbon nanotube composite organogels
[40][41][42][88,89,90] in 1,2-dichlorobenzene (40
wt%) via non-covalent interactions
[39][85].
Scheme 4. Chemical structures of polymer
F3, pillar[5]arene P10, pillar[5]arene-based polymer
P11 and PEG derivative
G4 [39][85].
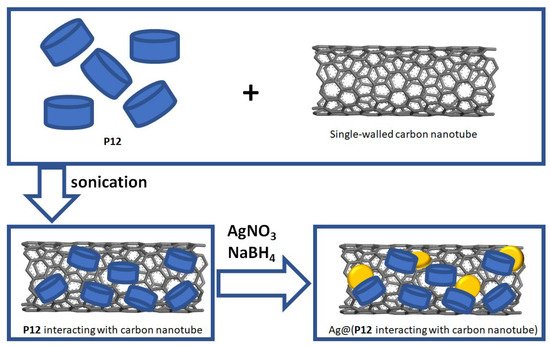
Besides the organic and polymeric composition, diverse inorganic materials were also employed for fabricating different functional, carbon nanotube-based, hybrid materials. For example
[43][86], the water-soluble phosphate-perfunctionalized pillar[6]arene
P12 (
Scheme 5 and Table 1) could decorate the surface of a single-walled carbon nanotube at room temperature via π–π stacking interactions between the carbon nanotube and benzene moieties of
P12 by sonicating in aqueous solutions, as confirmed by zeta potentials and Fourier transform infrared (FTIR) spectroscopy. Particularly, Ag nanoparticles could be further well dispersed on the surface of the carbon nanotube due to the coordination environment provided by the cavity of
P12. Thus, the obtained hybrid materials containing Ag nanoparticles, carbon nanotubes and
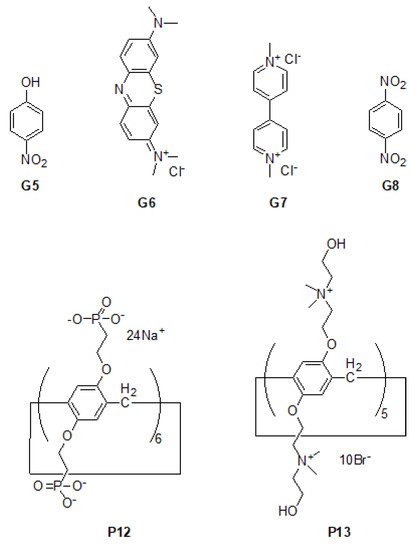
P12 exhibited strong catalytic activity towards a series of guest molecules such as 4-nitrophenol (
G5,
Scheme 5), methylene (
G6,
Scheme 5) and paraquat (
G7,
Scheme 5), paving the way for efficient electrochemical sensing of highly toxic herbicides.
Scheme 5. Chemical structures of pillar[6]arene P12 and pillar[5]arene
P13, as well as guests such as
G5–
G8 [43][44][86,87].
Figure 4. Illustration of the fabrication of hybrid materials Ag@(
P12 non-covalently interacting with carbon nanotubes)
[43][86].
Except for loading Ag nanoparticles, Au nanoparticles
[45][46][47][91,92,93] could also be introduced into carbon nanotube-based hybrid materials via the assistance of pillar[n]arene. For example, water-soluble hydroxyl pillar[5]arene
P13 (
Scheme 5) was also used for dispersing single-walled carbon nanotubes in aqueous solutions via non-covalent interactions, and further assisted in promoting the formation of Au nanoparticles on the surface of carbon nanotubes, leading to the formation of hybrid materials Au@(P13 interacting with carbon nanotubes)
[44][87]. It has been revealed that such hybrid materials had reasonable performances in catalyzing an ethanol oxidation reaction (EOR), as well as sensing
p-dinitrobenzene (
G8,
Scheme 5) because of pillar[5]arene cavities.