Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Shahzad Rafiq.
Houttuynia cordata Thunb (H. cordata) is a rhizomatous, herbaceous, and perennial plant widely distributed in Asia. It has multiple chemical constituents, such as alkaloids, essential oils, phenolic acids, and flavonoids used against various health problems. The essential oils and flavonoids are the main components of H. cordata that play an essential role in disease treatment and traditional health care. Moreover, the leaves and stems of H. cordata have a long medicinal history in China. In addition, H. cordata is used against several health issues, such as cold, cough, fever, pneumonia, mumps, and tumors, due to its anti-inflammatory, anti-bacterial, anti-viral, anti-oxidant, and anti-tumor effects.
- Houttuynia cordata Thunb
- anti-inflammatory
- anti-viral
- anti-bacterial
- immunomodulatory
- anti-tumor
1. Chemical Components of H. cordata
H. cordata has a variety of chemical constituents with characteristic medicinal properties and belonging to different chemical groups, such as alkaloids, essential oils, and flavonoids [21][1]. The alkaloids consist of aristolactam A, 3,4-dimethoxy-N-methyl aristolactam, lysicamine, noraritolodione, norcepharadione B, 3,5-didecanoyl-pyridine, 7-chloro-6-demethyl-cepharadione B, cis-N-(4-Hydroxystyryl) benzamide and trans-N-(4-Hydroxystyryl) benzamide, 2-nonyl-5-decanoylpyridine, 3,5-Didecanoyl-4-nonyl-1,4-dihydropyridine, cepharadione B, splendidine, piperolactam A, 3-decanoyl-4-nonyl-5-dodecanoyl-1,4-dihydropyridine, aristolactam B, 3,5-didodecanoyl-4-nonyl-1,4-dihydropyridine, 7-chloro-6-demethylcepharadione B, 3-nonylpyrazole, N-methyl-5-methoxy-pyrrolidin-2-one, phenanthrolactam compounds [22,23][2][3]. The flavonoid compounds included quercetin, rutin, hyperin, afzelin, quercitrin, isoquercitrin, kaempferol, quercetin hexoside, avicularin, apigenin, isorhamnetin, phloridzin, quercetin-3-O-β-D-galactoside-7-O-β-D-glucoside, and polyphenols include chlorogenic acid, vanillic acid, protocatechuic acid, catechin, p-hydroxy-benzoic acid methyl ester, chlorogenic acid methyl ester, cryptochlorogenic acid, neochlorogenic acid, procyanidin B, quinic acid, caffeic acid, cis-methyl ferulate, trans-methyl ferulate, methyl vanillate, vanillin, houttuynamide A, and houttuynoside A [24,25,26][4][5][6]. The main components of the essential oil are houttuynin, decanal, trans-caryophyllene, decanoic acid, camphene, β-pinene, lauraldehyde, α-pinene, limonene, nonanol and linalool bornyl acetate, methyl n-nonyl ketones, beta myrcene, monoterpene, 4-terpineol, caryophyllene oxide, phenylpropene derivatives, sesquiterpenes, and oxidized diterpenes [27][7].
H. cordata has many components, and alkaloids are abundant ingredients [28][8]. Essential oil and flavonoids are known to be major components that exert pharmacological activities. Moreover, decanoyl acetaldehyde in H. cordata has a fishy smell called Yu-Xing-Cao, and is a herb in traditional Chinese medicine [22][2]. It has anti-bacterial effects and is easily transformed to 2-undecanone at higher temperatures [29][9]. Steam distillation extracts of H. cordata contain some important oils, which consist of oxidized diterpenes, monoterpenes, sesquiterpenes, and oxidized diterpenes [30][10]. Others present in H. cordata include bornyl acetate (0.4–8.61%), ketones (2.10–40.36%), and β-myrcene (2.58–18.47%) [27][7]. Eleven ingredients have been isolated from leaves of H. cordata, and seven have been isolated from the roots and are not present in the leaves [7][11]. It is also reported that H. cordata from various areas has various anti-bacterial effects [31][12]. Flavonoids in H. cordata, such as quercetin, quercitrin, and hyperoside, are mostly combined with rhamnose in glycosides [26][6]. A new form of hyperoside and houttuynia has been isolated from flavonoid compounds in H. cordata [32][13]. Other new components are houttuynamide A and houttuynoside A [25][5]. Caffeic acid derivatives, quinic acid derivatives, chlorogenic acid, neochlorogenic acid, and cryptochlorogenic acid are considered the essential components of H. cordata [24][4]. Alkaloids such as phenanthrolactam, piperolactam, and aristololactam are key components of H. cordata and play an essential role in pharmacological effects [23][3].
2. Anti-Inflammatory Effects and Immunomodulatory Activity of H. cordata
Inflammation is a protective response of the body against offending agents such as viruses, bacteria, toxic chemicals, and damaged cells. There are two forms of inflammation. One is acute inflammation and the other is chronic inflammation [44][14]. All extracts have good anti-inflammatory activity. It has been found that the anti-inflammatory effects of water extract are better than those of ethnolic extract. Fresh H. cordata extracts showed better pharmacological activity than dry H. cordata extract [9][15]. Cells involved in body immunity are basophils, eosinophils, mast cells, lymphocytes, and neutrophils. They play vital immune functions. Antibodies, tumor necrosis factors, interferons, and interleukin also play an essential role in body immunity. Abnormal immune functions result in microcirculation, anaphylactic shock, and central nervous system disorders [46][16]. The polyphenols present in H. cordata show anti-allergic effects. Due to H. cordata extracts, decreased activity of iIgE and FcεRI expression on basophilic cells was observed. Moreover, mRNA activity associated with γ-chains and FcεRI was decreased, and histamine secretions were inhibited [47][17]. It was observed that H. cordata extract decreased cutaneous anaphylaxis in vivo in mice. The level of cAMP present in mast cells is enhanced by using H. cordata, which shows that H. cordata can speed up the recovery from allergic reactions. HCP-2 polysaccharides extracted from H. cordata regulate the expression of T cells with a dosage of 0.1–25 μg/mL. It increases tumor necrosis factor-α (TNF-α), immune molecule interleukin-1β, and macrophage inhibitory protein-1α and -1β, which increases body immunity. It has been recorded that H. cordata reduces Th2-mediated immune disorders. Ethanol extract of H. cordata decreases the migration of T cells, which ultimately strengthens immune response [10][18]. H. cordata extract helps in the regulation of immune mediators. After 18 h of treating vaginal epithelial cells with H. cordata, levels of leukocyte protease inhibitor mRNA and human β-defensin 2 were increased. Moreover, an increase in IL-2 and IL-6 and a decrease in CCL5 were observed. These findings show an increase in the overall immune response. H. cordata has the same effects on oral immune mediators by expressing human β-defensin 2, IL-8, CCL20, and secretory leukocyte protease inhibitor. In this manner, H. cordata regulates oral immune response [48][19].3. Effect of H. cordata on Different Organs
Lung inflammation is one of the most important signs during lung infection. H. cordata has an anti-inflammatory property that plays a significant role in treating lung inflammation. Quercetin obtained from HC, when administered orally at a dose rate of 100 mg/mL in an LPS-induced model, significantly decreased the production of NO and inflammatory mediators such as cytokines [35][20]. Researchers compared the effect of different dosage levels of flavonoid glucoside extract of H. cordata at 50, 100, and 200 mg/kg compared with ribavirin 100 mg/kg with the use of acute injury of lung tissues by the H1N1 virus. At 14 days, they found a lower lung index and less weight loss [37][21]. The oxidative lung damage caused pulmonary fibrosis. In rats, when pulmonary fibrosis was induced by bleomycin, H. cordata aqueous extracts showed a better and stronger anti-oxidant property than vitamin E by decreasing concentration of hydroxyproline, superoxide dismutase, and malondialdehyde [60][22]. The intestinal barrier is a structure that allows uptake of essential nutrients, while restricting pathogenic molecules and bacteria. The microflora present in the intestine also play a vital role in protecting the intestine [61][23]. The constituents of H. cordata are polysaccharides, and sodium houttuyfonate is instrumental in reducing or regulating the production of mucus from the goblet cells and wart formation of Secretory IgA (antibodies in the secretions and excretions). Moreover, the protein ZO-1, which forms a gap junction between the intestinal cells, is up-regulated or enhanced to compact intestinal, mechanical, and immunological barriers [62][24]. Intestinal inflammation induced by Salmonella typhimurium is dampened by sodium houttuyfonate in the form of up-regulating tight junction proteins between the mucosal cells of the intestine and the signaling pathway that leads to interleukin production [63][25]. These studies showed that H. cordata has a therapeutic effect on the GIT system. H. cordata protects the intestine various barriers (mucosal barriers, chemical, mechanical, biological, and immune barriers) are present in the intestine. Moreover, intestinal flora also play an essential role in protecting the intestines. Recently, it has been found that sodium houttuyfonate and polysaccharides extracted from H. cordata decrease the expression of sIgA, intestinal goblet cells, and tight junction protein present in the intestines. Sodium houttuyfonate is also involved in reducing inflammation caused by Salmonella typhimurium. Regulation of bacteria, such as Vibrio and Bacillus, also includes polysaccharides made up of galactose, glucose, rhamnose, and arabinose at a 40 mg/kg dosage. These findings show that sodium houttuyfonate and polysaccharides of H. cordata have protective activity by inhibiting NF-κB and regulating intestinal flora in the intestines [69][26]. Many natural extracts from plants have effective results in preventing and treating various liver ailments. For instance, the chemical components of extracts such as terpenoids, glycosides, coumarins, and alkaloids prevent liver fibrosis. Cholestasis is a common problem inhibited by compounds such as quercetin and rutin. Liver cells are very sensitive to oxidative stress. H. cordata ethyl acetate extract reduces liver damage through its anti-oxidant activity. Ethyl acetate of H. cordata extract at a dose rate of 1000 mg/kg causes an increase in superoxide dismutase, glutathione, and catalase enzymes, and a decrease in malondialdehyde and serum transaminase resulting in liver protection. The mixture of ethanol and water extract of H. cordata at a dosage of 300 mg/kg/day for seven days reduces oxidative factors in the liver [38][27]. Oxidative damage, inflammation, and infections caused by various pathogenic organisms are the major factors involved in kidney problems. It was observed that 1 to 2% of H. cordata water extract reduced the level of serum creatinine and blood urea nitrogen and oxidative factors in the kidney. Moreover, 2% extract of H. cordata causes inhibition of membrane-anchored receptor made up of end products (RAGE) and glycation, which activate mitogen-activated protein kinase. They induce intracellular reactive oxygen species generation and are involved in renal protection. Sodium houttuyfonate present in H. cordata causes a decrease in expression of MCP-1 and nuclear NF-κB at a dosage of 60–120 mg/kg. It protects against renal glomerulonephritis and kidney oxidative stress [66][28]. Anti-oxidants such as catechin and procyanidin B present in H. cordata intervene in remodeling of the heart. The use of 2% H. cordata water extract was found to down-regulate cardiac activity related to oxygen, interleukin-6, inflammatory factors, and protein carbonyl. Moreover, 1 and 2% of H. cordata water extract block expression of NF-κB p65, p47phox, and p-p38 in the mouse heart. Sodium houttuyfonate shows activity against myocardial hypertrophy induced by isoprenaline with a dosage of 90 and 180 mg/kg up to 1 week. Cyclic adenosine, left ventricular weight index, heart weight index, and angiotensin 2 were also decreased using sodium houttuyfonate. Moreover, the cross-sectional area of cardiomyocytes and expression of hydroxyproline was also reduced [70][29]. Sodium houttuyfonate with 50 and 100 mg/kg dosages causes down-regulation of renin-angiotensin-aldosterone, which involves controlling blood pressure. Sodium houttuyfonate is also associated with NF-κB pathway inhibition and adenosine monophosphate-activated protein kinase at the same dosage. It also reduces heart fibrosis and myocardial inflammatory factors. H. cordata reduces the release of inflammatory mediators of the heart and oxidative damage to the heart. Sodium houttuyfonate present in H. cordata also affects the sympathetic nervous system and the renin-angiotensin system by reversing hypertrophy and remodeling of myocardium. Sodium houttuyfonate treatment elevated the activation of adenosine monophosphate-activated protein kinase (AMPK) on post-infarct heart and post-hypoxia H9C2. AMPK did not suppress NF-κB signaling directly; its inhibition of NF-κB was realized indirectly via its downstream mediators, e.g., Sirtuin-1 (SIRT1), Forkhead box O (F oxO) family, and peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α). Therefore, AMPK activation and suppression of NF-κB and inflammatory cytokines was critically involved in the anti-remodeling effect of SH post-myocardial infarction. [71][30].4. Anti-Tumor Activity of H. cordata
In a study of mice with lung tumors induced by benzo-pyrene, it was found that the active components of H. cordata, such as 2-undecanone, had an anti-tumor effect that may be due to Nrf2-HO-1/NQO-1 pathway activation, which reduces inflammation of lung cells and damage of DNA. In addition, no signs of systemic toxicity were recorded [73][31]. Moreover, the polysaccharides present in H. cordata exhibited anti-tumor potential. The polysaccharide HCA4S1 inhibited proliferation of tumor cells by cancer cell cycle/A549 lung tumor arrest and apoptosis. Similarly, after HCA4S1 treatment, the activities of cyclin B1 and cleaved caspase3 in cells dramatically reduced [74][32]. The extracts of H. cordata with the concentration of 0 to 80 µg/mL caused a decrease in lipid accumulation in HepG2 cells when HepG2 cells were merged with a high level of glucose [75][33]. The ethanolic extract of H. cordata had anti-cancer effects against the colon cancer cell line HT-29. Cancer cell apoptosis was induced when treated with 450 µg/mL extract, which also resulted in lower mitochondrial membrane potential and increased reactive oxygen [76][34]. H. cordata also has activity against breast cancer. The development and progression of tumors are significantly influenced by the overexpression of the HER2/neu (receptors on breast cells) receptor. With an IC50 of 5.52 µg/mL, Houttuyninum suppressed HER2 phosphorylation in a dose-dependent manner in MDA-MB-453 cells without altering the expression of the HER2/neu protein. Additionally, HER2/neu-mediated signal transduction pathway downstream molecules ERK1/2 and AKT were blocked by houttuyninum from becoming activated [77][35]. At the concentration of 100 to 500 µg/mL, the ethanolic extract of H. cordata promotes apoptosis in breast cancer cells [78][36]. These studies showed that H. cordata has anti-tumor activity (Figure 21).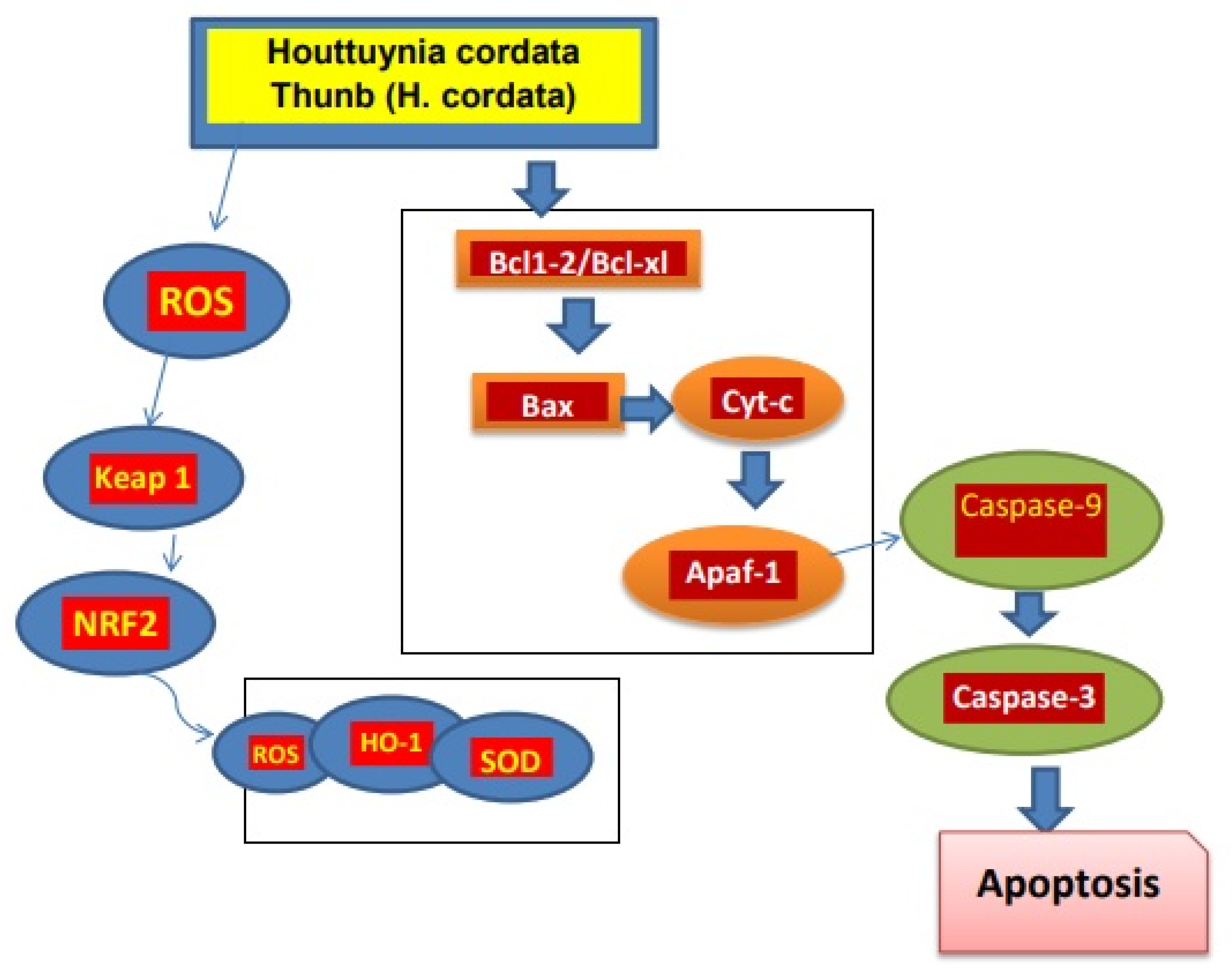
Figure 21.
Mechanism of
H. cordata
acts as an anti-tumor agent.
H. cordata suppresses cancerous cells by blocking NRF2/Bcl1, Bcl-xl signaling pathway and leading to apoptosis of cancerous cells [7].
suppresses cancerous cells by blocking NRF2/Bcl1, Bcl-xl signaling pathway and leading to apoptosis of cancerous cells [11].
5. Effect of H. cordata on Viruses
The research on plants for the treatment of AIDS has made significant progress over the last ten years. Many plants and their products, such as H. cordata, have been found to have anti-HIV properties [79][37]. In vitro, the steam distillate and three main components from H. cordata manifested virucidal effects against HSIV-1 and influenza. The pretreatment with the distillate for 2 and 6 h, respectively, resulted in the inactivation of 20% and 40% of HIV-1 at two-fold dilution [80][38]. H. cordata aqueous extract has immunomodulatory and anti-SARS properties. H. cordata causes an increase in the spread of mouse splenic lymphocytes. According to flow cytometry, H. cordata enhanced the fraction of CD4+ and CD8+ T cells. Furthermore, it increased the interleukin 2 and interleukin 10 releases by mouse splenic cells. Regarding anti-viral activity, H. cordata inhibited the 3C-like protease of the SARSCOV and RNA-dependent RNA polymerase [81][39]6. Anti-Bacterial Effect of H. cordata
Staphylococcus aureus is a food-borne, gram-positive bacterium that can cause infection of the skin, nasal cavity, GIT, and other human parts. MRSA was synergistically inhibited by sodium houttuyfonate and EDTA-Na2. Mice infected with MRSA were given sodium houttuyfonate combined with EDTA-Na2. After 28 days of MRSA infection, the survival rate of mice with sodium houttuyfonate treatment combined with EDTA-Na2 was 75 percent. It was significantly higher than the 43.75 and 50 percent survival rates of mice treated independently with EDTA-Na2 and sodium houttuyfonate, respectively [93][40]. At doses of 500 and 50 mg/mL, the aqueous H. cordata extracts demonstrated anti-bacterial effects against isolates of MDR E. coli, with the maximum and minimum zone diameters of inhibition of 29 and 13 mm, respectively. These findings suggest that H. cordata water extract (HCWE) has anti-microbial action against MDR E. coli in vitro [20][41] Pseudomonas aeruginosa is a Gram-negative bacterium that infects deep wounds of the body and causes systemic illness. It was reported that sodium houttuyfonate had anti-bacterial activity against pseudomonas aeruginosa. The biosynthesis of alginate, a key ingredient for PA biofilm development, was suppressed, and is linked to sodium houttuyfonate’s down-regulation of algD and algR genes. Simultaneously, electron microscope observations showed that the bacteria’s shape changed after treatment, and the amount of alginate present in bacterial biofilm decreased [94][42]. Water extracts of H. cordata were found to have anti-bacterial effects against salmonellosis. It was observed that, after 8 h, the anti-bacterial activity of H. cordata increased with concentrations of 25 to 100 mg/mL. Bacterial absorption and morphologic alterations of body cells showed that there was no significant difference in the replication of bacteria. H. cordata showed a decrease in the pathogenicity of salmonella bacterium. The death rate of bacteria at the 7th day in the untreated group was 100%, and with a dose rate of 25, 50, and 100 μg/mL of H. cordata, the extract group lived up to 11, 17, and 23 days, respectively. It was recorded that H. cordata water extract is effective and safe in treating salmonella bacteria infections and various replicating pathogens [95][43].7. Toxicity of H. cordata
H. cordata is an edible plant. Therefore, the toxic level of this plant is mostly ignored. However, it has been reported in some studies that aristolactams and aristolochic acid present in H. cordata can cause cancer [60][22]. Increased levels of aristolochic acid in liver cells also cause toxicity of proximal tubule epithelial cells present in the kidney. Aristolochic acid is also toxic in vivo because of its mutagenicity. A study revealed that 95% ethanol extracts from H. cordata show potential toxicity to zebrafish. A single dose of 2000 mg/kg of H. cordata with oral use had no harmful effects in mice during 14 days of treatment. However, oral administration of H. cordata with a dosage of 500–1000 mg/kg/day for 28 consecutive days led to some rats’ death. The histopathological examination showed inflammatory cell infiltration and vacuum degeneration of liver tissue, and focal necrosis of epithelial cells in kidneys. However, H. cordata has shown a very weak potential for toxicity. There is no evidence that H. cordata causes long-term toxicity. Nonetheless, H. cordata leaves and rhizomes are consumed in South China as an agricultural vegetable [96][44]. Research was conducted in 2018 to evaluate the toxicological effect of fermented Houttuynia cordata juice (FHJ) in a rodent model. FHJ was prepared by fermentation of Houttuynia cordata for 30 days and its active ingredients were evaluated. Due to lactic acid production, it has a lower pH of 3.63. Rats were fed with FHJ for 60 days and toxicological effects were evaluated using different biochemical, hematological, and histological tests. These revealed that there was no significant biochemical, histological, or hematological change in rats when compared with the control group. Therefore, it was postulated that FHJ did not have any toxicological effect in rats; hence, experiments should be conducted with humans in safety and toxicological studies. [97][45].References
- Bauer, R.; Pröbstle, A.; Lotter, H.; Wagner-Redecker, W.; Matthiesen, U. Cyclooxygenase Inhibitory Constituents from Houttuynia Cordata. Phytomedicine 1996, 2, 305–308.
- Ma, Q.; Wei, R.; Wang, Z.; Liu, W.; Sang, Z.; Li, Y.; Huang, H. Bioactive Alkaloids from the Aerial Parts of Houttuynia Cordata. J. Ethnopharmacol. 2017, 195, 166–172.
- Probstle, A.; Bauer, R. Aristolactams and a 4,5-Dioxoaporphine Derivative from Houttuynia Cordata. Planta Med. 1992, 58, 568–569.
- Nuengchamnong, N.; Krittasilp, K.; Ingkaninan, K. Rapid Screening and Identification of Antioxidants in Aqueous Extracts of Houttuynia Cordata Using LC-ESI-MS Coupled with DPPH Assay. Food Chem. 2009, 117, 750–756.
- Chou, S.C.; Su, C.R.; Ku, Y.C.; Wu, T.S. The Constituents and Their Bioactivities of Houttuynia Cordata. Chem. Pharm. Bull. 2009, 57, 1227–1230.
- Xu, X.; Ye, H.; Wang, W.; Yu, L.; Chen, G. Determination of Flavonoids in Houttuynia cordata Thunb. and Saururus chinensis (Lour.) Bail. by Capillary Electrophoresis with Electrochemical Detection. Talanta 2006, 68, 759–764.
- Lu, H.; Wu, X.; Liang, Y.; Zhang, J. Variation in Chemical Composition and Antibacterial Activities of Essential Oils from Two Species of Houttuynia THUNB. Chem. Pharm. Bull. 2006, 54, 936–940.
- Ahn, J.; Chae, H.S.; Chin, Y.W.; Kim, J. Alkaloids from Aerial Parts of Houttuynia Cordata and Their Anti-Inflammatory Activity. Bioorganic Med. Chem. Lett. 2017, 27, 2807–2811.
- Chen, J.; Wang, W.; Shi, C.; Fang, J. A Comparative Study of Sodium Houttuyfonate and 2-Undecanone for Their in Vitro and in Vivo Anti-Inflammatory Activities and Stabilities. Int. J. Mol. Sci. 2014, 15, 22978–22994.
- Řebíčková, K.; Bajer, T.; Šilha, D.; Houdková, M.; Ventura, K.; Bajerová, P. Chemical Composition and Determination of the Antibacterial Activity of Essential Oils in Liquid and Vapor Phases Extracted from Two Different Southeast Asian Herbs-Houttuynia cordata (Saururaceae) and Persicaria odorata (Polygonaceae). Molecules 2020, 25, 2432.
- Wu, Z.; Deng, X.; Hu, Q.; Xiao, X.; Jiang, J.; Ma, X.; Wu, M. Houttuynia Cordata Thunb: An Ethnopharmacological Review. Front. Pharmacol. 2021, 12, 714694.
- Verma, R.S.; Joshi, N.; Padalia, R.C.; Singh, V.R.; Goswami, P.; Kumar, A.; Iqbal, H.; Verma, R.K.; Chanda, D.; Chauhan, A.; et al. Chemical Composition and Allelopathic, Antibacterial, Antifungal, and Antiacetylcholinesterase Activity of Fish-Mint (Houttuynia cordata Thunb.) from India. Chem. Biodivers. 2017, 14, 10.
- Chen, S.D.; Gao, H.; Zhu, Q.C.; Wang, Y.Q.; Li, T.; Mu, Z.Q.; Wu, H.L.; Peng, T.; Yao, X.S. Houttuynoids A-E, Anti-Herpes Simplex Virus Active Flavonoids with Novel Skeletons from Houttuynia Cordata. Org. Lett. 2012, 14, 1772–1775.
- Kumar, R.; Clermont, G.; Vodovotz, Y.; Chow, C.C. The Dynamics of Acute Inflammation. J. Theor. Biol. 2004, 230, 145–155.
- Fu, J.; Dai, L.; Lin, Z.; Lu, H. Houttuynia cordata Thunb: A Review of Phytochemistry and Pharmacology and Quality Control. Chin. Med. 2013, 4, 101–123.
- Marshall, G.D. Challenges in Allergy Immunology Practice: Solutions Needed for Persistent Patient Problems. Ann. Allergy Asthma Immunol. 2018, 121, 647–648.
- Shim, S.Y.; Seo, Y.K.; Park, J.R. Down-Regulation of FcεRI Expression by Houttuynia Cordata Thunb Extract in Human Basophilic KU812F Cells. J. Med. Food 2009, 12, 383–388.
- Li, G.Z.; Chai, O.H.; Lee, M.S.; Han, E.H.; Kim, H.T.; Song, C.H. Inhibitory Effects of Houttuynia Cordata Water Extracts on Anaphylactic Reaction and Mast Cell Activation. Biol. Pharm. Bull. 2005, 28, 1864–1868.
- Satthakarn, S.; Chung, W.; Promsong, A.; Nittayananta, W. Houttuynia Cordata Modulates Oral Innate Immune Mediators: Potential Role of Herbal Plant on Oral Health. Oral Dis. 2015, 21, 512–518.
- Lee, J.H.; Ahn, J.; Kim, J.W.; Lee, S.G.; Kim, H.P. Flavonoids from the Aerial Parts of Houttuynia Cordata Attenuate Lung Inflammation in Mice. Arch. Pharm. Res. 2015, 38, 1304–1311.
- Ling, L.-J.; Lu, Y.; Zhang, Y.-Y.; Zhu, H.-Y.; Tu, P.; Li, H.; Chen, D.-F. Flavonoids from Houttuynia Cordata Attenuate H1N1-Induced Acute Lung Injury in Mice via Inhibition of Influenza Virus and Toll-like Receptor Signalling. Phytomedicine 2020, 67, 153150.
- Ng, A.W.T.; Poon, S.L.; Huang, M.N.; Lim, J.Q.; Boot, A.; Yu, W.; Suzuki, Y.; Thangaraju, S.; Ng, C.C.Y.; Tan, P.; et al. Aristolochic Acids and Their Derivatives Are Widely Implicated in Liver Cancers in Taiwan and throughout Asia. Sci. Transl. Med. 2017, 9, eaan6446.
- Lu, L.; Li, W.; Chen, L.; Su, Q.; Wang, Y.; Guo, Z.; Lu, Y.; Liu, B.; Qin, S. Radiation-Induced Intestinal Damage: Latest Molecular and Clinical Developments. Futur. Oncol. 2019, 15, 4105–4118.
- Zhu, H.; Lu, X.; Ling, L.; Li, H.; Ou, Y.; Shi, X.; Lu, Y.; Zhang, Y.; Chen, D. Houttuynia Cordata Polysaccharides Ameliorate Pneumonia Severity and Intestinal Injury in Mice with Influenza Virus Infection. J. Ethnopharmacol. 2018, 218, 90–99.
- Zhang, L.; Lv, H.; Li, Y.; Dong, N.; Bi, C.; Shan, A.; Wu, Z.; Shi, B. Sodium Houttuyfonate Enhances the Intestinal Barrier and Attenuates Inflammation Induced by Salmonella Typhimurium through the NF-ΚB Pathway in Mice. Int. Immunopharmacol. 2020, 89, 107058.
- Chen, M.Y.; Li, H.; Lu, X.X.; Ling, L.J.; Weng, H.B.; Sun, W.; Chen, D.F.; Zhang, Y.Y. Houttuynia Cordata Polysaccharide Alleviated Intestinal Injury and Modulated Intestinal Microbiota in H1N1 Virus Infected Mice. Chin. J. Nat. Med. 2019, 17, 187–197.
- Tian, L.; Shi, X.; Yu, L.; Zhu, J.; Ma, R.; Yang, X. Chemical Composition and Hepatoprotective Effects of Polyphenol-Rich Extract from Houttuynia Cordata Tea. J. Agric. Food Chem. 2012, 60, 4641–4648.
- Pan, P.; Wang, Y.J.; Han, L.; Liu, X.; Zhao, M.; Yuan, Y.F. Effects of Sodium Houttuyfonate on Expression of NF-KappaB and MCP-1 in Membranous Glomerulonephritis. J. Ethnopharmacol. 2010, 131, 203–209.
- Gao, J.P.; Wang, Y.; Lü, J.; Gu, W.L.; Chen, C.X. Effect of Sodium Houttuyfonate on Myocardial Hypertrophy in Mice and Rats. J. Pharm. Pharmacol. 2009, 61, 677–683.
- Tu, X.; Deng, Y.P.; Chen, J.; Hu, Q.; He, C.S.; Jordan, J.B.; Zhong, S. Screening Study on the Anti-Angiogenic Effects of Traditional Chinese Medicine–Part I: Heat-Clearing and Detoxicating TCM. J. Ethnopharmacol. 2016, 194, 280–287.
- Lou, Y.; Guo, Z.; Zhu, Y.; Kong, M.; Zhang, R.; Lu, L.; Wu, F.; Liu, Z.; Wu, J. Houttuynia Cordata Thunb. and Its Bioactive Compound 2-Undecanone Significantly Suppress Benzo(a)Pyrene-Induced Lung Tumorigenesis by Activating the Nrf2-HO-1/NQO-1 Signaling Pathway. J. Exp. Clin. Cancer Res. 2019, 38, 242.
- Han, K.; Jin, C.; Chen, H.; Wang, P.; Yu, M.; Ding, K. Structural Characterization and Anti-A549 Lung Cancer Cells Bioactivity of a Polysaccharide from Houttuynia Cordata. Int. J. Biol. Macromol. 2018, 120, 288–296.
- Kang, H.; Koppula, S. Houttuynia Cordata Attenuates Lipid Accumulation via Activation of AMP-Activated Protein Kinase Signaling Pathway in HepG2 Cells. Am. J. Chin. Med. 2014, 42, 651–664.
- Routhier, A.; Astuccio, M.; Lahey, D.; Monfredo, N.; Johnson, A.; Callahan, W.; Partington, A.; Fellows, K.; Ouellette, L.; Zhidro, S.; et al. Pharmacological Inhibition of Rho-Kinase Signaling with Y-27632 Blocks Melanoma Tumor Growth. Oncol. Rep. 2010, 23, 861–867.
- Zhou, N.N.; Tang, J.; Chen, W.D.; Feng, G.K.; Xie, B.F.; Liu, Z.C.; Yang, D.; Zhu, X.F. Houttuyninum, an Active Constituent of Chinese Herbal Medicine, Inhibits Phosphorylation of HER2/Neu Receptor Tyrosine Kinase and the Tumor Growth of HER2/Neu-Overexpressing Cancer Cells. Life Sci. 2012, 90, 770–775.
- Subhawa, S.; Chewonarin, T.; Banjerdpongchai, R. The Effects of Houttuynia Cordata Thunb and Piper Ribesioideswall Extracts on Breast Carcinoma Cell Proliferation, Migration, Invasion and Apoptosis. Molecules 2020, 25, 1196.
- Sandip, B.J.J.M.A.P.S. Medicinal Plants with Anti-HIV Potential. Clin. Exp. Pharmacol. Physiol. 2003, 25, 427–440.
- Hayashi, K.; Kamiya, M.; Hayashi, T. Virucidal Effects of the Steam Distillate from Houttuynia Cordata and Its Components on HSV-1, Influenza Virus, and HIV. Planta Med. 1995, 61, 237–241.
- Lau, K.M.; Lee, K.M.; Koon, C.M.; Cheung, C.S.F.; Lau, C.P.; Ho, H.M.; Lee, M.Y.H.; Au, S.W.N.; Cheng, C.H.K.; Lau, C.B.S.; et al. Immunomodulatory and Anti-SARS Activities of Houttuynia Cordata. J. Ethnopharmacol. 2008, 118, 79–85.
- Huang, W.; Duan, Q.; Li, F.; Shao, J.; Cheng, H.; Wu, D. Sodium Houttuyfonate and EDTA-Na2 in Combination Effectively Inhibits Pseudomonas Aeruginosa, Staphylococcus Aureus and Candida Albicans in Vitro and in Vivo. Bioorganic Med. Chem. Lett. 2015, 25, 142–147.
- Li, J.; Rehman, M.U.; Zhang, H.; Iqbal, M.K.; Mehmood, K.; Huang, S.; Nabi, F.; Sciences, A. Antibacterial Effect of the Water Extract of Houttuynia Cordata Water Extract Against Multi-Drug Resistant. Southeast Asian J. Trop. Med. Public Health 2017, 48, 1260–1266.
- Wu, D.Q.; Cheng, H.; Duan, Q.; Huang, W. Sodium Houttuyfonate Inhibits Biofilm Formation and Alginate Biosynthesis-Associated Gene Expression in a Clinical Strain of Pseudomonas Aeruginosa in Vitro. Exp. Ther. Med. 2015, 10, 753–758.
- Kim, G.S.; Kim, D.H.; Ju, L.J.; Lee, J.J.; Han, D.Y.; Lee, W.M.; Jung, W.C.; Min, W.G.; Won, C.G.; Rhee, M.H.; et al. Biological and Antibacterial Activities of the Natural Herb Houttuynia Cordata Water Extract against the Intracellular Bacterial Pathogen Salmonella within the RAW 264.7 Macrophage. Biol. Pharm. Bull. 2008, 31, 2012–2017.
- Chen, H.; Sha, X.; Luo, Y.; Chen, J.; Li, X.; Wang, J.; Cao, G.; Peng, X. Acute and Subacute Toxicity Evaluation of Houttuynia Cordata Ethanol Extract and Plasma Metabolic Profiling Analysis in Both Male and Female Rats. J. Appl. Toxicol. 2021, 41, 2068–2082.
- Chaiyasut, C.; Sivamaruthi, B.S.; Duangjitcharoen, Y.; Kesika, P.; Sirilun, S.; Chaiyasut, K.; Peerajan, S. Assessment of Subchronic Toxicity of Fermented Houttuynia Cordata Thunb. Using Rodent Model System. Asian J. Pharm. Clin. Res. 2018, 11, 307–311.
More
