You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Thangavel Chellappagounder and Version 3 by Catherine Yang.
Prostate cancer (PCa) is the second-leading cause of cancer-related deaths in men. PCa cells require androgen receptor (AR) signaling for their growth and survival. Androgen deprivation therapy (ADT) is the preferred treatment for patients with locally advanced and metastatic PCa disease. Bone-metastasis-induced early changes in the bone that proceed the osteoblastic response in the bone matrix are monitored and detected via modern magnetic resonance and PET/CT imaging technologies.
- prostate cancer
- androgen receptor
- castration-resistant prostate cancer
1. Bone Metastatic Castration-Resistant Prostate Cancer
The bone is one of the most-common metastatic sites for many solid tumors, including breast and prostate cancer. Bone metastases are common in men with mCRPC occurring in 30% of patients within 2 years of castration-resistant PCa and in >90% of patients over the course of the disease [1][31]. Patients with mCRPC-induced bone metastasis develop symptoms associated with skeletal-related events (SREs). The extent of bone involvement in mCRPC has been found to be associated with patient survival; the 5-year survival rate for these patients is 47% [2][3][4][32,33,34]. Previous studies have shown that both osteolytic, pro-osteoclastogenic factors, and osteoblastic components contribute to prostate cancer bone metastases [5][35]. The detection of bone metastases starts with Tc99m methylene diphosphate (MDP) bone/skeletal scintigraphy (SS), supported by plain film correlation, followed by magnetic resonance imaging (MRI), computerized tomography (CT), and position emission tomography (PET) to identify the early changes in the bone marrow that proceed the osteoblastic response in the bone matrix [6][36]. In addition, whole-body imaging modality is a bone scan currently being used to detect osseous metastases (a symptomatic bone metastases) induced by advanced prostate, lung and breast cancers [7][37].
2. Pathogenesis of Prostate Cancer Bone Metastasis and Its Deleterious Impacts on Vital Organs
PCa is a largely hormone-driven cancer, so androgen deprivation therapy (ADT) is increasingly used for the treatment of hormone-resistant PCa. However, ADT is possibly associated with numerous side effects, including an increased therapy-related sarcopenic obesity, mental depression/health, weaker lung and heart muscles, bone weakness, bone fracture, cardiovascular mortality, and altered expressions of hormone-dependent drug-metabolizing enzymes (human cytochrome p450). The deregulation of CYP450 enzymes leads to altered drug metabolism, drug resistance [8][38], CRPC and mCRPC development. The androgen receptor (AR) signal is considered to be the key driver of CRPC and mCRPC [9][39]. Approximately 80–90% of men with advanced PCa will develop bone metastasis, and most CRPC promotes bone metastasis [10][11][40,41]. In addition, a growing body of literature suggests that mixed-lineage protein kinase 3 (MLK3) signaling initiates WNT signaling and in turn WNT signaling promotes bone metastases in ADT-resistant PCa cells [12][13][42,43]. Advanced PCa-induced metastasis leads to organ failure and acute respiratory disorders [14][44]; the follow-up from the PCa initiation and the 1-, 3-, and 5-year survival rates of metastatic patients are 89.1%, 76.9% and 49.8%, respectively, however the 1-year survival rate of metastatic PCa without bone incidence is 87% and with bone incidence is 47% [2][3][4][15][16][32,33,34,45,46] (Figure 12A). While breast and other cancers form osteolytic lesions, bone metastasis of prostate cancer is characterized by an osteoblastic appearance which is caused by the deregulation of bone resorption and bone formation [17][18][19][20][47,48,49,50]. The researchers also identified osteoblastic bone metastasis with calcification from deidentified PCa-induced bone metastatic biopsies via H&E staining (bright field microscopic view, (Figure 12B)) and these results correlated with earlier reports [21][22][51,52]. Deidentified clinical samples were obtained from Thomas Jefferson University Hospital in accordance with Institutional Review Board Standards and in compliance with federal regulations governing research on deidentified specimens as described [23][53]. Bone metastasis causes strong bone pain, compression of the spinal cord, hypercalcemia, and increases mortality.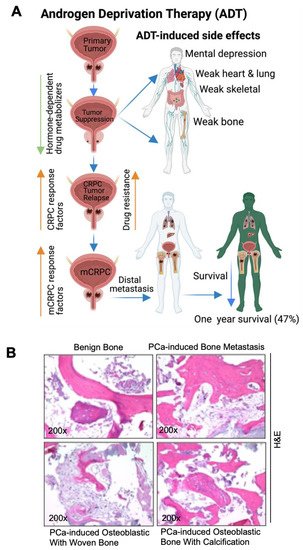
Figure 12. Pathogenesis of prostate cancer bone metastasis and its deleterious impacts in vital organs. (A) The schematic diagram shows that ADT suppresses PCa growth and simultaneously impairs hormone (androgen)-regulated drug metabolizers (cytochrome p450s). Additionally, ADT promotes mental depression, weakness in the bones, heart, lung, and skeletal muscles. ADT also promotes CRPC via AR-dependent and AR-independent mechanisms, drug resistance, and metastatic CRPC (mCRPC). mCRPC promotes local and distal metastasis, poor prognosis, and organ failure. (B) Bright field microscopic image of H&E-stained biopsy samples from (1) a male benign bone PCa (top left), (2) prostate-cancer-induced bone metastasis (top right), (3) prostate-cancer-induced bone metastasis with osteoblastic with woven bone (bottom left), (4) prostate-cancer-induced bone metastasis with osteoblastic bone with calcification (bottom right), magnification 200×. The up-arrow indicates increase and the down arrow indicates decrease. This diagrammatic illustration was created with BioRender.com agreement # CY23NSMIZH.
3. Bone Stromal and Prostate Cancer Cell Interaction Promotes Bone Metastasis
Changes in the stromal tissue associated with tumors and changes in the tumor cells themselves contribute to the ability of metastatic cancer cells to escape from tumor sites. Fibroblasts are associated with solid tumors or cancer-associated fibroblasts (CAFs) frequently acquire an activated myofibroblast phenotype in response to growth factors and the tumor microenvironment. The activated fibroblast produces elevated levels of matrix metalloproteinases (MMPs) and remodels the extracellular matrix (ECM) of tumors [24][54]. Cancer cells and the activated fibroblasts secrete high levels of VEGF-growth factor and the CXCL-family of chemokines, which recruits leukocytes and endothelial cells into the tumor microenvironment [24][54]. Tissue remodeling creates a microenvironment permissive of cancer cell escape and a tumor microenvironment (TME) with changes in the genetics of transformed cells possibly promotes metastasis. PCa patients often develop skeletal metastases; the establishment of metastases within the bone is a complex-multistep process, including colonization, dormancy, reactivation of dormant cancer cells, development, and reconstruction. Initially, the circulating cancer cells enter into the bone marrow, adapt to the bone microenvironment and remain in dormant status. The dormant cancer cells subsequently get reactivated to an active proliferative state and change the original/native bone structure and function [25][26][55,56].4. Mechanisms of Prostate-Cancer-Induced Bone Metastasis
Tumor-escaped PCa cells can reach the bone by traveling from local to the regional sites via the blood stream and establish molecular interactions with bone stromal cells, osteoblasts, osteoclast cells, and promote bone metastases [24][27][28][54,57,58]. PCa and bone resident cells interact with each other in response to various growth factor signals [24][29][54,59]. Bone marrow contains the hematopoietic stem cells (HSCs), osteoblasts, osteoclasts, and mesenchymal stem cells (MSCs). Previous studies have demonstrated that PCa cells influence bone resorption by osteoclasts and bone formation by osteoblasts by secreting monoamine oxidase A and eventually promote cancer cell progression. Hematopoietic stem cells (HSCs) serve as a foothold for PCa during their metastases to bone and mesenchymal stem cells (MSCs) and are found to increase the metastatic ability of the PCa cells by modulating AR signaling. PCa cells secrete active molecules that promote osteoblast differentiation, proliferation, including endothelin-1, and transforming growth factor β (TGF-β) [30][60]. In turn, enhanced osteoblast activity drives tumor progression by releasing interleukins 6 and 8 (IL-6 and IL-8) and insulin growth factor (IGF-1) [31][61]. PCa cells express angiogenic and bone-resorbing factors that induce cancer cell growth within the bone microenvironment [24][28][54,58]. Additionally, prostate cancer cells interact with bone stromal cells and promote the expression of cytokines, chemokines, and growth factors (Figure 23). Most importantly, growth factors, such as transforming growth factor, fibroblast growth factors, platelet-derived growth factors (PDGF), and bone morphogenetic proteins (BMP) are found in the bone matrix [32][33][34][62,63,64] and all these growth factors have been reported to participate in PCa metastasis. Osteoclasts are capable of degrading bone and tissue and releasing minerals, calcium, magnesium, and phosphate, and also degrading bone collagens into the blood stream and promoting hypercalcemia [35][65]. Bone calcium is removed from the bone via an osteolytic process and this causes bones to become weaker, leading to bone fractures and osteoporosis [36][66]. Continuous hypercalcemia promotes mental depression, weaker heart muscles, weaker bones (Figure 3), and nephrocalcinosis/kidney disorder [37][67]. Advanced prostate-cancer-induced bone metastases and bone-metastases-induced skeletal events (SREs) can be managed by lifestyle, nutritional, and pharmaceutical interventions as shown in Figure 23.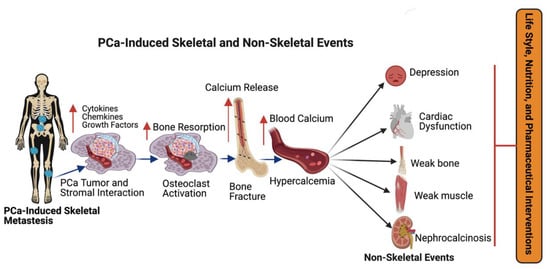
Figure 23. The impact of metastatic prostate cancer on bone health and bone-metastasis-induced skeletal and non-skeletal events. Prostate cancer often promotes bone metastasis (skeletal metastasis). The prostate cancer cells in the bone interact with bone stromal cells and promote the expression of cytokines, chemokines, growth factors, and osteoclast cell activation. Osteoclast activation initiates bone fracture and calcium release into the blood stream. Excess calcium in the blood stream promotes mental depression, cardiac dysfunction, bone, and muscle weakness, and nephrocalcinosis (kidney disorder) and skeletal and non-skeletal events are managed with lifestyle, nutritional and pharmaceutical interventions. This diagrammatic illustration was created with BioRender.com agreement # JO23NSOQ0.
5. Therapeutic Options for PCa-Induced Bone Metastases and Bone Management
The treatment options for metastatic prostate cancer are androgen deprivation therapy (ADT), surgery, bone marrow cell transplants, chemotherapy (taxanes and bisphosphonates), personalized medicine (T-cell therapy), radiotherapy or radiopharmaceuticals or a combination of chemo-radio therapy and immunotherapy (Figure 34A).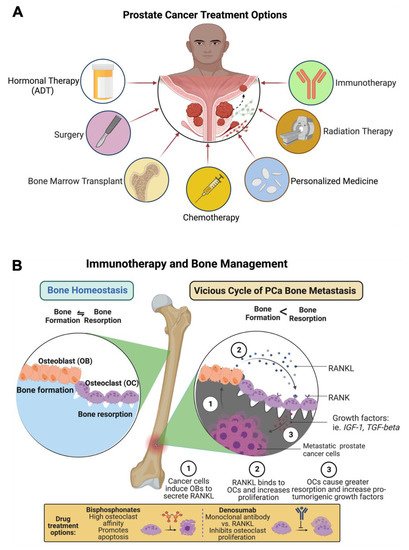
Figure 34. Treatments options for PCa-induced bone metastasis and bone management. (A) Patients with PCa-induced bone metastasis are treated with surgery, hormonal therapy (androgen deprivation therapy, ADT), bone marrow transplant, chemotherapy (taxanes, bisphosphonate), anti-androgen therapy (enzalutamide), personalized medicine (T-cell therapy), radiation therapy (radium-223), and immunotherapy (denosumab). (B) Prostate cancer treatment, including immunotherapy and bone management, is presented in the figure as a schematic diagram. The diagram shows that bisphosphonate reduces osteoclast numbers by promoting apoptosis. Anti-RANKL monoclonal antibody denosumab therapy inhibits the development and activity of osteoclasts, followed by the suppression of bone resorption. This diagrammatic illustration was created with BioRender.com agreement # ET23NSSNDQ and TC23NSPRCC.
