The ketogenic diet (KD), a low-carbohydrate and high-fat diet, has been widely prescribed in weight loss programs for metabolic abnormalities. Furthermore, research has investigated the effects of KD on the treatment of numerous diseases, including cardiovascular disease (CVD) and cancer, due to its role in promoting ketolysis, ketogenesis, and modifying many other metabolic pathways with potential favorable health effects.
1. History
Over the centuries, fasting has been used as the primary treatment for epilepsy
[1][12]. In the early twentieth century, Guepa and Maria used fasting to treat epilepsy in France
[2][13]. Ten years later, in 1921, Wilder suggested that this treatment for epilepsy may be due to body ketones
[3][14] and hence introduced KD, which produced similar biochemical changes as fasting
[4][15]. In the same year, Woodiat stated that in conditions of starvation or low carbohydrates, ketone bodies appeared in the blood
[5][16]. KD consists of a significant amount of fats, low carbohydrates, and limited protein, which leads to altered energy metabolism and an increase in body ketones in the blood, which eventually forces the body to use them to produce energy
[6][7][17,18]. The recommended ratio of fat to protein plus carbohydrates varies between 4:1 and 2:1 by weight
[4][15]. Later, Wilder and Teperman described this diet as 1 g and 10–15 g per kilogram of body weight of protein and carbohydrates, respectively, and the rest of the required energy from fat
[5][16].
Table 1 shows different versions of KD.
Table 1.
Different types of ketogenic diet (KD).
2. Classification
In general, four types of KD have been characterized. The first type is the classical or traditional type, with a 4:1 ratio of fat to protein plus carbohydrates. Thus, in this diet, fat intake provides 90% of the energy, which may be difficult and intolerant to the public
[8][19]. The second type contains medium-chain-triglyceride (MCT) like caprylic acid, capric acid, caproic acid, and lauric acid
[9][20], with 70% of energy uptake from fat including 10% long chain triglyceride (LCT) fat and 60% MCT fat, 20% from carbohydrate, and 10% from protein. Due to MCT’s faster membrane diffusion, the absorption process occurs earlier. Since the production rate of ketone bodies is higher than the previous type, for its equal formulation, less fat intake is required
[10][21]. The third type of KD with a 1.1:1 ratio of 65% fat, 10% carbohydrate, and 25% protein was developed in 1970 by Dr. Robert Atkins based on carbohydrate restriction for weight loss. It is characterized by a higher carbohydrate restriction and high-fat intake with no restrictions on calories and protein. This diet was called the Atkins modified diet
[11][22]. The fourth type is called the low glycemic index (GI) diet, which recommends food with a GI lower than 50 with fat to carbohydrate ratio of 6:1
[12][23].
3. Physiology and Metabolism
A drop in blood glucose levels through fasting or starvation stimulates the liver to produce glucose by breaking down glycogen stores and through gluconeogenesis
[13][24]. Therefore, persistent low blood glucose leads to the preferential breakdown of fat, with a major contribution to increased lipolysis due to lowered insulin levels. However, under these conditions, energy metabolism can hardly continue beyond the production of acetyl-coenzyme A (CoA). This is due to the limited availability of oxaloacetate for oxidation in the tricarboxylic acid (TCA) cycle in hepatocytes. This way, the liver produces ketone bodies as metabolic energy for extrahepatic tissues. Ketosis is caused by the preferential breakdown of fats for energy production due to an insufficient amount of carbohydrates, causing low systemic insulin levels
[14][25].
This process of ketone production is controlled by the sensitive lipase hormone, acetyl-CoA carboxylase, and 3-hydroxy-3-methylglutaryl (HMG) CoA synthase, all of which are regulated by three hormones: adrenaline, insulin, and glucagon
[15][26]. By acting on lipase-sensitive hormones, glucagon leads to the production of fatty acids and thus increases ketogenesis while inhibiting acetyl-CoA carboxylase, which prevents fatty acids from entering the liver mitochondria and increases ketogenesis. In the process of ketogenesis, glucagon accelerates ketogenesis by stimulating HMG CoA synthase
[16][17][27,28]. Unlike glucagon, insulin has an inhibitory effect on ketogenesis by inhibiting lipolysis and increasing lipogenesis, leading to a reduction in available fatty acids
[16][27].
The amount of ketone bodies in KD is between 0.5 and 3 mmoL, which is called nutritional or physiological ketosis
[18][19][29,30]. Reducing carbohydrate intake to less than 50 g per day and suppressing subsequent insulin secretion leads to reduced glucose and fat storage, increases the triacylglycerol lysis, and the formed fatty acids are subsequently converted to ketone bodies
[18][29]. The glycogen of the liver and skeletal muscle is depleted during carbohydrate restriction for 24 h and one week, respectively, in the ketogenesis process to supply energy to the mitochondrial matrix of the liver
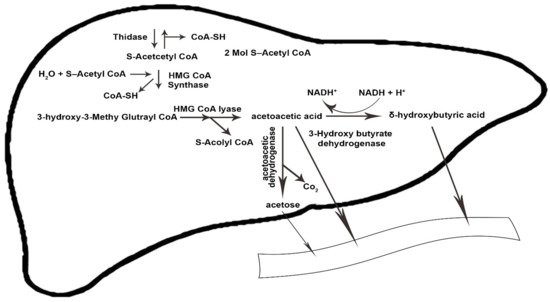
[20][21][31,32]. The condensation of β-oxidation-derived acetyl-coenzyme (acetyl-CoA) into acetoacetate (AcAc) is initiated by the sequential need of mitochondrial enzymes 3-hydroxy-3-methylglutaryl CoA synthase and 3-hydroxy-3-methylglutaryl CoA lyase. Acetoacetate forms acetone through spontaneous decarboxylation, which is a volatile substance. By reducing the ketone fractions, it becomes beta-hydroxybutyrate, which is the circulating form of ketone bodies in the human body
[22][23][33,34].
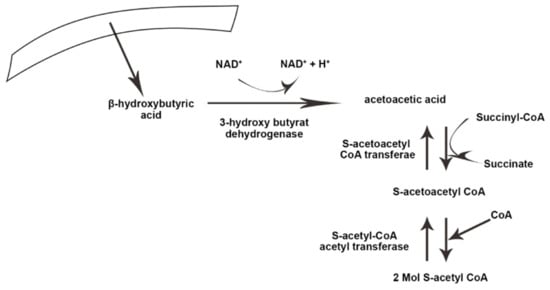
Ketolysis occurs in almost all extrahepatic tissues
[24][35]. Once in the bloodstream, monocarboxylic acid carriers transport ketone bodies
[25][36] and are absorbed by the brain and other tissues. The key enzyme in the ketolysis process is succinyl CoA transferase, which is the most active in the heart, muscle, kidneys, nervous system, and skeletal muscles
[26][37]. First, β-hydroxybutyrate (BOHB) is converted back to AcAc by NAD+ oxidation. It is finally converted to acetyl-CoA by BDH1, succinyl-CoA:3-oxoacid CoA transferase (SCOT), and mitochondrial thiolase, which are used in the Krebs cycle
[1][12].
Figure 1 and
Figure 2 illustrate the process of ketogenesis and ketolysis in the ketogenic diet.
Figure 1. The ketogenesis process in the body. CoA-SH, coenzyme A-SH; CoA, coenzyme A; H2O, water; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; NADH+, nicotinamide adenine dinucleotide + hydrogen; H+, Hydrogen; Co2, carbon dioxide. The arrows indicate the direction of changes in the process, as well substrates and products.
Figure 2. The process of ketolysis in the body. NAD+, Nicotinamide adenine dinucleotide. The arrows indicate the direct ion of changes in the process, as well substrates and products.