There is a critical need for new diagnostic and prognostic biomarkers with high specificity and sensitivity in patients with colorectal cancer (CRC). Liquid biopsy could represent the new era for biomarkers detection: the term “liquid biopsy” refers to the isolation of cancer-derived components, such as circulating tumor cells (CTC), circulating tumor DNA (ctDNA), microRNAs (miRNAs), long non-coding RNAs (lncRNAs) and proteins, from peripheral blood or other body fluids (i.e., ascites, urine, pleural effusion, and cerebrospinal fluid), and their genomic or proteomic assessment. Furthermore, exosomes (EXOs) which are membrane-bound extracellular vesicles containing proteins and nucleic acids released in the bloodstream by cancer cells, could represent potential biomarkers.
- liquid biopsy
- colorectal cancer
- biomarkers
- circulating tumor cells
- circulating tumor DNA
1.
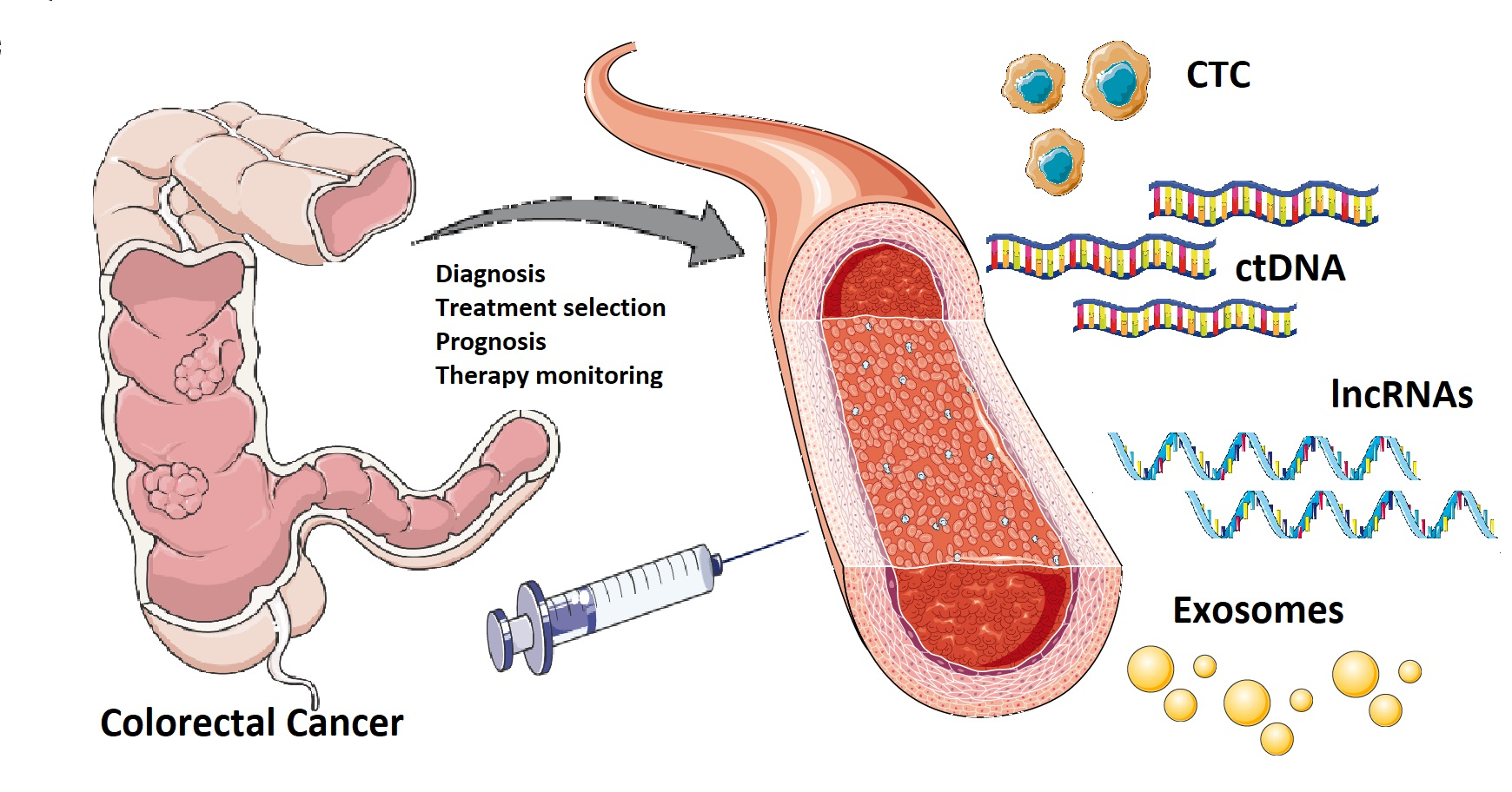
Figure 1. Currently, there is a crucial need for novel diagnostic and prognostic biomarkers with high specificity and sensitivity in patients with colorectal cancer. A “liquid biopsy” is characterized by the isolation of cancer-derived components, such as circulating tumor cells, circulating tumor DNA, microRNAs, long non-coding RNAs, and proteins, from peripheral blood or other body fluids and their genomic or proteomic assessment. The liquid biopsy is a minimally invasive and repeatable technique that could play a significant role in screening and diagnosis, and predict relapse and metastasis, as well as monitoring minimal residual disease and chemotherapy resistance in colorectal cancer patients. However, there are still some practical issues that need to be addressed before liquid biopsy can be widely used in clinical practice. Potential challenges may include low amounts of circulating tumor cells and circulating tumor DNA in samples, lack of pre-analytical and analytical consensus, clinical validation, and regulatory endorsement
