Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Hasan CAN.
Plants interact with diverse microbial communities and share complex relationships with each other. The intimate association between microbes and their host mutually benefit each other and provide stability against various biotic and abiotic stresses to plants. Endophytes are heterogeneous groups of microbes that live inside the host tissue without showing any apparent sign of infection.
- endophyte
- plant-microbe interaction
- molecular aspect of colonization
1. Introduction
The biology of endophytic microorganisms has been gaining momentum in the last few years due to better colonization efficacy and acclimatization potential against biotic and abiotic stress. In the last few years, endophytes, bacteria, or fungi have been frequently applied in sustainable agricultural practices as biofertilizers to meet nutrient requirements. Biocontrol agents have been used to prevent pathogen invasion or disease control and to mitigate various abiotic stresses, including salinity, drought, etc. The prospect of an endophytic microbiome has been reported in various review papers, which have been published recently [1[1][2],2], while molecular aspects of plant–endophyte interactions have been not covered extensively [3].
Plant-microbe interaction is a complex process in which the plant system interacts with diverse heterotrophic microorganisms and can share an intimate relationship from symbiotism to parasitism [4,5,6][4][5][6]. The intimate association of microbes with plants has a long history, and it is assumed that both have co-evolved together since the time of plants’ origin [7]. This inseparable relationship is also referred to as second genomes or holobionts, which play a significant role in maintaining plant health and fitness [8]. The term holobionts is also used as collective term for the microbiome associated with the host and referred as a single entity, which provides genomic reflection and stability to plants under various biotic and abiotic stresses [9]. The functional attributes of a plant as it secretes a range of sugars, metabolites organic or volatiles compounds are also dependent on the associated microorganism. Still, their exact mechanism is unclear at the community level. However, the study of synthetic communities and their outcome can be used to explore the colonization and assembly pattern of microorganism, which can be used to control pathogen invasion and biotic stress management [10,11][10][11]. The interaction of plants with microbes is mediated through various organic metabolites or signalling molecules. Their secretions include organic compounds such as amino acids, lipids, polysaccharides, flavonoids etc., that attract the microbial strains for colonization. For example, Steinkellner et al. [12] reported the functional role of root exudates, flavonoids, and strigolactones in the root colonization and hyphal growth differentiation of various Fusarium species and also their role as signaling molecules during symbiotic and pathogenic plant-fungus interactions. Similarly, Oku et al. [13] reported the role of amino acids in the root colonization of Pseudomonas fluorescens Pf0-1 to tomato plants.
The plant’s rhizosphere is a hot spot of microbial communities and is considered as the favourable site of plant-microbe interaction due to its abundantly present root exudates. Their composition depends upon plant genotypes, development stages, and the surrounding environmental conditions of the rhizospheric microbiota [14]. However, some of the rhizospheric microbes enter the host tissue through natural openings such as stomata, pores, wounds, and hydathodes, acting as endophytes. The entry and establishment of endophytes is a complex process accomplished by various signalling molecules and colonization processes.
2. An Overview of Microbial Endophytes
Endophytes are a heterogeneous group of microbes that live inside the host tissue without showing any external signs of infections. De Bary [15] firstly used the term endophytes for the microbes residing in the host tissue. However, later authors modified the concept and defined endophytes per their observation. Now, in general, any microbes living inside the host tissue for at least a part of their life cycle are considered an “endophyte”, and every plant species have some endophytes in their life cycle [16,17][16][17]. However, with the advent of the latest forms of omics and various kinds of technology, extensive research has been carried out on the endophytic microbiome. Initially, only cultivable microbial species isolated from the surface-sterilized tissue have been screened as endophytes. However, the latest next-generation sequencing technology frequently reported the endophytic microbiome of any tissue or host plant, including the non-cultivable microbial species even present in a small number of samples. Endophytes comprise diverse microbial communities, including various groups of fungi, bacteria, actinomycetes, etc. These endophytic microbial communities classified into different groups based on the host specificity, colonization pattern, transmission mechanism, and evolutionary relationship [18]. The most common fungal endophytes have been generally comprised of two groups: clavicipitaceous, comprising the endophytes harbored by grasses, and non-clavicipataceous, which colonize angiosperm, gymnosperm, and nonvascular plants. However, Epichloe (formely Neotyphodium), Claviceps (Clavicipitaceae), Cladosporium (Cladosporiaceae), Colletotrichum (Glomerellaceae), Piriformospora (Sebacinaceae), Stemphylium (Pleosporaceae), Acremonium and Trichoderma (Hypocreaceae) are the some common genera, and Glomeromycota followed by Ascomycota and Basidiomycota are the dominant endophytic fungal phyla. Pseudomonas, Bacıllus, Acinetobacter, Brevibacterium, and Rhizobium are the dominant bacterial genera, and Proteobacteria followed by Actinobacteria, Firmicutes, and Bacteroides are the dominant bacterial phyla reported as endophytes in most of the plant species [19].3. Entry and Transmission of Endophytes into Plant Tissue
The colonization of endophytic microbial strain to the host tissue is a series of consecutive events mediated by various secretory products and signalling molecules. However, attaching microbial strains to the host tissue is a primary step for endophyte colonization. The secretory product, such as lipopolysaccharides, and exopolysaccharides, directly or indirectly help the adhesion. In contrast, the structural components such as flagella, fimbriae, and pilli help in the movement of bacterial strains towards the host tissue during colonization. However, plants respond differentially after attachments of microbial strains to their surface especially in the gene expression patterns. In a study Bodilis and Barray [20] discussed the outer membrane porin F (OprF) proteins present on the surface of Pseudomonas and their role in the attachment with the host surfaces. Similarly the arabinogalactan proteins present on the plant cell wall help in the initial colonization of endophytic microorganisms [21]. After attachment, microbial strains start penetrating to enter the host tissue, which can be mediated through either active or passive processes. The passive penetration taken part at the cracks present on the site of root surface caused by the deleterious organism, while active penetration involved the structural components and secretory products in the entry or multiplication inside the host tissue [22]. The aerial parts such as the stomata, wounds, and cotyledons are the typical entry sites of endophytes [23]. However, significant differences between mutualistic and pathogenic strains have been observed during the process of entry and penetration. For example, the pathogens secrete higher amount of cell wall degrading enzymes in compared to the mutualistic microorganisms [24]. Therefore, mutualistic endophytes and hosts play a deeper and more precise modulation of molecular signalling compared to the pathogens during colonization of plant tissues. The transmission of endophytes within the host may be through different modes (e.g., horizontal, which is mediated through environments; vertical, referred to as transmission via parents to the offspring through seeds/pollen grains; or mixed ones, which either follows the horizontal and vertical or both modes of transmission) [25]. In a previous study, vertical modes of transmission were well documented in the fungal strains through the isolation of endophytes from seeds, cotyledons and leaves of forb species that grew under sterile conditions [26]. However, the horizontal transmission mode in fungal species has also been reported by various authors. This observation is based on some ubiquitous fungal sporophytes such as Alternaria and Cladosporium, which generally sporulate on the dead leaf and soil but are frequently reported as endophytes [27,28][27][28]. Similarly, bacterial species generally prefer the horizontal, vertical and mixed types of transmission. In another study, the author reported that a seed growing under aseptic conditions has lower bacterial diversity than one grown under normal environmental conditions. This observation suggests the prevalence of the horizontal transmission mode [29,30,31][29][30][31]. The successful colonization of endophytes depends upon several factors such as host genotypes, the nature of microbial strains, and the availability of nutrient sources [32,33,34][32][33][34]. The endophytes, during colonization and transmission, followed an unique path. For example, the strain Paraburkholderia phytofirmans PsJN made an entry to the host tissue through the exodermis or cortical cells and reached to the central zone of the roots and moved towards the upper zone or above ground tissue by the xylem vessels [35]. However, endophytic microorganisms commonly prefer unsuberized cells to enter the apical root [36]. A detailed overview of endophyte biology and their mode of transmission is discussed in Figure 1.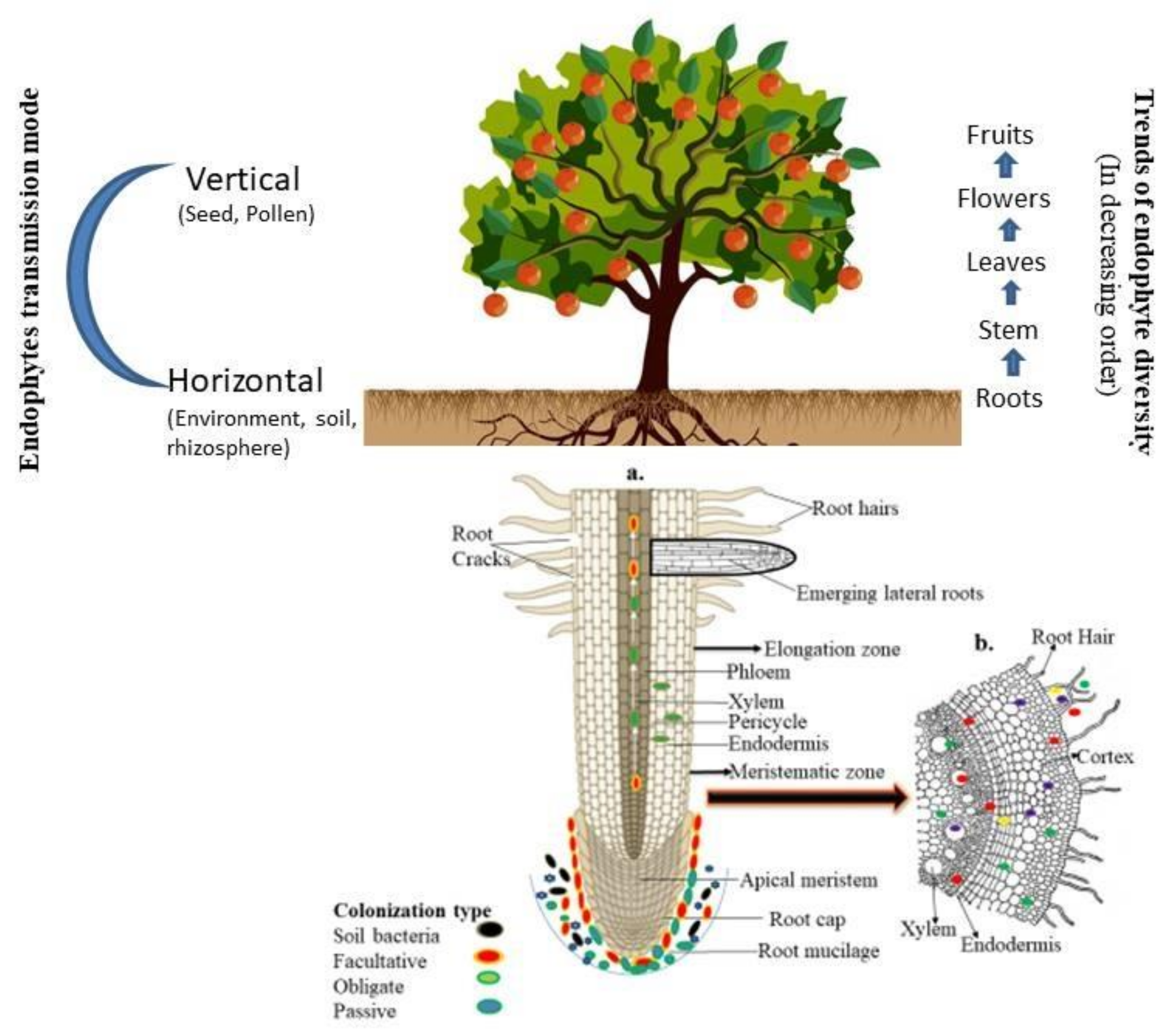
Figure 1. The detailed overview of endophyte biology and their mode of transmission: (a) entry of microbial endophytes to the plant tissue by different part of root zones. Arrows show the movement of endophytes; (b) occurrence of endophytes either at the entry site or in the intercellular spaces. Part of a figure has been taken from Kumar et al. [32].
References
- Brader, G.; Company, S.; Mitter, B.; Trognitz, F.; Sessitsch, A. Metabolic potential of endophytic bacteria. Curr. Opin. Biotechnol. 2014, 27, 30–37.
- Strobel, G. The Emergence of Endophytic Microbes and Their Biological Promise. J. Fungi. 2018, 4, 57.
- Skiada, V.; Avramidou, M.; Bonfante, P.; Genre, A.; Papadopoulou, K.K. An endophytic Fusarium-legume association is partially dependent on the common symbiotic signalling pathway. New Phytol. 2020, 226, 1429–1444.
- Reinhold-Hurek, B.; Hurek, T. Living inside plants: Bacterial endophytes. Curr. Opin. Plant Biol. 2011, 14, 435–443.
- Singh, L.P.; Gill, S.S.; Tuteja, N. Unraveling the role of fungal symbionts in plant abiotic stress tolerance. Plant Signal. Behav. 2011, 6, 175–191.
- Iqbal, J.; Nelson, J.A.; McCulley, R.L. Fungal endophyte presence and genotype affect plant diversity and soil-to-atmosphere trace gas fluxes. Plant Soil 2013, 364, 15–27.
- Redecker, D.; Kodner, R.; Graham, L.E. Glomalean fungi from the Ordovician. Science 2000, 289, 1920–1921.
- Kiers, E.T.; Heijden, M.G.V.D. Mutualistic stability in the arbuscular mycorrhizal symbiosis: Exploring hypotheses of evolutionary cooperation. Ecology 2006, 87, 1627–1636.
- Rosenberg, E.; Zilber-Rosenberg, I. Microbes drive evolution of animals and plants: The hologenome concept. MBio 2016, 7, e01395-15.
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621.
- Gong, T.; Xin, X.F. Phyllosphere microbiota: Community dynamics and its interaction with plant hosts. J. Integ. Plant Biol. 2021, 63, 297–304.
- Steinkellner, S.; Lendzemo, V.; Langer, I.; Schweiger, P.; Khaosaad, T.; Toussaint, J.-P.; Vierheilig, H. Flavonoids and Strigolactones in Root Exudates as Signals in Symbiotic and Pathogenic Plant-Fungus Interactions. Molecules 2007, 12, 1290–1306.
- Oku, S.; Komatsu, A.; Tajima, T.; Nakashimada, Y.; Kato, J. Identification of chemotaxis sensory proteins for amino acids in Pseudomonas fluorescens Pf0-1 and their involvement in chemotaxis to tomato root exudate and root colonization. Microbes Environ. 2012, 27, ME12005.
- Chagas, F.O.; de Cassia Pessotti, R.; Caraballo-Rodriguez, A.M.; Pupo, M.T. Chemical signaling involved in plant-microbe interactions. Chem. Soc. Rev. 2018, 47, 1652–1704.
- De Bary, A. Morphologie und Physiologie der Pilze, Flechten und Myxomyceten; W. Engelmann: Leipzig, Germany, 1866.
- Petrini, O. Fungal Endophytes of Tree Leaves. In Microbial Ecology of Leaves; Andrews, J.H., Hirano, S.S., Eds.; Brock/Springer Series in Contemporary Bioscience; Springer: New York, NY, USA, 1991; pp. 179–197.
- Peters, A.F. Field and culture studies of Streblonema—Macrocystis new species Ectocarpales Phaeophyceae from Chile, a sexual endophyte of giant kelp. Phycologia 1991, 30, 365–377.
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, A.R.A. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330.
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320.
- Bodilis, J.; Barray, S. Molecular evolution of the major outer-membrane protein gene(oprF) of Pseudomonas. Microbiology 2006, 152, 1075–1088.
- Nguema-Ona, E.; Vicré-Gibouin, M.; Gotté, M.; Plancot, B.; Lerouge, P.; Bardor, M.; Driouich, A. Cell wall O-glycoproteins and N-glycoproteins: Aspects of biosynthesis and function. Front. Plant Sci. 2014, 5, 499.
- Böhm, M.; Turek, T.; Reinhold-Hurek, B. Twitching motility is essential for endophytic rice colonization by the N2-fixing endophyte Azoarcus sp. strain BH72. Mol. Plant-Microbe Interact. 2007, 20, 526–533.
- Suárez-Moreno, Z.R.; Devescovi, G.; Myers, M.; Hallack, L.; Mendonça-Previato, L.; Caballero-Mellado, J.; Venturi, V. Commonalities and differences in regulation of N-acyl homoserine lactone quorum sensing in the beneficial plant-associated Burkholderia species cluster. Appl. Environ. Microbiol. 2010, 76, 4302–4317.
- Reinhold-Hurek, B.; Maes, T.; Gemmer, S.; Van Montagu, M.; Turek, T. An endoglucanase is involved in infection of rice roots by the not-cellulose-metabolizing endophyte Azoarcus sp. strain BH72. Mol. Plant-Microbe Interact. 2006, 19, 181–188.
- Frank, A.C.; Saldierna Guzmán, J.P.; Shay, J.E. Transmission of Bacterial Endophytes. Microorganisms 2017, 5, 70.
- Hodgson, S.; de Cates, C.; Hodgson, J.; Morley, N.J.; Sutton, B.C.; Gange, A.C. Vertical transmission of fungal endophytes is widespread in forbs. Ecol. Evol. 2014, 4, 1199–1208.
- Hayes, A.J. The microbiology of plant litter decomposition. Sci. Prog. (1933-) 1979, 66, 25–42.
- Marchisio, V.F.; Airaudi, D. Temporal trends of the airborne fungi and their functional relations with the environment in a suburban site. Mycologia 2001, 93, 831–840.
- Bright, M.; Bulgheresi, S. A complex journey: Transmission of microbial symbionts. Nat. Rev. Microbiol. 2010, 8, 218–230.
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial seed endophytes: Genera, vertical transmission and interaction with plants. Environ. Microbiol. Rep. 2015, 7, 40–50.
- Faeth, S.H. Are endophytic fungi defensive plant mutualists? Oikos 2002, 98, 25–36.
- Kumar, A.; Droby, S.; Singh, V.K.; Singh, S.K.; White, J.F. Entry, colonization, and distribution of endophytic microorganisms in plants. In Microbial Endophytes; Elsevier: Cambridge, MA, USA, 2020; pp. 1–33.
- Kobayashi, D.Y.; Palumbo, J.D. Bacterial endophytes and their effects on plants and uses in agriculture. In Microbial Endo-Phytes; CRC Press: Boca Raton, FL, USA, 2000.
- Singh, M.; Kumar, A.; Singh, R.; Pandey, K.D. Endophytic bacteria: A new source of bioactive compounds. 3Biotech 2017, 7, 315.
- Company, S.; Kaplan, H.; Sessitsch, A.; Nowak, J.; Ait Barka, E.; Clément, C. Endophytic colonization of Vitis vinifera L. by Burkholderia phytofirmans strain PsJN: From the rhizosphere to inflorescence tissues. FEMS Microbiol. Ecol. 2008, 63, 84–93.
- James, E.K.; Gyaneshwar, P.; Mathan, N.; Barraquio, W.L.; Reddy, P.M.; Iannetta, P.P.; Olivares, F.L.; Ladha, J.K. Infection and colonization of rice seedlings by the plant growth-promoting bacterium Herbaspirillum seropedicae Z67. Mol. Plant-Microbe Interact. 2002, 15, 894–906.
More
