Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yong-Zhong Du and Version 2 by Rita Xu.
Photodynamic therapy (PDT) has become a promising method of cancer treatment due to its unique properties, such as noninvasiveness and low toxicity. The efficacy of PDT is significantly reduced by the hypoxia tumor environments, because PDT involves the generation of reactive oxygen species (ROS), which requires the great consumption of oxygen. Moreover, the consumption of oxygen caused by PDT would further exacerbate the hypoxia condition, which leads to angiogenesis, invasion of tumors to other parts, and metastasis.
- photodynamic therapy
- hypoxia
- oxygen supply
1. Introduction
The tumor is characterized by the extremely uncontrolled growth of a series of cells, which can spread to other parts of bodies and bring threatening consequences to bodies. The tumor consists of cellular components and the tumor microenvironment (TME). The TME, which is composed of extracellular matrix, stromal cells, and immune cells, has been found to play a significant role in tumor progression [1]. Hypoxia, which arises when there is an imbalance between oxygen supply and consumption and is characterized by the O2 pressure < 10 mm Hg, is one of the important factors of tumor microenvironments [2][3][2,3]. Hypoxia-inducible factors (HIF) are transcription factors of downstream hypoxia-inducible genes, composed of oxygen-labile subunit HIF-α and a constitutively expressed HIF-β [4]. Among them, HIF-1α regulates the expression of genes that are involved in tumor progression, aggressiveness, and metastasis [5]. Under the hypoxic state, HIF-1α is activated and highly expressed. As a result, the hypoxic state in tumor leads to the aggressive phenotype, induces tumor progression, promotes tumor spreading, and causes angiogenesis [6][7][8][6,7,8]. Additionally, a great deal of evidence has also proved that hypoxia induces tumor resistance to various therapies via decreased drug-induced senescence [9][10][9,10]. Therefore, tumor cells exposed to hypoxia are much more aggressive and resistant.
Photodynamic therapy (PDT) has progressively played solid roles in cancer treatment owing to its inherent merits, such as low toxicity, minor side effects, and accurate target treatment without damage to adjacent normal tissues [11][12][13][14][11,12,13,14]. Fundamentally, PDT is composed of three components: light, a photosensitizer (PS), and oxygen [15]. Under laser irradiation, the PS is activated to generate reactive oxygen species (ROS) through the type I mechanism based on radical reaction or through the type II mechanism, where the energy is directly transferred to triplet ground state oxygen (3O2) to generate reactive singlet oxygen (1O2) [16]. The generated ROS, which is cytotoxic, could further induce cytotoxicity, cell damage, or death. While PDT possesses various advantages, it still has several limitations, such as the need for ideal PS and tumor hypoxia environments [17][18][17,18]. Until now, much research has been carried out to develop the ideal PS for phototherapies, such as gold nanoparticles (AuNPs) [19][20][21][19,20,21], chlorin e6 (Ce6) [22], and indocyanine green (ICG) [23][24][23,24]. Among the limitations of PDT, the hypoxia environment is one of the most significant factors. Since the PDT process involves especially high consumption of oxygen, the hypoxic character of tumor dramatically reduces PDT efficacy, and the hypoxia also deteriorates simultaneously. Consequently, the increased tumor hypoxia induces cancer progression and metastasis, leading to the higher risk of PDT resistance [25].
In spite of challenges in alleviating tumor hypoxia, significant efforts have been made to address the limitation of PDT caused by hypoxia in recent years [26][27][28][26,27,28]. These strategies are mainly divided into several directions, including (1) the direct transport of oxygen by O2 carriers, (2) in situ O2 generation by catalase or O2 self-supply materials, (3) a decrease in oxygen consumption, and (4) other strategies, such as increasing blood flow and downregulating HIF-1 (Figure 1).
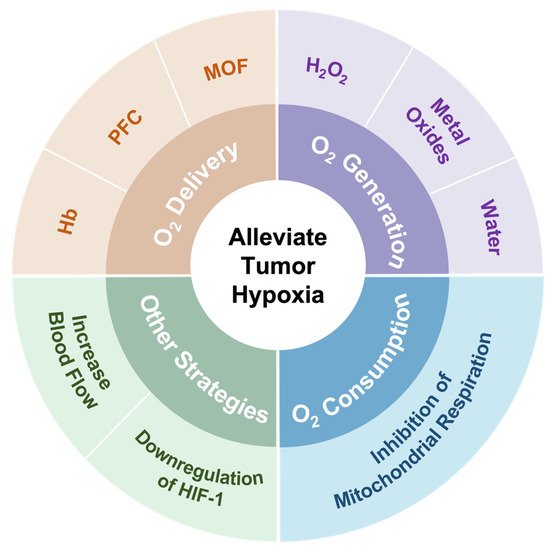
Figure 1. Summary of strategies employed to address hypoxia in the tumor microenvironment.
2. Direct Transport of Exogenous Oxygen to Tumors
To alleviate the tumor hypoxia in PDT, the transportation of exogenous oxygen to the tumor is a straight way. Hemoglobin, perfluorocarbon groups, and metal–organic frameworks are common O2 carriers used to deliver O2 to the tumor cells. These O2 carrier-contained nanoplatforms are summarized in Table 1.
Table 1. Summary of recent studies on direct transport of oxygen by O2 carriers.
| O | 2 | -Carrier | Nanosystems | PS | Strategy | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Hemoglobin | Gd@Hb | Ce6-PEG | Ce6 | Hb increased tumor oxygenation and MR imaging-guided enhanced PDT. | [29] | |||
| SPN-Hb@RBCM | SPNs | Prolonged circulation time and accumulation of nanoparticles in tumor by RBCM enhanced PDT treatment efficacies. | [30] | |||||
| HbTcMs | TCPP | HbTcMs could produce singlet oxygen and kill 4T1 cells under irradiation. | [31] | |||||
| Hb/ICG loaded artificial red cells | ICG | Hb supplied sufficient oxygen for PDT and self-monitoring PDT was performed through FL/PA dual-modal imaging. | [32] | |||||
| Perfluorocarbon | PFP, ICG and BPD contained nanodroplets | ICG | The systems could produce photoacoustic contrast, deliver oxygen, and improve PA-guided PDT efficacy. | [33] | ||||
| CCm-HSA-ICG-PFTBA | ICG | The nanoprobe was targeted to tumor tissues, mitigated hypoxia, and enhanced the PDT efficacy. | [34] | |||||
| O | 2 | @PFOB@PGL NPs | Porphyrin | The nanoparticles alleviated hypoxia under fluorescence/CT imaging guidance. | [35] | |||
| Ce6-PFOC-PEI-M | Ce6 | PFOC provided high oxygen-carrying ability, enhancing higher PDT efficacy. | [36] | |||||
| MOFs | CuTz-1-O | 2 | @F127 | CuTz-1 | CuTz-1@F127 could then deliver O | 2 | generated by PS to tumor cells, alleviating hypoxia. | [37] |
| O | 2 | @UiO-66@ICG@RBC | ICG | UiO-66 MOFs could store O | 2 | and release it upon light irradiation, relieving hypoxia. | [38] |
2.1. O2 Carriers Based on Hemoglobin Groups
In human bodies, red blood cells are fundamental tools for oxygen transportation under the help of hemoglobin. The hemoglobin molecule consists of four heme groups where an iron ion is ligated at the center of a porphyrin ring. Each of the four heme groups can strongly bind to one molecular O2 molecule, which can be carried from the lungs to other tissues by the hemoglobin inside red blood cells in human bodies, exhibiting the excellent O2 capacity of Hb. To mitigate the hypoxia environment in tumor, many strategies have been made to increase the endogenous oxygen content by utilizing the hemoglobin-based oxygen carriers. Although hemoglobin is known for its excellent oxygen loading and releasing capability, its relatively short lifetime and instability cause side effects to patients [39]. Thus, to address its shortcomings, Hb has been modified by physical encapsulation or chemical conjugation [40][41][42][40,41,42].
For instance, PEGylation of Hb can improve the biocompatibility, enhance the stability, and prolong the half-life of hemoglobin during the circulation. Shi et al. designed the nanostructures named Gd@HbCe6-PEG with PDT function through protein-mediated biomimetic synthesis, which is an eco-friendly technology used in the biomedical field and is a simpler process compared with complexed chemical synthesis. Firstly, Hb was PEGylated, followed by the cooperation of Gd nanoparticles, photosensitizer Ce6, and oxygen. Significantly, Hb in this system not only showed excellent biocompatibility and intrinsic oxygen-carrying capability, but also provided the reaction region for the biomimetic chemistry of Gd nanoparticles. The in vitro experiments in this study showed that the viability of 4T1 cells incubated with Gd@HbCe6-PEG and oxygenated Gd@HbCe6-PEG and laser irradiation decreased to 34% and 18%, respectively, indicating efficient killing of cancer cells. The results of PA imaging and HIF-1α protein expression analysis demonstrated that this system could efficiently deliver O2 and alleviate tumor hypoxia [29].
Since hemoglobin carries O2 inside the red blood cells (RBCs), RBCs have also been widely used as drug carriers. Nanoscale RBCs or red blood cell membrane (RBCM)-modified nanoparticles (NPs) are an effective way to ensure tumor permeability or internalization [43]. For example, Ding et al. constructed a red blood cell biomimetic nanovesicle (SPN-Hb@RBCM), as shown in Figure 2. In this system, the oxygen-supplying hemoglobin was linked with semiconducting polymer nanoparticles (SPN), which was a theranostic agent for near-infrared (NIR)-II fluorescence imaging and singlet oxygen (1O2) production for PDT. The resulting SPN-Hb was further coated with RBCM to form SPN-Hb@RBCM. Notably, this RBCM coated system promoted longer circulation time and nanoparticle accumulation in vivo, allowing for enhanced treatment efficacies. The in vitro experiment result showed that the overall oxygen-carrying capacity of SPN-Hb@RBCM nanoparticles was determined and calculated to be 0.142 μg of O2 per 1 mg NPs. The cell-killing efficacy was 90.3% in the SPN-oxy-Hb@RBCM group and was only 45.1% in the SPN@RBCM groups, showing that the SPN-oxy-Hb@RBCM with oxygen self-supply ability could retain its great PDT efficiency under hypoxia environments. Although this strategy could improve hypoxia to a certain extent, the oxygen loading content of the nanomaterial was limited, which may restrict the practical application [30].
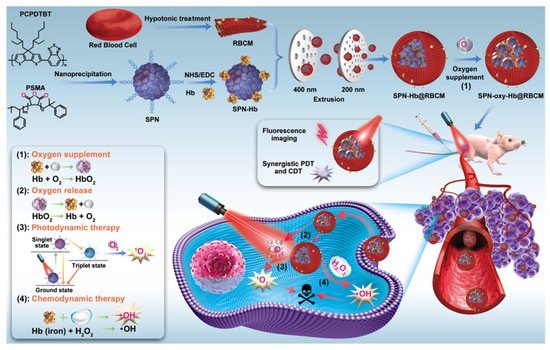
Figure 2. Illustration for the preparation of the SPN-oxy-Hb@RBCM theranostic nanoagent and its application in fluorescence imaging (FI)-guided chemo- and photodynamic therapy of solid hypoxic tumors. Adapted with permission from Ref. [30]. Copyright 2021, American Chemical Society.
2.2. O2 Carriers Based on Perfluorocarbon Groups
Since hemoglobin has several limitations, such as susceptible conformation changes and a short circulation time, perfluorocarbon (PFC) is an alternative oxygen carrier. Perfluorocarbons (CnF2n+2), show extremely strong O2 affinity due to their high electronegative fluorine, biocompatibility, and high stability, thus representing good oxygen carriers [44][45][44,45]. Significantly, PFCs can dissolve an enormously large amount of oxygen compared with water. For example, the molecular ratio of dissolved O2 is 5:1 in perfluorodecalin (PFD), but 1:200 in water, which means PFD shows the 1000 times increased molecular solubility compared with water [46]. In addition, it is also reported that the lifetime of singlet oxygen in PFC is longer than in the intracellular environment or in water [47]. Hence, in these years, numerous research studies have been carried out to deal with tumor hypoxia and improve PDT efficacy by introducing PFC-based O2 carriers.
Xavierselvan et al. designed nanodroplets that were composed of O2-saturated perfluoropentane, indocyanine green (ICG) that has peak light absorption in the NIR range, as well as benzoporphyrin derivative (BPD). This research showed the 9.1-fold increase in oxygen after the administration and irradiation of nanodroplets, proving the outstanding oxygen supply capability to the hypoxia site. Both in vitro and in vivo murine tumor model studies conducted in this research suggested that these PFP containing nanodroplets loaded with photosensitizer have shown to be able to enhance photoacoustic contrast, deliver oxygen, combat hypoxia, and increase PDT efficacy [33].
Although ICG can be utilized for deep tissue penetration, fluorescence quenching may occur due to the aggregation and short time of blood circulation. To solve this obstacle, Fang et al. has constructed an oxygen delivery nanoprobe, HSA-ICG-doped perfluorotributylamine, coated by the cancer cell membrane (CCm–HSA–ICG–PFTBA), to target and alleviate the hypoxia in tumor tissues, as shown in Figure 3a. Human serum albumin (HSA) was used to stabilize the photosensitizer ICG and prolong the circulation time. The coating with cancer cell membrane could stabilize nanoparticles and had the ability to perform homologous targeting. The in vivo 18F-fluoromisonidazole (18F-FMISO) positron emission tomography/computed tomography (PET/CT) imaging was performed in this research through the scheme in Figure 3c. The imaging and quantitative analysis results (Figure 3b,e) showed that the value of SUVmax significantly decreased after the treatment of CCm–HSA–ICG–PFTBA. Moreover, the ex vivo immunofluorescence staining imaging results shown in Figure 3d,f indicated that the hypoxia area was prominently reduced from 87.4% to 8.3% after the injection of the nanosystem. These results indicated the high oxygen level delivers the capability of PFTBA, relieving tumor hypoxia and improving PDT efficacy [34].
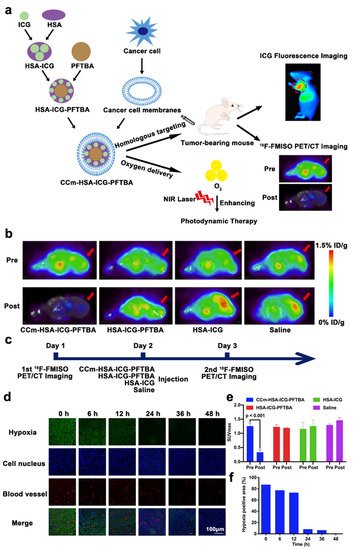
Figure 3. Summary of the design of CCm–HSA–ICG–PFTBA and the results of hypoxia improvement at tumor sites. (a) Illustration of CCm–HSA–ICG–PFTBA for homologous targeting and improving oxygen concentration at tumor sites. (b) In vivo transverse 18F-FMISO PET/CT images of TNBC xenografts before and after the injection of the CCm–HSA–ICG–PFTBA, HSA–ICG–PFTBA, HSA–ICG, and saline. (c) Scheme of the PET/CT imaging. (d) Immunofluorescence images of tumor slices stained by the hypoxyprobe. (e) The quantitative analysis of SUVmax at tumor sites in the pre and post 18F-FMISO PET/CT imaging. (f) Quantification of tumor hypoxia densities for different time points. Adapted with permission from Ref. [34]. Copyright 2021, BioMed Central Ltd.
2.3. O2 Carriers Based on Metal-Organic Frameworks
Metal–organic frameworks (MOFs) are composed of metal ions coordinating with organic linkers via coordination bonds. MOFs have been widely investigated in the application of sensors, catalysis, and storage due to their porous network, which is capable of trapping and loading diverse molecules and compounds via covalent or noncovalent interactions [48][49][48,49]. In recent years, MOFs have been applied in cancer therapy and showed various advantages, such as increased drug loading capability due to the large surface areas and high porosities, as well as high biodegradability because of the weak coordination bonds [50]. By changing the pore geometry and size of MOFs, MOFs which possess oxygen storage ability have also been synthesized [51]. Therefore, MOF-based O2 carriers have aroused attractive attention to supply sufficient O2 to tumors, overcoming hypoxia.
The Cu-based MOFs possess high O2 storage capability owing to the coordinatively unsaturated Cu sites [51]. Therefore, Cai et al. designed an O2-loaded CuTz-1@F127 MOF therapeutic system (CuTz-1-O2@F127), which is biocompatible and biodegradable. As a light-activated photosensitizer, the CuTz-1 MOF could generate hydroxyl radicals and O2 in the presence of H2O2 through type I PDT. The in vitro study revealed that at standard atmospheric pressure and room temperature, the oxygen-carrying capacity was adsorbed up to 400 μmol O2 g−1 of CuTz-1-O2@F127 (12.8 μg of O2 per 1 mg,), which is much higher than the previously mentioned SPN-Hb@RBCM NPs. The CuTz-1@F127 could then deliver O2 into cancer cells, thus alleviating intracellular hypoxia. At the same time, the CuTz-1@127 could absorb overexpressed intracellular glutathione (GSH), which would significantly reduce the cytotoxicity of ROS during the treatment of cancers. The cell study showed that the cell viability of the group treated with CuTz-1@127 reduced to approximately 20%, while the cell viability was 100% for the control group. Thus, in this research, the efficiency of PDT was greatly enhanced both through the increased O2 supply and reduced level of GSH [37].
In addition, a zirconium (IV)-based MOF, UiO-66, has been widely used for O2 storage due to its tunable pore sizes and high specific surface areas [51]. Inspired by this, Gao et al. fabricated a biomimetic O2-evolving PDT nanoplatform (O2@UiO-66@ICG@RBC), where the ICG was anchored on the surface of the UiO-66 and the NPs were encapsulated by RBC membranes. Under the 808 nm laser irradiation, ICG could generate singlet oxygen through energy transfer and thus degrade RBC membranes. The oxygen stored by UiO-66 would be released by the heat converted from NIR light, overcoming hypoxia. In this research, PA imaging was used to evaluate the ability of O2@UiO-66@ICG@RBC to induce oxygenation by measuring oxygenated hemoglobin (HbO2). The results showed that the signal intensity of HbO2 was significantly increased in the group treated with O2@UiO-66@ICG@RBC, while the signal intensity was not obvious for the groups treated with UiO-66@ICG@RBC or saline. The in vivo PDT also showed efficient tumor growth inhibition owing to the excellent tumor accumulation and oxygen capacity of O2@UiO-66@ICG [38].
Despite the fact that a great number of excellent research has been carried out successfully to develop the NPs to carry oxygen, the ratio of the amount of PSs to the amount of transported O2 is not given in these studies. This value should be studied and included in future O2-carrying PDT research, as it is an essential parameter to suggest the efficiency of the PS in transporting O2 in order to alleviate the hypoxia. In addition, the biocompatibility and biosafety of MOFs should be carefully assessed for clinical application, as some metal ions may have harmful effects on human bodies.
