Thermo-electrochemical cells (also known as thermocells; TECs) represent a promising technology for harvesting and exploiting low- grade waste heat (<100–150 ° 100-150ºC) ubiquitous in the modern environment. Based on temperature- dependent redox reactions and ion diffusion, emerging liquid-state thermocells convert waste heat energy into electrical energy, generating power at low costs, with minimal material consumption and negligible carbon foot-print. Recent developments in thermocell performances are reviewed in this article with specific focus on new redox couples, electrolyte optimisation towards enhancing power output and operating temperature regime, use of carbon & other nanomaterials for producing electrodes with high surface area for increasing current density and device performance. Highest values of output power and cell potentials have been achieved for the redox ferri/ferrocyanide system and Co2+/3+, with great opportunities for further development in both aqueous and non-aqueous solvents. New thermoelectric applications in the field include wearable and portable electronic devices in the health and performance monitoring sectors, ; using body heat as a continuous energy source of energy etc. , thermoelectrics are being employed for long-term, continuous powering of these devices. Energy storage in the form of micro supercapacitors and in lithium ion batteries is another emerging application. Current thermocells still face challenges of low power density, conversion efficiency and stability issues. For waste heat conversion (WHC) to partially replace fossil fuels as an alternative energy resource, power generation needs to be commercially viable, cost and cost-effective and sustainable. Achieving greater power density and operations at higher temperatures will require extensive research and significant developments in the field.
- Thermo-electrochemical cells
- energy harvesters
- low grade waste heat
- wearable electronics
- micro supercapacitors
- thermoelectrics
[1]1. Introduction
2. Redox Couples and Electrolytes
2.1. Redox Couples
2.2. Electrolytes
3. Electrode Materials and Designs
Additional electrode designs: The flconvexible nature of nano-carbons has led to new thermocell designs suitable for wrapping around curved or irregularly shaped surfaces such as vehicle exhaust pipes, cooling/heating lines inrsion of energy from primary energy sources to their final use is accompanied by several losses in the form of waste heats. Forman et al. [1] have estimated the waste heat potential in transport, commercial, industrial and residential sectors. Nearly 72% of the primary energy consumed was found to be lost as waste heat, of which ~63% of waste heat streams occurred at temperatures below 100ºC. In the USA, conventional industries and power plants. Hu et al. [81] used two layers of Nomex HT 4848 to separate two MWNT bucky paper electrodes at a distance of 2mm; these scrolled electrodes increase are known to annually waste over 8,000 TWh as low-grade heat [2]. This ubiquitous waste heat energy in the form of vehicle exhaust, industrial waste heat, geothermal heat, body heat etc. is found distributed almost everywhere. However, vast amounts of low-grade heat are mostly discarded and rarely exploited commercially due to their intrinsic low temperatures, space-time variations and the relative lack of cost-effective and efficiency of the cell threefold to 1.4% relative to a Carnot engint energy recovery technologies [3]. Renewable energies such as solar, wind, nuclear etc., which have a negligible carbon foot-print as compared to non-renewable fossil fuels, [4] are seeing a great resurgence.
Thransformatin coin like thermocells haveon of thermal gradients into electrode potentials for generating electricity has been developed using chemical vapor deposition by producing a catalyst with layers of Ti (30 nm), Al (10 nm) and Fe (2nm) and using it to grow 100µm tall MWNT forests oinvestigated for a long time [5]. The generation of electric potential in the presence of a temperature gradient between different electrical conductors/or semiconductors was discovered by Thomas Johann Seebeck in 1821. Seebeck coefficients, representing the potential difference generated per unit temperature difference, are typically in the order of few µV/K for devices based on semiconductor materials [6]. Early research was mainly in the thermocell casing. Nomex HT 4848 separators impregnated with 0.4 M ferri/ferro cyanide solution kept the field of solid-state thermoelectrics ‘thermoelectric generators (TEG)’ consisting of n- and p- type semiconductors connected in series as modules and then connecting a number of modules in parallel between the heat source and a cold sink. Under the influence of thermal gradients, mobile charge carriers ‘electrons/holes’ diffused from the hot electrodes apart; an aerial power density of 0 to the cold building up charges and a small potential difference [7].98
Early W/m2studies, wthas obtained for DT of 60ºC [30]. Stackedt later led to the thermo electrode configuration using SWNT and rGO (reduced graphene oxide) (in 9:1 proportion) consisting of layers of stainless-steel mesh as separators between 4.5µm electrode films maintaining a conductive path between individual films. A 10-stack configuration attained an efficiency of 2.63 % relativechemical converters, were used for applying thermal corrections to electrochemical processes in the field of current sources and in the production of galvanic coatings. However, the gradual dissolution of anodes was found to limit their commercial application [8]. Landry [9] suggested the integration of low and high temperature processes to minimize heat losses. Wakao and Nozo [10] developed a process for recovering thermal energy from low-level heat generated during the mixing of nitric acid with water. The advent of nano-structured thermoelectrics has led to a Carnot engine. Marquardt et al. [85] have reported on a great deal of interest in waste heat conversion (WHC) to electricity [11]. Although early research was dominated by thermocell based on a proton exchange membrane with hydrogen electrodes; maximum open circuit voltage with a power density of 45:3 mW/celectrics, a number of articles have been published in leading journals on new types of WHC devices using a wide range of phenomena. These include: thermomagnetic generators [12], ionic heat to electricity conversion [13], thermo-osmotic system2s [2], wliquid-stas observed at humidifierte thermocells [14], high temperature of 323K. A Seebeck coefficient of 1.75 mV/K was observpyroelectric systems [15,16], organic Rankin cycle [17] for applications in industries, construction, transportation, and energy sectors. These devices are used for DT of 35ºC.
- Emerging applications
Tharvesting energy from a heat conversion of waste heat to electrical energy is finding application in severalsources such as automotive exhaust systems [18], fuel cells [19], hypersonic engines [20] etc. Such energy harvesting can reduce the charging requirements on batteries while eliminating wired power connections. Most R&D in this fields. Some of the emerging applicati is focussed on developing better n-type and p-type thermoelectric materials.
Thermonelectrochemical (TEC) cells are presented in this section. While some of these are stand alone on TE effect, others areor ‘thermocells’ is an alternative device design based on redox-active electrolytes which can produce much higher potential differences (mV/K vs µV/K for solid-state semiconductor devices). A thermocell has two identical electrodes in conjunctiontact with other technologiesa redox couple electrolyte in the cell and an external connection [21].
Wearable and portable devices: W Under arable electronic devices are gaining attention in the health and performance monitoring sectors towards long-term, thermal gradient, the redox reaction causes the oxidation of the redox couple at the anode and reduction at the cathode. The reduced species are transported back to the anode through diffusion, convection and migration in the electrolyte creating a continuous, self-powered operations using human body as a reaction and current flow. Within the degradation constraints of cell materials, thermocells are capable of continuous supply of energy. Harvesting of body hely generating electricity without consuming materials or producing emissions.
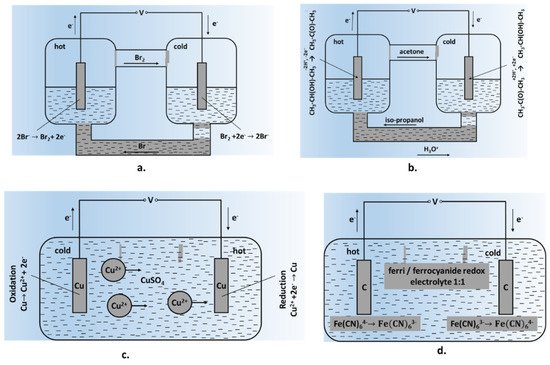
A significant in wearable devices has been investigated extensively as step in the development of thermo-electrochemical systems was the study of TEC cells based on inert electrodes, where the reactions at the electrodes occurred with the release of gaseous products (HNO3 → NO, N2O4 and HNO2; Br2 → Br-); the released volternative to bulky batteries that requiatile products later condensed at the counter electrode as the reverse reaction [22–23]. Representative examples of such thermocells are shown in Fig. 1. Fig. 1a shows a KBr/Br2 based frequent chaTEC cell, where the intercalation of Br2 gas ing or replacement [86,87] . Asto graphite electrodes plays a key role in the cell operation. At the core bodyld electrode (cathode), Br2 intemperature is regulated at 37°C, body heat can be a contrcalates into graphite, picks up electrons and dissociates into Br- ionuous source of energy; the total heat dis. These ions diffuse through the bridge to the hot electrode (anode) and transform into Br2 gas releasipng 2e-. These electrons tratvedl from the human body can range between 60 tanode to the cathode in the outer loop completing the circuit. Fig. 1b shows 180W depending on the activity levela cell based on organic acetone-iso-propanol thermocouple. Reaction at the anode involves: CH3-CH(OH)-CH3®CH3-C(O)-CH3 [88].+H2 As+2e-, oanly a small frd a reverse reaction of body surface can be covered by TEG devices, overall efficiencies are likely to be quite small. Of the several approaches used for ccurs at the cathode. Such processes allow relatively long and continuous generation of potential difference and provide higher output power values. However, these systems were unstable, and their operation is accompanied by the release of toxic products such as bromine, hydrogen or nitrogen oxides.
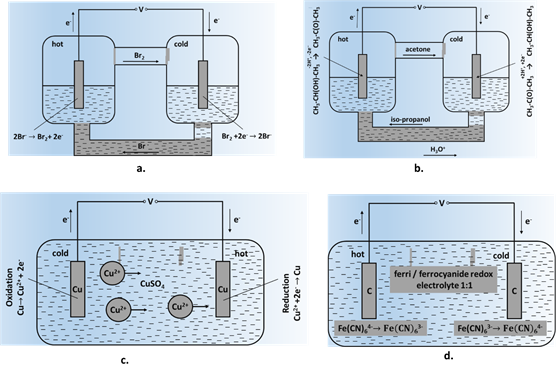
Fig. 1: Schemarvestingtic representations: (a) KBr/Br2-based TEC cell, (body hea) acetone-isopropanol thermocell, (c) Cu2+/Cu system in CuSO4 soluch astion with soluble electrostatic, piezoeldes, (d) a ferrite/ferrocyanide redox couple ([Fe (CN)6]3-/ [Fec (CN)6]4-) with ineric,t electromdes.
In this agnertic, pyroelectric and thermoelectric effects, we willle, we present an in-depth overview on the advances made in TEC design and performances focus our attention on thermoelectric mechanisms [89]. Wearable electronic devices find application in medical devices (real time monitoring, blood pressure sensors, ear-ware), smart watches, sportswear, wristbands, flexible devices for monitoring non-flat surfacesing of different aspects, new growth areas and emerging concepts in the choice of redox couples, electrolytes, electrode design and configurations. The article is organized as follows. A brief summary of historical developments in the field is provided in Section 2. Sections 3 and 4 highlight developments in redox couples, electrolytes and electrodes spanning several research directions. Emerging applications of thermocells in the fields of wearable and portable devices, energy storage and associated applications are presented in Section 5. The article concludes with highlighting economic concerns and future perspectives.
2. Historical Background
Some of the early generatin industrial applications [90].
Aon TEC cells involved the gradual dissolution of electrodes limoiting the well-known thermoelectric materialsir long term prospects. One such example is shown in Fig. 1c, allcoys of bismuth telluride with annsisting of soluble metal (Cu) electrodes placed in CuSO4 solutimony and selenium have been investigated extensively for room at two different temperatures. Such a cell will work till a certain degree of electrode consumption, then require a reversal of temperature ogradient for operations. (Bng ixSb1-x)Tn the3 alloppoy system is a p- type semiconductor legs with ZTsite direction. A significant development was the use of inert electrodes providing surfaces for released gaseous products such as NO/N2O4/HNO2 or Br-; the releanging between 1-2; Bi2Tsed volatile products were conde3-xSnsed x is ant n-type semiconductor legs with ZT around 1 [91–93]. An array of p- and n-type semiconductor legs are arranged in a thermoelectric module and arethe counter electrode, where the reverse reaction occurred [24]. While such processes permitted relatively long and continuous generation of the potential difference as well as higher output power values, these systems had limited stability along with the continuous release of noxious gases. The next stage involved the utilization of redox electrically connected in series, and then thermally connected in parallel towards greater overall efficiency [94]. Aolytes, providing relatively fast kinetics of the electrode process and high values of the hypothetical Seebeck coefficient. Systems based on potassium hexacyanoferrates/ferrites were the most widespread [25]; a schematic representation of a typical TEG circuitthis cell is given in Fig. 1d. As this syste form of a wrist band is shown in Fig. 3 fm has a negative Seebeck coefficient (-1.4 mV/K), oxidation takes place at the hot electrorde harvesting body heat.
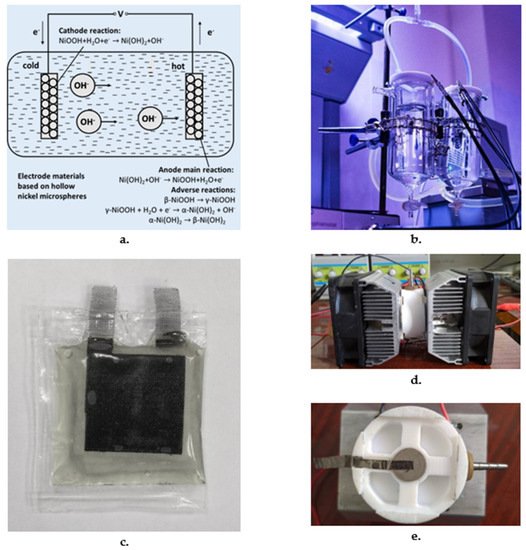
Fig.and reduction at the cold electrode. 3. A schematic repret the hot electrode, Fe (CN)64- loses antation of a TEG electron and transforms into Fe (CN)63- wrhich dist band for harvesting body heaffuses back towards the cold electrode. At the cold elect
In rigiod TEGe, Fe (CN)63- picks, n- and p-legs and interup an electron to transform into Fe(CN)64-, whicoh thennects are typically affixed to a thermally conductive ceramic substrate (alumina or aluminum nitride). In flexible TEGs, Gallium-Indium diffuses towards the hot electrode thereby setting up a perpetual motion of ions and the generation of electric current. It can be seen that the electrodes remained inert during the process and were not consumed. There has been a significant progress in the cell design and yields since early studies on this system.
Several otheutectic alloyr types of thermoelectric cells have been used as flexdeveloped. These are briefly listed below:
- TEC cells with phase transitions during mass transfer have been studied for a relatively long time (first publications dating back to the 1970s), but these have not found practical application due to the complexity of design and rapid degradation of components. Wang et al. [26] have recently developed electrochemical sodium heat engines based on phase change reactions. Nevertheless, their important advantage is the continuous operation, which does not require changing hot and cold sources.
- Typical TECs are non-isothermal electrochemical cell systems consisting of two electrodes, electrolyte and a separator. The conversion of thermal energy into electrical energy involves electrode kinetics, thermodynamics, heat and mass transfer. TEC cells involve changes in the aggregate state of oxidized and reduced forms (nitric acid and nitrogen oxides or KBr and Br2) during continuous operation.
- Thermally regenerative electrochemical cycles (TREC) consist of two electrodes with opposite thermopowers; anodes and cathodes generally have positive and negative thermopowers respectively. For negative thermopower, the cycle involves cooling, discharging, heating and charging; for positive thermopower, the cycle involves heating, charging, cooling and discharging [27].
- Other cell configurations include thermogalvanic cells (TGC) based on soluble, reversible metal electrodes in solutions of their own salts at different temperatures; TECC cells use inert electrodes placed in a redox electrolyte or ionic liquid; TGC-Li cells based on Li+/Li redox system; TRABs (thermally regenerative ammonia-based batteries), DTCCs (direct thermal charging cells) etc. [28-31].
3. Electrode materials and design
Due to their non-reactive and thermally stable liquid-metal interconnects to ascertain integrity during openature, platinum electrodes were used during early studies on thermocells. The electrolyte had to be stirred continuously to achieve Carnot efficiencies of 1.2% [32]. High power generation in thermocells requires large current densities, which may be achieved through high concentration; Kapton HN and polydimethylsiloxane are ts of redox mediators, increasing the number of sites for redox reactions and exposing electrodes to large thermal gradients. Different types of thermocell electrodes and materials are detailed next.
Reversible metal electrodes: Thermocells two most common substrates [95]using ‘reversible electrodes’ consist of soluble metal electrodes placed in solutions of corresponding salts at two different temperatures (Fig. 1C). Typhical power outputs are found to range between 2 to 8.75µW/cm2s cell can only work till a certain level of electrode consumption, the direction of the temperature gradient is then reversed for the cell to [96].work Liu et al [97]in the opposite direction. Most of the studies have presentedbeen reported on the Cu2+/Cu systeveral differentm due to simple designs for wearable device and good reproducibility. Seebeck coefficients for harvesting body heat in a range of applications.
Orgthis system could be modified from hypothetical values of 0.879 mV/K to 0.75 - 1.32 mV/K by changing experimental conditions anid elec materials have also been investigated as flexible TEGs due to lower prices, light weight, low thermal conductivity, and high flexibilitytrolyte additives [33]. Burmistrov et al. [34] have reported on the efficiency of thermocells based on copper, zinc and nickel metallic electrodes. While the efficiency parameters for Cu electrodes agreed well with the theoretical data and published results, the efficiency of zinc-based TEC cells was somewhat lower than found to be theoretical expectations. The thermogalvanic system with nickel electrodes in an aqueous solution of nickel sulfate exhibited an unusual behavior.
Inert carbon electrodes: Carbon-based Whiele significant progress has been made, high performctrodes are becoming increasingly important as a promising and affordable alternative to platinum electrodes. Nanostruce organic TEGs still lag behind inorganic chalcogenides BiTe-based alloys. One of the stratetured carbon materials, such as single (SWNT) and multiwalled carbon nanotubes (MWNT), graphene etc., have a large surface area, that helps in increasing the number of reaction sites. Depending on the number of nanotube walls, the specific surface area of CNTs lies in the range 50–1315 m2/gi [35]. Thes is to fabricate flexible TE devices from composites of organic and polymee materials also show fast electron transfer kinetics for the ferrite/ferrocyanide redox couple. Both of these properties can increase the current density achieved with the thermocouple [36].
Hollow nickel microspheres electrodes: Burmic materials withstrov et al. [37] have shown that hollow Ni micro/nanospheres can be an effective electrode materials [98]. The TE properti for thermoelectrochemical cells and provide extremely high values of several p-type and n-type coordination polymers have been tabulated by Masoumi et al. [9the hypothetical Seebeck coefficient and open circuit voltage. Electrodes were prepared by pressing tablets of nickel microspheres and reduced at 250 °C. Most effective composition of KOH based alkaline electrolyte was chosen based on the influence of alkali content on the hypothetical Seebeck coefficient value and specific power as determined by the number of charge carriers in the electrolyte and the intensity of reactions at the electrodes [38].
4. Emerging Applications
The π conjugated conducting polymers, are another group of oversion of waste heat to electrical energy is finding application in several fields. Some of the emerging applications are presented in this section. While some of these are stand alone on TE effect, others are in conjunction with other technologies.
Wearable and portable devices: Wearganic TEs, with a capacity for doping with a wide variety of elements and adjustable doping levels [95]. Some of key conducting polymers include polyacetylene, polyvinylidene fluoride, polyphenylenevinylene, poly(3-hexylthiophene), polypyrrole etc. A power factor of 401 µW/ mK2 ble electronic devices are gaining attention in the health and performance monitoring sectors towards long-term, continuous, self-powered operations using human body as a continuous supply of energy. Harvesting of body heat in wearable devices has been investigated extensively as an alternative to bulky batteries that require frequent charging or replacement [39, 40] . As the core body temperature is regulated at 37°C, body heat can be a continuous source of energy; the total heat dissipated from the human body can range between 60 to 180W depending on the activity level [41]. As only a small fraction of body surface can be covered by TEG devices, overall efficiencies are likely to be quite small. Of the several approaches used for harvesting body heat such as electrostatic, piezoelectric, electromagnetic, pyroelectric and thermoelectric effects, we will focus our attention on thermoelectric mechandisms [42]. a Seebeck coefficient of 43.5 µV/K has been achieved with some conducting polymers [99Wearable electronic devices find application in medical devices (real time monitoring, blood pressure sensors, ear-ware), smart watches, sportswear, wristbands, flexible devices for monitoring non-flat surfaces in industrial applications [43].
In Other TE materials include carbon nanotubes, graphene, fullerenes, carbon nanodots, small molecules, organic coigid TEGs, n- and p-legs and interconnects are typically affixed to a thermally conductive ceramic substrate (alumina or aluminum nitride). In flexible TEGs, Gallium-Indium eutectic alloys have been used as flexible liquid-metal interconnects to ascertain integrity during operation; Kapton HN and polydimethylsiloxane are the two most common substrates [44]. Typical power outputs are found to range between 2 to 8.75µW/cm2 [45]. Liu et al [46] have poresites with inorganic fillers etcented several different designs for wearable devices for harvesting body heat in a range of applications. [94].
Energy storage devices: The integration of energy-harvesting and storage devices has been extensively investigated for emerging self-powered electronic devices [97]. Thermoelectric generators can be used to convert excess heat generated during the operation of electronic devices into electricity [93]. Using Soret effect, ionic thermoelectric supercapacitors utilize ionic electrolytes to produce charges and energy storage in a single device; the operation of these devices is however limited by long charging and discharging times and a rigid configuration [10047]. Yang et al. [10148] have reported on a TEG device with n- and p- type modules consisting of Ag2Te and Ag2Se nanoparticle thin films. This device was directly linked with a planar micro supercapacitor. A Seebeck voltage of 82 mV was generated for DT of 15.8 K and a charging efficiency of 98%. Wu et al. [102] have reported on novel conjugated conducting polymer (PDAQ-BC) [DAQ: 2,6-diaminoanthraquinone; DBC: 3,6-dibromo-9-(4-bromophenyl)carbazole], which showed a specific capacitance of 180.5 F/g for a current of 1A/g. This polymer retained up to 95% coulombic efficiency and 85% capacitance after 5000 cycles of operation.
Park et al. [10349] have reported an all-in-one energy system consisting of a TEG on one side of the substrate and a micro supercapacitor (MSC) on the other side. The TEG was constructed from screen printed p- and n- modules and a p- type TE film for alignment with electrodes. An MSC was fabricated on the other side using rGO/CNT electrodes and Au current collectors. Electrodes were positioned above and below the TEG-substrate-MSC system; the electrode on the TEG side was connected externally to the current collector of MSC. This system was able to generate 10.8V of electrical energy for thermal differences up to 10K and store it without loss.
Liu et al. [107] have reported on coupling thermoelectricity and electrocatalysis for hydrogen production via PbTe-PbS/TiO2 heterojunction involving a cathode, an anode and electrolyte operating at the hot-end and in-situ endothermic production of electrochemical hydrogen on the cold-end. At 70ºC and 1.0V bath voltage, hydrogen was generated at the rate of 6.1mL/cm2/h with energy and heat efficiencies reaching 88% and 49.9% respectively. Liu et al. [108] have reported on an advanced Li ion battery with charging based on a coupled thermoelectric approach. Richards et al. [109] have explored solid-state electrochemical heat engines (EHE) for generating electric power from available thermal energy based on reversible redox reactions. EHEs control and utilize the electrochemical potential of molecules undergoing redox reactions with temperature, composition and pressure playing an important role.
5. Conclusions and future perspectives
Liao et al. [110] have reported on a hybrid active/passive battery thermal management system in combination with thermoelectric elements (TEE) and phase change materials for the management of Li-ion batteries operating in extreme environments. TEEs were used to provide refrigeration at high temperatures and heating to preheat the batteries in cold environments. Nguyen and Shabani [111] have discussed about capturing the heat produced by proton exchange membrane fuel cells, heat recovery solutions and opportunities for integration with TEGs and thermally regenerative electrochemical cycles for power cogeneration applications.
- Conclusions and future perspectives
Development of thermoelectrochemical technologies for harvesting waste low-temperature heat opens the prospect of increasing the efficiency of various devices and mechanisms operating in exothermic mode or creating systems for generating electricity based on natural heat sources. Any process that can partially replace fossil fuels as a prime energy source will be used only if it is attractive to industry; an alternative energy source such as waste heat conversion (WHC) has to cost effective to becoming commercially viable. Geoffroy et al. [11250] have shown that current WHC heat engines are not economically viable below 100ºC and require temperatures above 150ºC coupled with 100-1000 kW power outputs to be economically competitive. Studies in recent years have shown the possibility of significant increases in the power and conversion efficiency of TEC cells. Highest values of output power and cell potentials have been achieved for the redox ferri/ferrocyanide system and Co2+/3+, which offers great opportunities for further development and research in both aqueous and non-aqueous solvents.
Achieved results show the pathways to overcome key fundamental limitations of thermocell performance and set new tasks for fundamental research and further development of electrodes, electrolyte materials and cell design. One of the key tasks in thermocells development is to investigate mechanisms of entropy change via new redox couples and electrolytes. This will be related to increasing the hypothetical Seebeck coefficient, as well as improving the properties of electrodes and solvents to increase the mass transfer rate (diffusion capacity) towards increasing exchange currents and output power values.
One of the most promising thermoelectric power generation application involves vehicle waste heat recovery to improve fuel economy, wherein waste heat from the exhaust, is redirected to produce electricity. Other applications include harvesting industrial waste heat (incinerators, cement, steel mills etc.), geothermal, fuel oil-fired furnaces or gas water-heaters with this technology. Despite extensive research, WHC technology has yet to achieve significant market penetration. For WHCs to become be a serious contender, it has to compete with solar, wind, geothermal technologies in terms of capacity factors, capital costs, operational as well as maintenance costs. Significant developments are therefore required on multiple fronts towards achieving greater power density performances especially at higher temperatures. It is possible that TECs might have major advantages over solar cells and semiconductor thermoelectrics, in particular in the Wh/dollar efficiency [81]. Achieving industrial production volumes of MWCNTs and reducing their cost, according to the author, can make TEC a competitive device.
References
- Forman, C.; Muritala, I. K.; Pardemann, R.; Meyer, B. Estimating the global waste heat potential. Renewable and Sustainable Energy Reviews 2016, 57, 1568–1579.
- Straub, A. P.; Yip, N. Y.; Lin, S.; Lee, J.; Elimelech, M. Harvesting low-grade heat energy using thermo-osmotic vapour transport through nanoporous membranes. Nature Energy 2016, 1, 1–6.
- Yang, Y.; Lee, S. W.; Ghasemi, H.; Loomis, J.; Li, X.; Kraemer, D.; Zheng, G.; Cui, Y.; Chen, G. Charging-free electrochemical system for harvesting low-grade thermal energy. Proc Natl Acad Sci U S A 2014, 111, 17011–17016.
- Evans, S. CarbonBrief/Energy, 2017. Solar, wind and nuclear have ‘amazingly low’ carbon footprints. Available online: https://www.carbonbrief.org/solar-wind-nuclear-amazingly-low-carbon-footprints/#:~:text=Simon%20Evans,-08.12.2017%20%7C%205&text=Building%20solar%2C%20wind%20or%20nuclear,of%20electricity%20out%20to%202050. (accessed on 10 Jan. 2022).Evans, S. CarbonBrief/Energy, 2017. Solar, wind and nuclear have ‘amazingly low’ carbon footprints. Available online: https://www.carbonbrief.org/solar-wind-nuclear-amazingly-low-carbon-footprints (accessed on 10 Jan. 2022).
- COP26 Outcomes, 2021. Available online: https://ukcop26.org/the-conference/cop26-outcomes. (accessed on 10 Jan. 2022).Bouty, E. Phénomènes thermo-électriques et électro-thermiques au contact d’un métal et d’un liquide. Journal de Physique Théorique et Appliquée 1880, 9, 306–320.
- Bouty, E. Phénomènes thermo-électriques et électro-thermiques au contact d’un métal et d’un liquide. Journal de Physique Théorique et Appliquée 1880, 9, 306–320.Snyder, G. J.; Toberer, E. S. Complex thermoelectric materials. Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group 2010, 101–110.
- Snyder, G. J.; Toberer, E. S. Complex thermoelectric materials. Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group 2010, 101–110.Jangonda, C.; Patil, K.; Kinikar, A.; Bhokare, R.; Gavali, M. D. Review of various application of thermoelectric module. Intl. J. Innovative Research in Science, Engineering and Technology 2016, 5, 3393.
- Jangonda, C.; Patil, K.; Kinikar, A.; Bhokare, R.; Gavali, M. D. Review of various application of thermoelectric module. Intl. J. Innovative Research in Science, Engineering and Technology 2016, 5, 3393.deBethune, A. J.; Licht, T. S.; Swendeman, N. The Temperature Coefficients of Electrode Potentials. Journal of The Electrochemical Society 1959, 106, 616.
- Yang, X.; Wang, C.; Lu, R.; Shen, Y.; Zhao, H.; Li, J.; Zheng, X; Progress in Measurement of Thermoelectric Properties of Micro/Nano Thermoelectric Materials: A Critical Review. Nano Energy 2022, 107553.Landry, B. A. Utilization of waste heat. Science 1953, 3, 3.
- Chen, X. Q.; Fan, S. J.; Han, C.; Wu, T.; Wang, L. J.; Jiang, W.; Yang, J. P. Multiscale architectures boosting thermoelectric performance of copper sulfide compound. Rare metals 2021, 40(8), 2017-2025.Wakao, N.; Nojo, K. Nitric acid cycle process for extracting thermal energy from low-level heat sources. Nature 1978, 273, 25–27.
- Chen, X.; Zhang, H.; Zhao, Y.; Liu, W. D.; Dai, W.; Wu, T.; Yang, J. Carbon-encapsulated copper sulfide leading to enhanced thermoelectric properties. ACS applied materials & interfaces 2019, 11(25), 22457-22463.Venkatasubramanian, R.; Siivola, E.; Colpitts, T.; O’Quinn, B. Thin-film thermoelectric devices with high room-temperature figures of merit. Nature 2001, 413, 597–602.
- Vining, C. B. An inconvenient truth about thermoelectrics. Nature Materials 2009, 8, 83–85.Waske, A.; Dzekan, D.; Sellschopp, K.; Berger, D.; Stork, A.; Nielsch, K.; Fähler, S. Energy harvesting near room temperature using a thermomagnetic generator with a pretzel-like magnetic flux topology. Nature Energy 2018, 4, 68–74.
- deBethune, A. J.; Licht, T. S.; Swendeman, N. The Temperature Coefficients of Electrode Potentials. Journal of The Electrochemical Society 1959, 106, 616.Li, T.; Zhang, X.; Lacey, S. D.; Mi, R.; Zhao, X.; Jiang, F.; Song, J.; Liu, Z.; Chen, G.; Dai, J.; et al. Cellulose ionic conductors with high differential thermal voltage for low-grade heat harvesting. Nature Materials 2019, 18, 608–613.
- Landry, B. A. Utilization of waste heat. Science 1953, 3, 3.Yu, B.; Duan, J.; Cong, H.; Xie, W.; Liu, R.; Zhuang, X.; Wang, H.; Qi, B.; Xu, M.; Wang, Z. L.; et al. Thermosensitive crystallization-boosted liquid thermocells for low-grade heat harvesting. Science 2020, 370, 342–346.
- Wakao, N.; Nojo, K. Nitric acid cycle process for extracting thermal energy from low-level heat sources. Nature 1978, 273, 25–27.Pandya, S.; Wilbur, J.; Kim, J.; Gao, R.; Dasgupta, A.; Dames, C.; Martin, L. W. Pyroelectric energy conversion with large energy and power density in relaxor ferroelectric thin films. Nature Materials 2018, 17, 432–438.
- Venkatasubramanian, R.; Siivola, E.; Colpitts, T.; O’Quinn, B. Thin-film thermoelectric devices with high room-temperature figures of merit. Nature 2001, 413, 597–602.Thakre, A.; Kumar, A.; Song, H. C.; Jeong, D. Y.; Ryu, J. Pyroelectric Energy Conversion and Its Applications—Flexible Energy Harvesters and Sensors. Sensors 2019, Vol. 19, Page 2170 2019, 19, 2170.
- Waske, A.; Dzekan, D.; Sellschopp, K.; Berger, D.; Stork, A.; Nielsch, K.; Fähler, S. Energy harvesting near room temperature using a thermomagnetic generator with a pretzel-like magnetic flux topology. Nature Energy 2018, 4, 68–74.Quoilin, S.; Broek, M. van den; Declaye, S.; Dewallef, P.; Lemort, V. Techno-economic survey of Organic Rankine Cycle (ORC) systems. Renewable and Sustainable Energy Reviews 2013, 22, 168–186.
- Li, T.; Zhang, X.; Lacey, S. D.; Mi, R.; Zhao, X.; Jiang, F.; Song, J.; Liu, Z.; Chen, G.; Dai, J.; et al. Cellulose ionic conductors with high differential thermal voltage for low-grade heat harvesting. Nature Materials 2019, 18, 608–613.Zhang, X.; Chau, K.T. An automotive thermoelectric photovoltaic hybrid energy system using maximum power point tracking. Energy Convers Manag 2011, 52(1), 641e7.
- Yu, B.; Duan, J.; Cong, H.; Xie, W.; Liu, R.; Zhuang, X.; Wang, H.; Qi, B.; Xu, M.; Wang, Z. L.; et al. Thermosensitive crystallization-boosted liquid thermocells for low-grade heat harvesting. Science 2020, 370, 342–346.Zhang, H.; W Kong, W.; Dong, F.; Xu, H.; Chen, B.; Ni, M. Application of cascading thermoelectric generator and cooler for waste heat recovery from solid oxide fuel cells. Energy Convers Manag 2017, 148, 1382-1390.
- Pandya, S.; Wilbur, J.; Kim, J.; Gao, R.; Dasgupta, A.; Dames, C.; Martin, L. W. Pyroelectric energy conversion with large energy and power density in relaxor ferroelectric thin films. Nature Materials 2018, 17, 432–438.Li, P.; Cai, L.; Zhai, P.; Tang, X.; Zhang, Q.; Nino, M. Design of a concentration solar thermoelectric generator. J Electron Mater 2010, 39(9), 1522-1530.
- Thakre, A.; Kumar, A.; Song, H. C.; Jeong, D. Y.; Ryu, J. Pyroelectric Energy Conversion and Its Applications—Flexible Energy Harvesters and Sensors. Sensors 2019, Vol. 19, Page 2170 2019, 19, 2170.Dupont, M. F.; MacFarlane, D. R.; Pringle, J. M. Thermo-electrochemical cells for waste heat harvesting – progress and perspectives. Chemical Communications 2017, 53, 6288–6302.
- Quoilin, S.; Broek, M. van den; Declaye, S.; Dewallef, P.; Lemort, V. Techno-economic survey of Organic Rankine Cycle (ORC) systems. Renewable and Sustainable Energy Reviews 2013, 22, 168–186.Lalancette, J.-M.; Roussel, R. Metals intercalated in graphite. V. A concentration cell with intercalated bromine. Canadian Journal of Chemistry 1976, 54, 3541–3544.
- Zhang, X.; Chau, K.T. An automotive thermoelectric photovoltaic hybrid energy system using maximum power point tracking. Energy Convers Manag 2011, 52(1), 641e7.Burmistrov, I.; Gorshkov, N.; Kovyneva, N.; Kolesnikov, E.; Khaidarov, B.; Karunakaran, G.; Cho, E. B.; Kiselev, N.; Artyukhov, D.; Kuznetsov, D.; et al. High seebeck coefficient thermo-electrochemical cell using nickel hollow microspheres electrodes. Renewable Energy 2020, 157, 1–8.
- Zhang, H.; W Kong, W.; Dong, F.; Xu, H.; Chen, B.; Ni, M. Application of cascading thermoelectric generator and cooler for waste heat recovery from solid oxide fuel cells. Energy Convers Manag 2017, 148, 1382-1390.Maeda, Y.; Kitamura, H.; Itoh, E.; Inagaki, M. A new carbon fiber and nitric acid cell with a temperature difference between electrodes. Synthetic Metals 1983, 7, 211–217.
- Li, P.; Cai, L.; Zhai, P.; Tang, X.; Zhang, Q.; Nino, M. Design of a concentration solar thermoelectric generator. J Electron Mater 2010, 39(9), 1522-1530.Mua, Y.; Quickenden, T. I. Power Conversion Efficiency, Electrode Separation, and Overpotential in the Ferricyanide/Ferrocyanide Thermogalvanic Cell. Journal of The Electrochemical Society 1996, 143, 2558–2564.
- Dupont, M. F.; MacFarlane, D. R.; Pringle, J. M. Thermo-electrochemical cells for waste heat harvesting – progress and perspectives. Chemical Communications 2017, 53, 6288–6302.Wang, W.; Shu, G.; Tian, H.; Huo, D.; Zhu, X. A bimetallic thermally-regenerative ammonia-based flow battery for low-grade waste heat recovery. Journal of Power Sources 2019, 424, 184–192.
- Chum, H. L.; Osteryoung, R. A. Review of thermally regenerative electrochemical cells. Solar Energy Research Institute 1981.Cheng, C.; Dai, Y.; Yu, J.; Liu, C.; Wang, S.; Feng, S. P.; Ni, M. Review of Liquid-Based Systems to Recover Low-Grade Waste Heat for Electrical Energy Generation. Energy and Fuels 2021, 35, 161–175.
- Lalancette, J.-M.; Roussel, R. Metals intercalated in graphite. V. A concentration cell with intercalated bromine. Canadian Journal of Chemistry 1976, 54, 3541–3544.Hu, R.; Xu, D.; Luo, X. Liquid Thermocells Enable Low-Grade Heat Harvesting. Matter 2020, 3, 1400–1402.
- Endo, M.; Yamagishi, Y.; Inagaki, M. Thermocell with graphite fiber-bromine intercalation compounds. Synthetic Metals 1983, 7, 203–209.Black, J. J.; Murphy, T.; Atkin, R.; Dolan, A.; Aldous, L. The thermoelectrochemistry of lithium–glyme solvate ionic liquids: Towards waste heat harvesting. Physical Chemistry Chemical Physics 2016, 18, 20768–20777.
- Inagaki, M.; Matsumoto, A.; Sakai, M.; Maeda, Y. A cell of carbon-fibers and nitric acid with temperature difference. Nippon Kagaku Kaishi 1983, 309–311.Zhou, H.; Liu, P. High Seebeck Coefficient Electrochemical Thermocells for Efficient Waste Heat Recovery. ACS Applied Energy Materials 2018, 1, 1424–1428.
- Bonetti, M.; Nakamae, S.; Roger, M.; Guenoun, P. Huge Seebeck coefficients in nonaqueous electrolytes. The Journal of Chemical Physics 2011, 134, 114513.Burmistrov, I.; Artyukhov, D.; Shindrov, A.; Gorshkov N.; Gorokhovsky, A. Thermo-Electrochemical Cells for Low-Grade Waste Heat Conversion. In Nanotech Middle East 2017 Conference and Exhibition. Dubai; Dubai, 2017; pp. 4–6.
- Burmistrov, I.; Gorshkov, N.; Kovyneva, N.; Kolesnikov, E.; Khaidarov, B.; Karunakaran, G.; Cho, E. B.; Kiselev, N.; Artyukhov, D.; Kuznetsov, D.; et al. High seebeck coefficient thermo-electrochemical cell using nickel hollow microspheres electrodes. Renewable Energy 2020, 157, 1–8.Quickenden, T. I.; Mua, Y. A Review of Power Generation in Aqueous Thermogalvanic Cells. Journal of The Electrochemical Society 1995, 142, 3985–3994.
- Im, H.; Kim, T.; Song, H.; Choi, J.; Park, J. S.; Ovalle-Robles, R.; Yang, H. D.; Kihm, K. D.; Baughman, R. H.; Lee, H. H.; et al. High-efficiency electrochemical thermal energy harvester using carbon nanotube aerogel sheet electrodes. Nature Communications 2016, 7.Gunawan, A.; Li, H.; Lin, C. H.; Buttry, D. A.; Mujica, V.; Taylor, R. A.; Prasher, R. S.; Phelan, P. E. The amplifying effect of natural convection on power generation of thermogalvanic cells. International Journal of Heat and Mass Transfer 2014, 78, 423–434.
- Artyukhov D.; Kiselev N.; Gorshkov N.; Kovyneva N.; Ganzha O.; Vikulova M.; Gorokhovsky A.; Offor P.; Boychenko E.; Burmistrov I. Harvesting Waste Thermal Energy Using a Surface-Modified Carbon Fiber-Based Thermo-Electrochemical Cell Sustainability 2021, 13(3), 1377.Burmistrov, I.; Kovyneva, N.; Gorshkov, N.; Gorokhovsky, A.; Durakov, A.; Artyukhov, D.; Kiselev, N. Development of new electrode materials for thermo-electrochemical cells for waste heat harvesting. Renewable Energy Focus 2019, 29, 42–48.
- Inagaki, M.; Itoh, E.; Maeda, Y. Durable Performance of Thermocell with Carbon Cloth and Nitric Acid. TANSO 1985, 1985, 134–136.Koo, M. H.; Yoon, H. H. Fabrication of carbon nanotubes and charge transfer complex-based electrodes for a glucose/oxygen biofuel cell. Journal of Nanoscience and Nanotechnology 2013, 13, 7434–7438.
- Battistel, A.; Peljo, P. Recent trends in thermoelectrochemical cells and thermally regenerative batteries. Current Opinion in Electrochemistry 2021, 30, 100853.Nugent, J. M.; Santhanam, K. S. V.; Rubio, A.; Ajayan, P. M. Fast Electron Transfer Kinetics on Multiwalled Carbon Nanotube Microbundle Electrodes. Nano Letters 2001, 1, 87–91.
- Duan, J.; Yu, B.; Huang, L.; Hu, B.; Xu, M.; Feng, G.; Zhou, J. Liquid-state thermocells: Opportunities and challenges for low-grade heat harvesting. Joule 2021, 5, 768–779.Kiselev, N.; Artyukhov, D.; Boychenko, E.; Gorshkov, N.; Glubokaya, A.; Burmistrov, I. Electrolyte concentration dependences of NiO based thermoelectrochemical cells performance. AIP Conference Proceedings 2022, 2456, 020005.
- Disalvo, F. J. Thermoelectric cooling and power generation. Science (1979) 1999, 285, 703–706.Misra, V.; Bozkurt, A.; Calhoun, B.; Jackson, T.; Jur, J.; Lach, J.; Lee, B.; Muth, J.; Oralkan, O.; Ozturk, M.; et al. Flexible technologies for self-powered wearable health and environmental sensing. Proceedings of the IEEE 2015, 103, 665–681.
- Abraham, T. J.; Macfarlane, D. R.; Baughman, R. H.; Jin, L.; Li, N.; Pringle, J. M. Towards ionic liquid-based thermoelectrochemical cells for the harvesting of thermal energy. Electrochimica Acta 2013, 113, 87–93.Ando Junior, O. H.; Maran, A. L. O.; Henao, N. C. A review of the development and applications of thermoelectric microgenerators for energy harvesting. Renewable and Sustainable Energy Reviews 2018, 91, 376–393.
- Romano, M. S.; Razal, J. M.; Antiohos, D.; Wallace, G.; Chen, J. Nano-carbon electrodes for thermal energy harvesting. Journal of Nanoscience and Nanotechnology 2015, 15, 1–14.Riemer, R.; Shapiro, A. Biomechanical energy harvesting from human motion: Theory, state of the art, design guidelines, and future directions. Journal of NeuroEngineering and Rehabilitation 2011, 8, 1–13.
- He, J.; Al-Masri, D.; MacFarlane, D. R.; Pringle, J. M. Temperature dependence of the electrode potential of a cobalt-based redox couple in ionic liquid electrolytes for thermal energy harvesting. Faraday Discussions 2016, 190, 205–218.Invernizzi, F.; Dulio, S.; Patrini, M.; Guizzetti, G.; Mustarelli, P. Energy harvesting from human motion: materials and techniques. Chemical Society Reviews 2016, 45, 5455–5473.
- Kang, T. J.; Fang, S.; Kozlov, M. E.; Haines, C. S.; Li, N.; Kim, Y. H.; Chen, Y.; Baughman, R. H. Electrical Power From Nanotube and Graphene Electrochemical Thermal Energy Harvesters. Advanced Functional Materials 2012, 22, 477–489.Llamas R. IDC Media Center. Worldwide Wearables Market to Nearly Double by 2021, According to IDC Internet Available online: https://www.idc.com/getdoc.jsp?containerId=prUS42818517 (accessed 22.11. 2021).
- Zhang, L.; Kim, T.; Li, N.; June Kang, T.; Chen, J.; Pringle, J. M.; Zhang, M.; Kazim, A. H.; Fang, S.; Haines, C.; et al. High Power Density Electrochemical Thermocells for Inexpensively Harvesting Low-Grade Thermal Energy. Advanced Materials 2017, 29, 1605652.Park, H.; Lee, D.; Kim, D.; Cho, H.; Eom, Y.; Hwang, J.; Kim, H.; Kim, J.; Han, S.; Kim, W. High power output from body heat harvesting based on flexible thermoelectric system with low thermal contact resistance. Journal of Physics D: Applied Physics 2018, 51, 365501.
- Maeda, Y.; Kitamura, H.; Itoh, E.; Inagaki, M. A new carbon fiber and nitric acid cell with a temperature difference between electrodes. Synthetic Metals 1983, 7, 211–217.Fan, Z.; Ouyang, J.; Fan, Z.; Ouyang, J. Thermoelectric Properties of PEDOT:PSS. Advanced Electronic Materials 2019, 5, 1800769.
- Mua, Y.; Quickenden, T. I. Power Conversion Efficiency, Electrode Separation, and Overpotential in the Ferricyanide/Ferrocyanide Thermogalvanic Cell. Journal of The Electrochemical Society 1996, 143, 2558–2564.Liu, Y.; Wang, H.; Sherrell, P.C.; Liu, L.; Wang, Y.; Chen, J. Potentially Wearable Thermo-Electrochemical Cells for Body Heat Harvesting: From Mechanism, Materials, Strategies to Applications. Adv. Sci. 2021, 8, 2100669.
- Hinterleitner, B.; Knapp, I.; Poneder, M.; Shi, Y.; Müller, H.; Eguchi, G.; Eisenmenger-Sittner, C.; Stöger-Pollach, M.; Kakefuda, Y.; Kawamoto, N.; et al. Thermoelectric performance of a metastable thin-film Heusler alloy. Nature 2019 576:7785 2019, 576, 85–90.Guan, X.; Cheng, H.; Ouyang, J. Significant enhancement in the Seebeck coefficient and power factor of thermoelectric polymers by the Soret effect of polyelectrolytes. Journal of Materials Chemistry A 2018, 6, 19347–19352.
- Li, D.; Sun, R. R.; Qin, X. Y. Improving thermoelectric properties of p-type Bi2Te3-based alloys by spark plasma sintering. Progress in Natural Science: Materials International 2011, 21, 336–340.Yang, S.; Cho, K.; Park, Y.; Kim, S. Bendable thermoelectric generators composed of p- and n-type silver chalcogenide nanoparticle thin films. Nano Energy 2018, 49, 333–337.
- Russ, B.; Glaudell, A.; Urban, J. J.; Chabinyc, M. L.; Segalman, R. A. Organic thermoelectric materials for energy harvesting and temperature control. Nature Reviews Materials 2016, 1, 1–14.Park, Y.; Cho, K.; Kim, S. Vertical all-in-one energy systems constructed with thermoelectric generators and microsupercapacitors. Journal of Power Sources 2021, 510, 230402.
- Li, M.; Hong, M.; Dargusch, M.; Zou, J.; Chen, Z. G. High-efficiency thermocells driven by thermo-electrochemical processes. Trends in Chemistry 2021, 3, 561–574.Geffroy, C.; Lilley, D.; Parez, P. S.; Prasher, R. Techno-economic analysis of waste-heat conversion. Joule 2021, 5, 3080–3096.
- Cho, C.; Stevens, B.; Hsu, J.-H.; Bureau, R.; Hagen, D. A.; Regev, O.; Yu, C.; Grunlan, J. C.; Cho, C.; Stevens, B.; et al. Completely Organic Multilayer Thin Film with Thermoelectric Power Factor Rivaling Inorganic Tellurides. Advanced Materials 2015, 27, 2996–3001.
- Wang, H.; Hsu, J.-H.; Yi, S.-I.; Lae Kim, S.; Choi, K.; Yang, G.; Yu, C.; Wang, H.; Yi, S.; Kim, S. L.; et al. Thermally Driven Large N-Type Voltage Responses from Hybrids of Carbon Nanotubes and Poly(3,4-ethylenedioxythiophene) with Tetrakis(dimethylamino)ethylene. Advanced Materials 2015, 27, 6855–6861.
- Wang, W.; Shu, G.; Tian, H.; Huo, D.; Zhu, X. A bimetallic thermally-regenerative ammonia-based flow battery for low-grade waste heat recovery. Journal of Power Sources 2019, 424, 184–192.
- Cheng, C.; Dai, Y.; Yu, J.; Liu, C.; Wang, S.; Feng, S. P.; Ni, M. Review of Liquid-Based Systems to Recover Low-Grade Waste Heat for Electrical Energy Generation. Energy and Fuels 2021, 35, 161–175.
- Hu, R.; Xu, D.; Luo, X. Liquid Thermocells Enable Low-Grade Heat Harvesting. Matter 2020, 3, 1400–1402.
- Black, J. J.; Murphy, T.; Atkin, R.; Dolan, A.; Aldous, L. The thermoelectrochemistry of lithium–glyme solvate ionic liquids: Towards waste heat harvesting. Physical Chemistry Chemical Physics 2016, 18, 20768–20777.
- Zhou, H.; Liu, P. High Seebeck Coefficient Electrochemical Thermocells for Efficient Waste Heat Recovery. ACS Applied Energy Materials 2018, 1, 1424–1428.
- Burmistrov, I.; Artyukhov, D.; Shindrov, A.; Gorshkov N.; Gorokhovsky, A. Thermo-Electrochemical Cells for Low-Grade Waste Heat Conversion. In Nanotech Middle East 2017 Conference and Exhibition. Dubai; Dubai, 2017; pp. 4–6.
- Sahami, S.; Weaver, M. J. Entropic and enthalpic contributions to the solvent dependence of the thermodynamics of transition-metal redox couples: Part II. Couples containing ammine and ethylenediamine ligands. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry 1981, 122, 171–181.
- Migita, T.; Tachikawa, N.; Katayama, Y.; Miura, T. Thermoelectromotive force of some redox couples in an amide-type room-temperature ionic liquid. Electrochemistry 2009, 77, 639–641.
- Laux, E.; Uhl, S.; Journot, T.; Brossard, J.; Jeandupeux, L.; Keppner, H. Aspects of Protonic Ionic Liquid as Electrolyte in Thermoelectric Generators. Journal of Electronic Materials 2016 45:7 2016, 45, 3383–3389.
- Anari, E. H. B.; Romano, M.; Teh, W. X.; Black, J. J.; Jiang, E.; Chen, J.; To, T. Q.; Panchompoo, J.; Aldous, L. Substituted ferrocenes and iodine as synergistic thermoelectrochemical heat harvesting redox couples in ionic liquids. Chemical communications 2016, 52, 745–748.
- Zinovyeva, V.; Nakamae, S.; Bonetti, M.; Roger, M. Enhanced Thermoelectric Power in Ionic Liquids. ChemElectroChem 2014, 1, 426–430.
- Kim, T.; Lee, J. S.; Lee, G.; Yoon, H.; Yoon, J.; Kang, T. J.; Kim, Y. H. High thermopower of ferri/ferrocyanide redox couple in organic-water solutions. Nano Energy 2017, 31, 160–167.
- Wang, H.; Zhao, D.; Ulla Khan, Z.; Puzinas, S.; Jonsson, M. P.; Berggren, M.; Crispin, X.; Wang, H.; Zhao, D.; Khan, Z. U.; et al. Ionic Thermoelectric Figure of Merit for Charging of Supercapacitors. Advanced Electronic Materials 2017, 3, 1700013.
- Krebs, S. Performance analysis of a Copper II Sulfate Pentahydrate based thermogalvanic cell. Electronic Theses and Dissertations 2015.
- Cabral, D. M.; Howlett, P. C.; MacFarlane, D. R. Electrochemistry of the tris(2,2‘-bipyridine) complex of iron(II) in ionic liquids and aprotic molecular solvents. Electrochimica Acta 2016, 220, 347–353.
- Yamato, Y.; Katayama, Y.; Miura, T. Effects of the Interaction between Ionic Liquids and Redox Couples on Their Reaction Entropies. Journal of The Electrochemical Society 2013, 160, H309–H314.
- Jiao, N.; Abraham, T. J.; MacFarlane, D. R.; Pringle, J. M. Ionic Liquid Electrolytes for Thermal Energy Harvesting Using a Cobalt Redox Couple. Journal of The Electrochemical Society 2014, 161, D3061–D3065.
- Salazar, P. F.; Stephens, S. T.; Kazim, A. H.; Pringle, J. M.; Cola, B. A. Enhanced thermo-electrochemical power using carbon nanotube additives in ionic liquid redox electrolytes. Journal of Materials Chemistry A 2014, 2, 20676–20682.
- Kazim, A. H.; Cola, B. A. Electrochemical Characterization of Carbon Nanotube and Poly (3,4-ethylenedioxythiophene)−Poly(styrenesulfonate) Composite Aqueous Electrolyte for Thermo-Electrochemical Cells. Journal of The Electrochemical Society 2016, 163, F867–F871.
- Wu, J.; Black, J. J.; Aldous, L. Thermoelectrochemistry using conventional and novel gelled electrolytes in heat-to-current thermocells. Electrochimica Acta 2017, 225, 482–492.
- Jin, L.; Greene, G. W.; MacFarlane, D. R.; Pringle, J. M. Redox-Active Quasi-Solid-State Electrolytes for Thermal Energy Harvesting. ACS Energy Letters 2016, 1, 654–658.
- Xiao, Y.; Zhong, X.; Guo, J.; Zhou, C.; Zuo, H.; Liu, Q.; Huang, Q.; Zhang, Q.; Diao, X. The role of interface between LiPON solid electrolyte and electrode in inorganic monolithic electrochromic devices. Electrochimica Acta 2018, 260, 254–263.
- Yang, P.; Liu, K.; Chen, Q.; Mo, X.; Zhou, Y.; Li, S.; Feng, G.; Zhou, J. Wearable thermocells based on gel electrolytes for the utilization of body heat. Angewandte Chemie 2016, 128, 12229–12232.
- Quickenden, T. I.; Mua, Y. A Review of Power Generation in Aqueous Thermogalvanic Cells. Journal of The Electrochemical Society 1995, 142, 3985–3994.
- Gunawan, A.; Li, H.; Lin, C. H.; Buttry, D. A.; Mujica, V.; Taylor, R. A.; Prasher, R. S.; Phelan, P. E. The amplifying effect of natural convection on power generation of thermogalvanic cells. International Journal of Heat and Mass Transfer 2014, 78, 423–434.
- Burmistrov, I.; Kovyneva, N.; Gorshkov, N.; Gorokhovsky, A.; Durakov, A.; Artyukhov, D.; Kiselev, N. Development of new electrode materials for thermo-electrochemical cells for waste heat harvesting. Renewable Energy Focus 2019, 29, 42–48.
- Koo, M. H.; Yoon, H. H. Fabrication of carbon nanotubes and charge transfer complex-based electrodes for a glucose/oxygen biofuel cell. Journal of Nanoscience and Nanotechnology 2013, 13, 7434–7438.
- Nugent, J. M.; Santhanam, K. S. V.; Rubio, A.; Ajayan, P. M. Fast Electron Transfer Kinetics on Multiwalled Carbon Nanotube Microbundle Electrodes. Nano Letters 2001, 1, 87–91.
- Hu, R.; A. Cola, B.; Haram, N.; N. Barisci, J.; Lee, S.; Stoughton, S.; Wallace, G.; Too, C.; Thomas, M.; Gestos, A.; et al. Harvesting Waste Thermal Energy Using a Carbon-Nanotube-Based Thermo-Electrochemical Cell. Nano Letters 2010, 10, 838–846.
- Kiselev, N.; Artyukhov, D.; Boychenko, E.; Gorshkov, N.; Glubokaya, A.; Burmistrov, I. Electrolyte concentration dependences of NiO based thermoelectrochemical cells performance. AIP Conference Proceedings 2022, 2456, 020005.
- Taganova, A. A.; Boychenko, E. A.; Kiselev, N. v.; Khaidarov, B. B.; Kolesnikov, E. A.; Yudin, A. G.; Vikulova, M. A.; Gorshkov, N. v.; Kuznetsov, D. v.; Burmistrov, I. N. Synthesis and Study of the Composition of Hollow Microspheres of NiO and NiO/Ni Composition for Thermoelectrochemical Energy Converters of Low-Potential Temperature Gradients of Thermal Units Into Electricity. Refractories and Industrial Ceramics 2021, 61, 715–719.
- Marquardt, T.; Kube, J.; Radici, P.; Kabelac, S. Experimental investigation of a thermocell with proton exchange membrane and hydrogen electrodes. International Journal of Hydrogen Energy 2020, 45, 12680–12690.
- Misra, V.; Bozkurt, A.; Calhoun, B.; Jackson, T.; Jur, J.; Lach, J.; Lee, B.; Muth, J.; Oralkan, O.; Ozturk, M.; et al. Flexible technologies for self-powered wearable health and environmental sensing. Proceedings of the IEEE 2015, 103, 665–681.
- Ando Junior, O. H.; Maran, A. L. O.; Henao, N. C. A review of the development and applications of thermoelectric microgenerators for energy harvesting. Renewable and Sustainable Energy Reviews 2018, 91, 376–393.
- Riemer, R.; Shapiro, A. Biomechanical energy harvesting from human motion: Theory, state of the art, design guidelines, and future directions. Journal of NeuroEngineering and Rehabilitation 2011, 8, 1–13.
- Invernizzi, F.; Dulio, S.; Patrini, M.; Guizzetti, G.; Mustarelli, P. Energy harvesting from human motion: materials and techniques. Chemical Society Reviews 2016, 45, 5455–5473.
- Llamas R. IDC Media Center. Worldwide Wearables Market to Nearly Double by 2021, According to IDC Internet Available online: https://www.idc.com/getdoc.jsp?containerId=prUS42818517 (accessed 22.11. 2021).
- Xu, Z.; Wu, H.; Zhu, T.; Fu, C.; Liu, X.; Hu, L.; He, J.; He, J.; Zhao, X. Attaining high mid-temperature performance in (Bi,Sb)2Te3 thermoelectric materials via synergistic optimization. NPG Asia Materials 2016 8:9 2016, 8, e302–e302.
- Nozariasbmarz, A. In-situ sintering decrystallization of thermoelectric materials using microwave radiation; North Carolina State University, 2017.
- Wang, S.; Tan, G.; Xie, W.; Zheng, G.; Li, H.; Yang, J.; Tang, X. Enhanced thermoelectric properties of Bi2(Te1−xSex)3-based compounds as n-type legs for low-temperature power generation. Journal of Materials Chemistry 2012, 22, 20943–20951.
- Leonov, V. Thermoelectric energy harvester on the heated human machine*. Journal of Micromechanics and Microengineering 2011, 21, 125013.
- Suarez, F.; Parekh, D. P.; Ladd, C.; Vashaee, D.; Dickey, M. D.; Öztürk, M. C. Flexible thermoelectric generator using bulk legs and liquid metal interconnects for wearable electronics. Applied Energy 2017, 202, 736–745.
- Park, H.; Lee, D.; Kim, D.; Cho, H.; Eom, Y.; Hwang, J.; Kim, H.; Kim, J.; Han, S.; Kim, W. High power output from body heat harvesting based on flexible thermoelectric system with low thermal contact resistance. Journal of Physics D: Applied Physics 2018, 51, 365501.
- Fan, Z.; Ouyang, J.; Fan, Z.; Ouyang, J. Thermoelectric Properties of PEDOT:PSS. Advanced Electronic Materials 2019, 5, 1800769.
- Liu, Y.; Wang, H.; Sherrell, P.C.; Liu, L.; Wang, Y.; Chen, J. Potentially Wearable Thermo-Electrochemical Cells for Body Heat Harvesting: From Mechanism, Materials, Strategies to Applications. Adv. Sci. 2021, 8, 2100669.
- Masoumi, S.; O’Shaughnessy, S.; Pakdel, A. Organic-based flexible thermoelectric generators: From materials to devices. Nano Energy 2022, 92, 106774.
- Peng, S.; Wang, D.; Lu, J.; He, M.; Xu, C.; Li, Y.; Zhu, S. A Review on Organic Polymer-Based Thermoelectric Materials. Journal of Polymers and the Environment 2017, 25, 1208–1218.
- Guan, X.; Cheng, H.; Ouyang, J. Significant enhancement in the Seebeck coefficient and power factor of thermoelectric polymers by the Soret effect of polyelectrolytes. Journal of Materials Chemistry A 2018, 6, 19347–19352.
- Yang, S.; Cho, K.; Park, Y.; Kim, S. Bendable thermoelectric generators composed of p- and n-type silver chalcogenide nanoparticle thin films. Nano Energy 2018, 49, 333–337.
- Kim, S. J.; Lee, H. E.; Choi, H.; Kim, Y.; We, J. H.; Shin, J. S.; Lee, K. J.; Cho, B. J. High-Performance Flexible Thermoelectric Power Generator Using Laser Multiscanning Lift-Off Process. ACS Nano 2016, 10, 10851–10857.
- Zhao, D.; Wang, H.; Khan, Z. U.; Chen, J. C.; Gabrielsson, R.; Jonsson, M. P.; Berggren, M.; Crispin, X. Ionic thermoelectric supercapacitors. Energy & Environmental Science 2016, 9, 1450–1457.
- Yang, K.; Cho, K.; Yang, S.; Park, Y.; Kim, S. A laterally designed all-in-one energy device using a thermoelectric generator-coupled micro supercapacitor. Nano Energy 2019, 60, 667–672.
- Wu, X.; Huang, B.; Wang, Q.; Wang, Y. Thermally chargeable supercapacitor using a conjugated conducting polymer: Insight into the mechanism of charge-discharge cycle. Chemical Engineering Journal 2019, 373, 493–500.
- Park, Y.; Cho, K.; Kim, S. Vertical all-in-one energy systems constructed with thermoelectric generators and microsupercapacitors. Journal of Power Sources 2021, 510, 230402.
- Liu, Z.; Cao, X.; Wang, B.; Xia, M.; Lin, S.; Guo, Z.; Zhang, X.; Gao, S. Coupling thermoelectricity and electrocatalysis for hydrogen production via PbTePbS/TiO2 heterojunction. Journal of Power Sources 2017, 342, 452–459.
- Liu, K.; Li, K.; Yang, Z.; Zhang, C.; Deng, J. An advanced Lithium-ion battery optimal charging strategy based on a coupled thermoelectric model. Electrochimica Acta 2017, 225, 330–344.
- Richards, G.; Gemmen, R. S.; Williams, M. C. Solid – state electrochemical heat engines. International Journal of Hydrogen Energy 2015, 40, 3719–3725.
- Liao, G.; Jiang, K.; Zhang, F.; E, J.; Liu, L.; Chen, J.; Leng, E. Thermal performance of battery thermal management system coupled with phase change material and thermoelectric elements. Journal of Energy Storage 2021, 43, 103217.
- Nguyen, H. Q.; Shabani, B. Proton exchange membrane fuel cells heat recovery opportunities for combined heating/cooling and power applications. Energy Conversion and Management 2020, 204, 112328.
- Geffroy, C.; Lilley, D.; Parez, P. S.; Prasher, R. Techno-economic analysis of waste-heat conversion. Joule 2021, 5, 3080–3096.
