Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Laura Vergani and Version 2 by Sirius Huang.
Numerous plants, plant extracts, and plant-derived compounds are being explored for their beneficial effects against overweight and liver diseases. Many herbs of the Lamiaceae family are widely employed as food and spices in the Mediterranean area, but also in folk medicine, and their use for the management of metabolic disorders is well documented.
- Lamiaceae
- medicinal plants
- phytochemicals
- nutraceutics
- obesity
1. Salvia Species
Salvia is the largest genus of the Lamiaceae, as it includes roughly 900 aromatic species of herbaceous, perennial, biennial, and annual plants [1][22]. This genus covers almost all continents including the Pacific Islands, Central Asia, the Mediterranean, tropical Africa, and America [1][22]. Common species include Salvia officinalis L. (common sage), Salvia hispanica L. (chia), Salvia miltiorrhiza Bunge (Chinese sage), and Salvia sclarea L. (clary sage), with the first two being the most studied ones.
Salvia officinalis L. is a perennial subshrub native to the Mediterranean area, but certainly naturalized in Southern France and Spain, widely used in both culinary and medicinal preparations. Its name “sage” originates from the old Latin word “salvarem” meaning ‘to heal’, and in fact S. officinalis is rich in bioactive compounds, mostly diterpenoids (abietane and labdane) and phenolic compounds (caffeic acid derivatives). The long history of sage as medicinal plant was confirmed by studies showing its promising potential in ameliorating heart diseases and obesity [2][23]. Sage was shown to also be effective in improving inflammation [3][24], hyperlipidemia, hypercholesterolemia, and T2DM [4][5][6][25,26,27]. In streptozotocin-induced diabetic rats, the alcoholic extract of sage leaves decreased blood glucose (GLU), TG, total cholesterol (Chol), cyclooxygenase-2 (COX2), urea, uric acid, creatinine, aspartate transaminase (AST), alanine transaminase (ALT) and increased insulin secretion [4][25], while the methanolic extract decreased blood GLU and insulin levels, and improved the lipid profile and the insulin sensitivity by reducing the plasmatic pro-inflammatory cytokines: tumor necrosis factor alpha (TNFα), interleukin-12 (IL-12), and increasing the anti-inflammatory cytokines: IL-2, IL-4, and IL-10 [7][28]. A study on male Wistar rats demonstrated the effects of sage leaves in regenerating/restoring Langerhans islets to their normal size [6][27]. Moreover, sage as a food supplement seems to act in the prevention of T2DM by inhibiting hepatic gluconeogenesis and/or glycogenolysis [8][29]. The oral administration of sage to diabetic rats up-regulated the expression of insulin and insulin-regulated glucose transporter (Glut-4), and inhibited α-glucosidase activity. Moreover, the sage leaves inhibited lipogenesis in adipocytes by reducing the accumulation of lipid droplets [7][28]. Figure 1 reports the different classes of the common phytochemical constituents of Lamiaceae plant species.
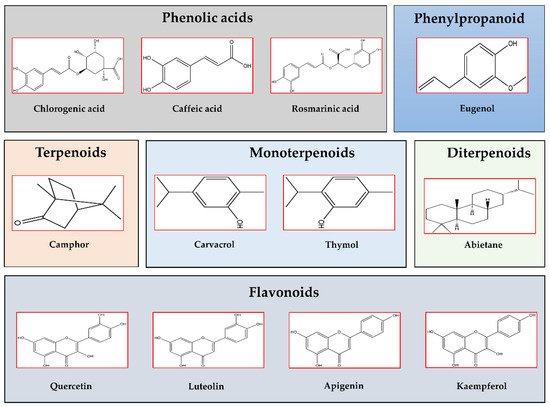
Figure 12. Chemical structures of the most abundant and common bioactive compounds found in Lamiaceae plant species (ChemDraw2020 software, PerkinElmer Informatics, Waltham, MA, USA). Phenolic acids: Chlorogenic acid, Caffeic acid, Rosmarinic acid; Phenylpropanoid: Eugenol; Terpenoids: Camphor; Monoterpenoids: Carvacrol, Thymol; Diterpenoids: Abietane; and Flavonoids: Quercetin, Luteolin, Apigenin, Kaempferol.
An in vivo study on ovariectomized rats revealed that sage leaves possess estrogenic activity that led to loss in the body weight and restored the plasma lipid levels to the normal profile by reducing total Chol, high-density lipoprotein (HDL-Chol), low-density lipoprotein (LDL-Chol), TG, total lipids, very-low-density lipoprotein (VLDL) [9][30] In humans, a double-blind clinical trial was carried out on 80 diabetic patients being divided into two groups which received S. officinalis tablets (150 mg extract) or placebo, three times a day for 90 days; both the plasma GLU and Chol levels significantly decreased in S. officinalis treated patients [10][31]. Another study demonstrated the efficacy and safety of incapsulated S. officinalis leaf ethanolic extract on 86 hyperlipidemic diabetic patients treated with placebo or 500 mg capsule every 8 h by the oral route for three months. An improvement of the glycemic and lipid profile with a reduction in the fasting GLU, glycated haemoglobin (HbA1c), total Chol, TG, and LDL-Chol and elevating HDL-Chol was observed [11][32]. A pilot trial was carried out on six female volunteers to investigate the effects of sage being consumed as tea and showed that sage tea improved the lipid profile, antioxidant defences, and lymphocyte 70kDa heat shock proteins (Hsp70) expression [5][26].
Salvia hispanica L. is an annual herbaceous plant, originally from Mexico and Guatemala, but recently has also been cultivated in Australia, Bolivia, Columbia, Peru, Argentina, America, and Europe [12][33]. It is commonly known as chia, a name that originates from the Spanish word “chian” meaning oily. Indeed, S. hispanica is extremely rich in ω-3/-6 fatty acids [12][33], α-linolenic acid in particular, which are precursors of prostaglandins, leukotrienes, and thromboxanes. Chia seeds are an ancient food that is tracked back to the pre-Columbian era, when they were also used in religion [13][34]. S. hispanica provides a balanced amount of nutrients composed of insoluble fibers, proteins with a high quality of amino acids, high content of antioxidants, such as phenolic compounds such as caffeic acid, chlorogenic acid, and quercetin. Many studies described its potential for treating obesity [14][35], diabetes [14][15][16][35,36,37], hypertension [15][36], cardiovascular disease [16][37], NAFLD [17][38], hyperlipidemia [18][39], inflammation and oxidative stress [19][20]. Table 1 summarizes the most abundant bioactive compounds found in each species[40, the corresponding applications, and the number of citations for each41].
Table 1. An overview summary on the most abundant bioactive compounds in each species, its applications, and the number of citations for each.
| List of Plants | Most Abundant Bioactive Compounds | Applications |
|---|---|---|
| Salvia officinalis L. | Diterpenoids: abietane and labdane Phenolic compounds: caffeic acid derivatives |
Folk medicine |
| Salvia hispanica L. | Caffeic acid, chlorogenic acid, and quercetin | Uses in food, folk medicine, primary cosmetics, and a part of religious rituals |
| Thymus species | Thymol, carvacrol apigenin, luteolin, thymusin, rosmarinic, and caffeic acid and derivatives | Traditional phytomedicine, food, food additive, spicy, and herbal tea |
| Rosmarinus officinalis L. | Carnosic acid, rosmarinic acid, camphor, caffeic acid, ursolic acid, betulinic acid, and carnosol | Traditional phytomedicine, food additives, and herbal tea |
| Mentha species | Menthol, luteolin, rosmarinic acid, Kaempferol, and hesperidin | Traditional phytomedicine, food, food additive, spicy, herbal tea |
| Melissa officinalis L. | Rosmarinic acid, geranial, neral, luteolin, naringin, hesperidin, and caffeic acid and derivatives | Traditional phytomedicine, food flavoring, and herbal tea |
| Leonurus sibiricus L. | Chlorogenic acid, caffeic acid, and quercetin | Herbal medicine |
| Thymbra spicata L. | Carvacrol and rosmarinic acid | Culinary ingredient: in salad and tea infusion Herbal medicine |
| Orthosiphon aristatus (Blume) Miq. | Rosmarinic acid | Folk medicine |
| Lycopus lucidus Turcz. ex Benth | Rosmarinic acid and derivatives Flavonoid: chrysoeriol, luteolin, quercetin, isoquercitrin, and rutin |
Traditional phytomedicine |
| Scutellaria baicalensis Georgi | Flavonoid: Baicalein, wogonoside, and wogonin | Traditional phytomedicine |
| Ocimum species | Eugenol, rosmarinic acid, apigenin, luteolin, β-sitosterol, and carnosic acid | Traditional phytomedicine, food additive, spicy, and fragrance agent |
| Mesona chinensis Benth. | Caffeic acid | Traditional phytomedicine, gelatin-type dessert, and herbal beverage |
| Leonotis leonurus (L.) R.Br. | Marrubin and premarrubin | Traditional phytomedicine |
The in vivo study on male Wistar rats [18][39] investigated the anti-steatotic effect of chia seeds by taking four rat groups fed DE (diet of standard food) and 4 groups fed DC (diet with added chia) for 4 weeks. After this period the groups were further divided into 2 control groups, two groups received tyloxapol to induce acute dyslipidemia, 2 groups received (CCl4) to induce acute steatohepatitis, and the last 2 groups were treated with tyloxapol + CCl4 to induce acute dyslipidemia along with NASH. The results showed that chia intake, partially or totally prevented cholestasis, liver damage, inflammation, oxidative stress, and markedly lowered the TG and Chol in both dyslipidemic and NASH groups compared to controls. It is possible that the hypolipidemic and hepatoprotective effects of chia may be correlated to its high content of α-linolenic acid. On the other hand, the chia intake improved antioxidant defence by increasing superoxide dismutase (SOD) expression and activity, peroxisome proliferator-activated receptor (PPARα) expression, catalase (CAT) activity, and HDL-Chol levels, and decreased the concentrations of total Chol, LDL-Chol, and the inflammatory markers IL-1β, as well as the lipid profile and liver indices [19][40]. Chia seed supplementation for rats fed a sucrose-rich diet reduced the abdominal and thoracic circumferences, carcass fat content, adipose tissue weights, and visceral adiposity index, and this was accompanied by an improvement in insulin sensitivity and plasma lipid profile through a reduction in both FAT/CD 36 plasma membrane and the fat synthesis enzyme activities: ATP-citrate lyase, FAS cell surface death protein (FAS), G6PD, and PEPCK [21][42]. The supplementation of chia seeds to Wistar rats fed a sucrose-rich diet improved both the activities of the antioxidant enzymes CAT, SOD, and glutathione peroxidase (GPx), as well as the mRNA expression of (Nrf2), and led to a decrease in xanthine oxidase (XO) activity and reactive oxygen species (ROS) contents, and in the plasma pro-inflammatory cytokines IL-6 and TNFα [22][43]. Moreover, chia supplementation increased the PPARγ expression and the ω-3/-6 fatty acid ratio of membranes, suggesting that chia seed may improve the dysfunction induced by a sucrose excess, and reduced adipocyte hypertrophy by improving lipogenic enzyme activities, and decreased lipid storage, GLU phosphorylation and oxidation in skeletal muscle [23][44]. Moreover, chia seeds seem to play a cardioprotective activity in normalizing the systolic blood pressure [24][45]. In a clinical trial a single arm experimental study investigated the effects of the daily intake (25 g/day) of chia seeds by 25 patients with NAFLD, showing a decrease in body weight, total Chol, non-HDL-Chol, and circulating free fatty acids (FFA) [17][38]. On the other hand, a double-blind, randomized, controlled trial with two parallel groups of 77 obese patients with T2DM [14][35] indicated the beneficial potential of chia seeds in promoting weight loss, and improving obesity-related risk factors such as a decrease in c-reactive protein (CRP) and an increase in plasma adiponectin. A single-blind study of over 20 subjects with T2DM evaluated the effect of the daily intake (37 ± 4 g/day) of chia seeds for 12 weeks and found that chia attenuated the a major cardiovascular risk factor [16][37]. In the same context, the daily consumption of 40 g of chia seeds over 12 weeks is more than enough to reduce the systolic blood pressure of diabetic patients [15][36].
The corresponding picture for each plant species of the Lamiaceae family is presented in Figure 2.

Figure 23. A Group of Lamiaceae plants (Available online: www.theplantlist.org, accessed on 5 August 2022) included. (A): Salvia officinalis L., (B): Salvia hispanica L., (C): Thymbra capitata (L.) Cav., (D): Thymus saturejoides Coss., (E): Rosmarinus officinalis L., (F): Mentha spicata L., (G): Melissa officinalis L., (H): Leonurus sibiricus L., (I): Thymbra spicata L., (J): Orthosiphon aristatus (Blume) Miq., (K): Lycopus lucidus Turcz. ex Benth., (L): Scutellaria baicalensis Georgi., (M): Ocimum species, (N): Mesona chinensis Benth., (O): Leonotis leonurus (L.) R.Br.
2. Thymus Species
The genus Thymus L. contains about 300 species of aromatic perennial herbaceous plants and subshrubs that are native to temperate regions in Eurasia, but now are cultivated throughout the world.
Thymus vulgaris L. (common thyme) is the most common herb of this genus. It is native to Italy and the western Mediterranean, where it is widely used as food and herbal medicine. It is slightly spicier than oregano and sweeter than sage. Fresh thyme is rich in rosmarinic acid and luteolin, but thymol is the most abundant phenolic compound present in thyme essential oil [25][46]. Many in vitro and in vivo studies reported the antioxidant and anti-inflammatory effects of T. vulgaris, where it was able to decrease a wide range of inflammatory mediators such as ROS, reactive nitrogen species (RNS), and pro-inflammatory cytokines [26][47]. In a mouse model of liver damage, T. vulgaris played hepatoprotective effects by decreasing oxidative stress and inflammatory markers [27][48], and in gentamicin-treated rats, it had hypolipidemic and anti-inflammatory effects by normalizing the plasmatic AST and ALT and bilirubin level, as by restoring the normal lipid parameters and ROS production [28][49].
T. saturejoides Coss. is a Moroccan perennial shrub locally known as “Azkouni” or “Zaitra”. It is largely employed in traditional medicine to treat hypertension, T2DM, cold, fever, dermatological and circulatory disorders [29][50]. In streptozotocin-treated rats (model of T2DM), T. saturejoides was reported to reduce blood GLU and body weight and improve GLU tolerance [30][51].
Other Thymus species seem to have anti-diabetic potential: T. schimperi Ronniger seems to lower the blood GLU in alloxan-insulted diabetic mice [31][52], and T. praecox Opiz restores GLU homeostasis, ameliorates insulin resistance, and improves pancreatic β-cell function in streptozotocin/nicotinamide-induced diabetic rats [32][53].
In humans, a randomized, controlled, double-blind, crossover human trial was carried out on hypercholesterolemic patients to investigate the effect of virgin olive oil enriched with thyme phenolic compounds (PC). The daily dietary intake for three weeks of a PC-enriched virgin olive oil decreased blood oxidized LDL [33][54], and improves the expression of chol efflux-related genes [34][55]. This cardioprotective effect could be mediated by the modulation of gut microbiota by the increase in populations of bifidobacteria together with increases in PC microbial metabolites with antioxidant activities.
3. Rosmarinus officinalis L.
R. officinalis L. (Rosemary) is native to countries in southern Europe such as Portugal, as well as Asian Mediterranean Countries (Lebanon, Syria, and Palestine) [35][56], and was largely used as a food and natural medicine for over a million of years. R. officinalis possesses many different biological properties that are mainly due to the presence of volatile and phenolic compounds [36][57]. Extraction of bioactive compounds from rosemary showed that rosmarinic, carnosol, and carnosic acids are the most abundant compounds [36][57]. A lot of in vitro studies reported the ability of R. officinalis extracts to exert antioxidant and antidiabetic activities (Table 2). Its leaves extract decreased the level of ROS in hydrogen peroxide (H2O2)-insulted Hela cells [37][58], stimulated the glycolysis and fatty acid oxidation through activating AMP-activated protein kinase (AMPK) and PPAR pathways in cultured human hepatocellular carcinoma cells (HepG2) subjected to hypoglycemic conditions [38][59]. Moreover, leave extract was able to modulate human adipocyte differentiation and to interfere with adipogenesis and lipid metabolism [39][60], to improve the insulin resistance in fatty acid-loaded L6 myotubes through stimulation of GLU uptake and AMPK phosphorylation, and to decrease the activation of c-Jun N-terminal kinases, mammalian targets of rapamycin and 70-kDa ribosomal protein S6 kinase [40][61].
Carnosic acid, a major component of leaves, is known for its antioxidant activity due to its ability as a 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenger [41][99]. It also shows anti-adipogenic activity as it decreases body weight, liver weight, blood and hepatic TG and total Chol levels, and through the activation of expression of lipolysis-related genes (CPT1) [42][43][100,101] and inhibiting α-Glucosidase enzyme [41][99]; however, its low solubility limits its applications [44][102].
The antioxidant and hepatoprotective potential of both leaves of essential oil [45][62] and leaves of ethanol extract [46][63] of R. officinalis were investigated in rats exposed to chromium and CCl4 to induce hepatotoxicity. The results demonstrated their ability in preventing oxidative damage and restoring the antioxidant enzymes levels to normal, decreasing lipid peroxidation and preventing hepatocytes necrosis and fibrosis. Moreover, the antidiabetic potential of different R. officinalis leaves extracts were summarized by decreased levels of total serum HbA1c, GLU, Chol and TG, and increased wound healing ability when they were investigated on different animal models with induced NAFLD [47][103], hyper Chol [48][64], T2DM [49][104], and obesity [50][105]. Lastly, the anti-inflammatory activity of R. officinalis was characterized by decreased levels of inflammatory biomarkers: COX2, prostaglandin E2, IL-1b, matrix metallopeptidase (MMP2), and nitric oxide (NO) when tested on carbon tetrachloride (CCI4)-induced rats [51][65]. In humans, a clinical study carried out on 40 adults (mean age 56 years) diagnosed with T2DM, the intake of rosemary tea (2 g/L of water per day) for 90 days was assessed in order to evaluate whether using rosemary tea instead of powder might have a similar therapeutic effect in the treatment of T2DM, since a large number of leaf powder capsules are required (10 capsules daily) [52][106]. The results indicate a decrease in mass index, waist-hip ratio, lipid peroxidation, insulin resistance, and the pancreatic β-cell function. It was concluded that shortening time and dose, as well as changing the formulation of the R. officinalis constitutes a promising treatment for drug-resistant T2DM patients.
4. Mentha Species
The genus Mentha (Mint) grows worldwide, especially in South Africa, Australia and the temperate regions of Eurasia [53][107], and includes 38 species of aromatic, almost exclusively perennial plants. While members of Mentha are known as the “true mints”, some other genera of Lamiaceae use mint in their common name. Mentha plants are cultivated for the aromatic essential oil contained in the stems and leaves, which is used for culinary, cosmetic and medicinal purposes [54][108]. Mint oils are currently one of the most valuable essential oils worldwide. Spearmint (Mentha spicata L.) and peppermint (Mentha piperita L.) are the most important commercial species.
Mentha spicata L. is known for its anti-diabetic potential. Oral administration of an aqueous extract of M. spicata leaves significantly reduced blood GLU level in diabetic rats [55][109]. On the other hand, M. spicata seems to possess hepatoprotective activity. In rats with nicotine-induced liver damage, M. spicata administration significantly reduced AST, Alkaline phosphatase (ALP), ALT, lactate dehydrogenase (LDH), and lipid peroxidation in rat liver [56][66].
Mentha piperita L. is widely consumed as a food ingredient, essential oil, and tea infusion. M. piperita extracts and oil have been used in traditional medicine [57][110]. Studies on cellular and animal models demonstrated a wide range of biological and pharmacological functions. While rosmarinic acid is the main phenolic compound in the aqueous extract, menthol and menthone are more abundant in the essential oils of M. piperita. The essential oil showed beneficial effects on diabetic rats: treatment with 40 mg/kg alleviated hyperglycemia, improved the antioxidant defence and led to the regeneration of the liver and pancreas tissues [58][111]. The hepatoprotective ability of M. piperita leaf extract against arsenic-induced hepatotoxicity was observed in Swiss albino mice, leading to a reduction in acyl carrier protein, ALP, serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), and lipid peroxidation levels [59][112]. Similarly, M. piperita essential oil exerted a protective effect against CCl4 -induced hepatotoxicity and renal failure in rats by reducing lipid peroxidation, ALT, AST, ALP, LDH, gamma glutamyl transferase, urea and creatinine, and enhanced antioxidant enzymes [60][67]. M. piperita essential oil also demonstrated antifibrogenic effects against CCl4-induced liver fibrosis by improving the antioxidant status, suppressing p53, and subsequently modulating transforming growth factor (TGF-β1) expression [61][68].
Mentha villosa Huds (the synonym of Mentha cordifolia) is another popular herb typically used both as flavour in Thai food and as herbal tea. It has been shown to improve lipid metabolism and glycemia in obese mice treated with M. villosa leaf extract for six weeks, leading to a reduction in GLU, insulin, leptin, TG levels, and in the inflammatory cytokines IL-6 and TNFα, and an increase in adiponectin level, accompanied with the activation of the AMPK signaling pathway [62][69].
M. longifolia (L.) L. is known for its potential in treating hypertension. In humans, a randomized, blind, placebo-controlled trial including 29 subjects with mild hypertension showed that the hydro-alcoholic extract of M. longifolia leaves led to a dose- and duration-dependent reductions in systolic and diastolic blood pressures as well as in mean arterial blood pressures, thus confirming an effective, safe and promising potential for this plant as a phyto-therapeutical for hypertension therapy [63][113].
5. Melissa officinalis L.
M. officinalis L., also known as lemon balm or honey balm, is a bushy herbaceous perennial herb that is typically grown in herb gardens and border fronts for its lemon-scented leaves. It is native to southern Europe and the Mediterranean region, West Asia, and North Africa [64][114]. Traditionally, it is consumed as an herbal tea to improve digestion, reduce gastrointestinal disorders, reduce sleep disturbances, and for its antispasmodic properties [65][115]. The bioactive components of M. officinalis are mainly found in the leaves; the essential oil is rich in volatile compounds, terpenoids (monoterpenes, sesquiterpenes, triterpenes), and polyphenolic compounds [rosmarinic acid, caffeic acid, protocatechuic acid, quercitrin, rhamnocitrin, luteolin]. For this reason, the use of M. officinalis essential oil is suggested for the prevention and management of diseases like hyperlipidemia, T2DM, and other metabolic syndromes [64][114].
Many studies described the antioxidant and antidiabetic potentials of different M. officinalis extracts. Both the radical scavenging activity [65][115] and the antioxidant potential in human endothelial cells (HUVECs)-insulted cells [66][70] were reported. In cellular models the M. officinalis extracts were able to decrease the expression of angiogenic factors, and of MMP-2 and MMP-9 in HUVEC cells [67][71], and to stimulate the expression of PPARα target genes acting in fatty acid β-oxidation and lipolysis [68][72] and AMPK phosphorylation [69][73] in HepG2 cells. In mice, M. officinalis extracts were able to reduce lipid peroxidation and total thiol levels in the brain of Mn-insulted mice [70][74]. Moreover, M. officinalis extracts played anti-diabetic and hepatoprotective effects in hyperlipidemic rats by lowering total Chol, total lipid, the serum level of ALT and AST and lipid peroxidation. In established animal models of T2DM, M. officinalis extracts increased the production of fatty acid-oxidizing enzymes (AMPKα2, ACOX, MCAD, and VLCAD) in the liver, and inhibited pancreatic inflammation by reducing the expression of inflammatory factors IL-6 and CD68 [71][75]. In NAFLD mice, M. officinalis extracts led to a reduction in hepatic fibrosis [69][73]. Furthermore, some clinical studies on diabetic and hyperlipidemic patients showed that the daily administration of M. officinalis capsules resulted in decreasing their LDL, TG, and HbA1c, as well as decreasing the inflammatory biomarker hs-CRP; however, further investigations are needed [64][72][114,116].
6. Leonurus sibiricus L.
L. sibiricus L., commonly called honeyweed or Siberian motherwort, is a ubiquitous aromatic herb native to Russia, Mongolia, and China [73][118]. It is largely used as a culinary ingredient and in folk medicine for treating T2DM [74][76], hypercholesterolemia and oxidative stress [75][77], and weight loss [76][78]. The medicinal properties of L. sibiricus are attributed mainly to the phenolic acids, iridoid and phenylpropanoid glycosides, flavonoids, alkaloids, and labdane diterpenoids [77][119].
An in vitro study reported that both aqueous and methanolic extracts from L. sibiricus aerial parts enhanced insulin secretion and insulinoma cell proliferation in rat insulinoma cells (INS-1E) through depolarization of the plasma membrane and an increase in the intracellular calcium concentration [74][76]. Additionally, an in vivo study using C57BL/6 mice showed reduced plasmatic chol levels, lipid peroxidation and protein carbonyls, and increased HDL-chol levels and activation of the hepatic antioxidant enzymes SOD, CAT, glutathione reductase, and GPx upon administration of the ethanolic extract of L. sibiricus [75][77]. On the other hand, the aqueous extract of L. sibiricus aerial parts exerted anti-adipogenic effects (inhibition of weight gain) in ovariectomized mice by decreasing the serum TG, total chol, and LDL-Chol levels and positively regulating the hormone-sensitive lipase and Adipose triglyceride lipase expression [76][78].
7. Thymbra Species
The genus Thymbra includes many thyme-like plants native to the Mediterranean region, mainly Lebanon, Turkey, and Greece [78][120]. Thymbra spicata L. (known in Lebanon as wild Za’atar) is traditionally used as a food and herbal tea, and in folk medicine as an antiseptic agent and to relieve headaches, toothaches, colds, asthma, and rheumatism [78][120], and this can be greatly attributed to its richness in phenolic compounds including phenolic acids (rosmarinic acid), phenolic monoterpenoids (carvacrol, thymol), and flavonoids (both glycosides and aglycones) [79][121].
The species T. spicata has gained much popularity as a remedy to combat hypercholesterolemia and oxidative stress [80][79]. In vivo studies conducted on high-fat diet (HFD) fed mice showed that both ethanolic and aqueous extracts of T. spicata aerial parts possess anti-hypercholesterolaemic, antioxidant, and anti-steatohepatitic potentials by reducing total Chol, LDL, TG, and malondialdehyde (MDA) concentrations, and increased HDL concentration and stimulated glutathione (GSH), SOD and CAT activities [80][81][79,80]. A recent in vitro study proved that both ethanolic and aqueous extracts of T. spicata aerial parts act as lipid lowering agents in a model of NAFLD by reducing the number of lipid droplets, the intracellular free radicals and lipid peroxidation [82][10].
Another species, Thymbra capitata (L.) Cav. (the synonym of Thymus capitatus (L.) Hoffmanns.), which is considered as a good ecological indicator of the dry Mediterranean area [83][122], is traditionally used in different European countries (Italy, Spain, and Portugal) for the treatment of cutaneous infections due to its powerful antiseptic properties [84][123]. Currently, the essential oil from T. capitata is greatly appreciated, especially in Portugal, and is endowed with several pharmacological properties such as antioxidant [85][124], anti-inflammatory [86][125], and anti-hyperglycemic activities by preventing lipid peroxidation, scavenging the peroxyl free radicals, and inhibiting lipoxygenase and α-amylase [86][125]. The essential oils of T. capitatus seem to improve liver damage in paracetamol-induced toxicity in rats by increasing SOD and GPx activities [87][81].
8. Orthosiphon aristatus (Blume) Miq.
O. aristatus (Blume) Miq. (synonym of Orthosiphon stamineus) is widely diffused in Southeast Asia regions such as Malaysia, Indonesia, and Thailand. It has been introduced as a culinary tea and was traditionally used for ameliorating rheumatism, hypertension, tonsillitis, epilepsy, menstrual disorders, gonorrhea, syphilis, renal calculus, hyperglycemia, and gallstones [88][126]. A phytochemical screening has identified the phenolic compounds isolated from this plant, including flavonol glycosides, lipophilic flavones, and caffeic acid derivatives (rosmarinic acid and 2,3-dicaffeoyltartaric acid) [89][127]. The first scientific studies have reported that methanolic and aqueous extracts from O. aristatus leaves have pharmacological activities such as anti-diabetic [90][128], hypolipidemic and anti-obesity [91][82] properties.
Using HFD-obese mice, Seyedan et al. observed that O. aristatus ethanolic extract could reduce body weight and also the serum TG, Chol, and LDL levels resulting in a significant reduction in fat accumulation, lipid peroxidation and stimulation of SOD activity in the liver [91][82]. Another study using diabetic rats showed that O. aristatus. aqueous extract possesses antihyperglycemic activity by boosting the expression level of Glucagon-like peptide 1 and ghrelin with the consequent reduction in glycemia and stimulation of insulin secretion [92][83].
9. Lycopus lucidus Turcz. ex Benth.
The genus Lycopus L. includes approximately 16 species, distributed in the Northern hemisphere and in the Eastern Asia. In Europe, we find the species Lycopus europaeus L. and Lycopus exaltatus L.f. The leaves are rich in flavonoids, coumarins, terpenoids, and tannins [93][129], and they have use in treating edema, wound healing, T2DM, and pain, especially in traditional Chinese medicine. L. lucidus has been suggested to play a protective role against obesity, NAFLD, and metabolic diseases. An in vitro study on HepG2 cells reported that the L. lucidus ethanolic extract significantly decreased the intracellular lipid accumulation in FFA-induced hepatic steatosis, and it was able to decrease the expression of lipogenic genes and increase β-oxidation [94][84]. An in vivo study showed that the administration of L. lucidus ethanolic extract to HFD mice significantly decreased body weight gain, serum ALT, LDL, Chol, GLU, insulin, leptin, and TNFα levels [94][84]. Moreover, both the liver weight and the hepatic TG and total Chol contents were significantly reduced, and these effects seem to be mediated by a down-regulation of sterol regulatory element-binding protein-1 (SREBP-1) and the activation of AMPK and PPARα. On the other hand, Lee et al. [95][85] observed that the aqueous extract of L. lucidus suppressed vascular inflammation in HUVEC exposed to high GLU through the attenuation of the crassulacean acid metabolism, and inhibited ROS production and suppressed the translocation and transcriptional activity of NFκB.
The hepatoprotective effect of a phenolic enriched extract from L. lucidus roots was also reported by an in vivo study using CCl4-induced hepatotoxicity [96][86]. In detail, the oral administration of this extract to mice significantly reduced the CCl4-induced increase of serum ALT, AST, ALP, TG, total Chol, and total bilirubin. The extract treatment also increased the hepatic GSH content and stimulated the activity of antioxidant enzymes SOD and CAT, decreased the hepatic MDA level, and prevented the deoxyribonucleic acid (DNA) fragmentation.
10. Scutellaria baicalensis Georgi
S. baicalensis Georgi, also called Baikal Skullcap, is a perennial herb that is widely distributed in East Asia, including China, Japan, and Mongolia, where it is traditionally employed for treating inflammation, jaundice, and liver disorders [97][130]. Its dried roots are used in traditional Chinese medicine. Modern pharmacological studies reported that the bioactive compounds of S. baicalensis plays has many pharmacological effects, including anti-oxidant [98][131] and anti-inflammatory ones [99][89].
In both fatty acid-loaded hepatic HepG2 cells, an in vitro model of NAFLD, and in NAFLD mice and rats, a water extract from S. baicalensis roots ameliorated fat-induced lipotoxicity through the AMPK-mediated SREBP signaling pathway [100][87]. Moreover, the S. baicalensis water extracts seem to reduce weight gain, hypertriglyceridemia, and hyperinsulinemia, and restored metabolic process and insulin signaling pathways in obese mice [101][88]. S. baicalensis can help in improving insulin resistance by decreasing the levels of fasting and postprandial GLU, fasting insulin, homeostatic model assessment for insulin resistance, TGs, and LDL-Chol, and preventing inflammation by lowering the expression of inflammatory gene (TNF-α, IFN-γ, and F4/80) in HFD-induced insulin-resistant mice [99][89]. On the other hand, S. baicalensis methanol extract was able to prevent liver fibrosis and reduce the levels of liver hydroxyproline and lipid peroxidation induced by bile duct ligation and scission or CCl4 in rats [97][130].
11. Ocimum Species
Ocimum genus, a widely grown herb native to areas in Asia and Africa, Central and South America, includes approximately 150 species with well-known therapeutic properties. The most important are O. gratissimum L., O. basilicum L., and O. tenuiflorum L. [102][132].
O. gratissimum L. ameliorated the estrogen deficiency-induced obesity in ovariectomized rats mimicking menopausal women [103][90]. O. gratissimum water extract significantly reduced body weight gain and adipocyte size, suggesting that O. gratissimum L. dietary supplements may be useful in controlling the body weight of menopausal women.
O. tenuiflorum L. (the synonym of Ocimum sanctum) as a tea infusion seems to improve liver disease and lipid metabolism in diet-induced obese rats by reducing hepatic lipid accumulation through the down-regulation of lipogenesis and the up-regulation of mitochondrial fatty acid uptake, ameliorating insulin resistance and oxidative damage by stimulating the activity of the hepatic antioxidant enzymes glutathione s transferase (GST), GPx, and CAT [104][91]. O. tenuiflorum aqueous extracts from leaves exerted a lipid-lowering and antioxidant effect in rats fed with a high-Chol diet [105][92], where it decreased lipid accumulation in the liver and hyperlipidemia in blood, as well as enhanced liver antioxidant defence enzymes. Similar outcomes were found in O. tenuiflorum essential oils [106][94], which displayed anti-diabetic effects, by lowering the blood GLU, the serum lipid profile, and the serum creatinine effect in diabetic rats [107][93]. On the other hand, O. tenuiflorum ethanolic extract in diabetic rats modulated glycogen and enzymes of carbohydrate metabolism such as Glucokinase (GK), hexokinase, and phosphofructokinase (PFK) [108][133].
Other plants of the Ocimum genus seem to have anti-diabetic potential in animal models. An anti-hyperlipidemic effect was reported for O. basilicum L. extract on rats [109][134]. In addition, O. basilicum extract showed anti-inflammatory activity on an in vitro model of obesity-induced inflammation [110][135]. Besides the anti-obesity and anti-diabetic effects, the antioxidant, anti-inflammatory, and anti-fibrosis effects of Ocimum spp. such as O. gratissimum, O. basilicum, and O. tenuiflorum have also been reported [111][112][113][136,137,138].
In humans, O. tenuiflorum consumption improved the serum total TG, total Chol, body mass index (BMI), plasma insulin, and insulin resistance in young overweight and obese subjects [114][139]. In NAFLD patients, O. basilicum seeds supplementation significantly reduced BMI [115][140].
12. Mesona chinensis Benth
M. chinensis Benth. is a perennial herbaceous plant widely distributed in Southeast Asia and China. Traditionally, it is consumed as an herbal drink and a gelatine dessert, but it has also been used for the treatment of heat-shock, hypertension, fever, inflammatory, T2DM and liver diseases [116][117]. Interests were focused on its polysaccharides, which include mainly galactose, GLU, rhamnose, arabinose, mannose, and uronic acid, as they possess multiple bioactivities, such as antioxidant activity, anti-diabetes activity, anti-hypertensionactivity, and the prevention of heat stroke [117][141].
An in vitro study reported a significant activity of M. chinensis aqueous extract against oxidative stress tested in terms of DPPH, superoxide and hydroxyl radical scavenging assays, the ferric reducing antioxidant power assay, oxygen radical absorbance capacity, and ferrous ion chelating activity [116][117]. In the same paper, the antioxidant and postprandial glycemia status after M. chinensis whole plant boiling water extract consumption in humans was studied, the results reported a decrease in postprandial plasma GLU, MDA, and serum TG levels, and an increase in plasma antioxidant capacity after the consumption of a high carbohydrate meal together with M. chinensis in 40 overweight participants of both genders and of different ages, suggesting that M. chinensis may have the potential for the prevention of chronic conditions and diseases associated with overweight and obesity [116][117]. Another study carried on mouse macrophage like cells (RAW) 264.7 cells and on cyclophosphamide-induced immune deficient mice showed an antioxidant activity of the polysaccharide extract from M. chinensis whole plant powder, which stimulated the antioxidant enzymes SOD, CAT and GPx compared to non-treated controls, while it also showed decreased levels of MDA, indicating less lipid peroxidation [118][95].
13. Lenotis leonurus (L.) R.Br.
L. leonurus is a shrub growing mainly in South Africa. The protective activities of chloroform, ethanol or acetone extracts from leaves and flowers of L. leonurus have been reported in several studies. The anti-inflammatory and anti-diabetic effects of a L. leonurus leaf extract were shown in two different rats’ models, where L. leonurus inhibited fresh egg albumin-induced paw edema, and played hypoglycemic effects in streptozotocin-induced diabetic rats [119][96]. Another study compared the effects of L. leonurus leaf organic extract with those played by murbiin, a diterpenoid labdane lactone abundant in L. leonurus leaves. To investigate and determine the mechanism of the hypoglycemic activity of this extract, the INS-1 rat cells cultured under hyperglycemic conditions or obese rats were treated with L. leonurus, the results showed increased insulin and GLUT2 gene expressions in INS-1 cells, and increase in respiratory rate and mitochondrial membrane potential. These extracts increased insulin secretion, HDL-Chol, restored total Chol, LDL-Chol, atherogenic index, IL-1β and IL-6 levels to their normal levels, suggesting the potential role of L. leonurus in the alleviation of diabetic symptoms [120][97]. Furthermore, the antidiabetic and anti-inflammatory effects of L. leonurus leaf extract were investigated in both cell models involving 3T3-L1 (fat), Chang (liver), C2C12 (muscle), and INS-1 (pancreatic) cells and in obese rats, thus proving that the mechanism of action is mainly at the adipose tissue level through increases in PPARγ, glucokinase, FAS and UCP2 gene expression [121][98].
