Diabetes mellitus (DM) is one of the major traditional cardiovascular (CV) risk factors promoting vasculogenic ED, together with hypercholesterolemia, hypertension, and cigarette smoking
[5][6]. According to multivariate analyses, DM determines the highest risk for ED among all CV risk factors
[6][7][7,8]. It has been reported that the prevalence of ED in the diabetic population is about three times greater than that in non-diabetic individuals
[8][9]. Among diabetic patients, ED is more common than retinopathy or nephropathy
[9][10]. In 12% of type 1 diabetic men, ED was the first symptom of DM
[10][11]. Moreover, ED also develops earlier in diabetic patients than in non-diabetic patients. In fact, within ten years of DM onset, more than 50% of patients develop ED
[11][12].
The pathogenesis of diabetes erectile dysfunction (DED) is much more complex than in non-diabetic men. In fact, DM accelerates endothelial dysfunction and the atherosclerotic process through several alterations in molecular pathways, resulting in an inability to vasodilate the small penile arterioles.
Decreased endothelial nitric oxide synthesis (eNOS)
[12][13][13,14], selective degeneration of NO-dependent nitrergic nerves
[14][15], increased advanced glycation end products (AGEs) and increased oxygen free radical content
[15][16], decreased NO/cGMP signalling
[16][17][17,18], increased endothelin B receptor binding sites
[18][19], and an upregulated RhoA/Rho pathway
[19][20] are just some of the mechanisms involved in the development of ED in diabetics. In addition to these known molecular pathways, NO mediates many of the antiatherogenic functions of the endothelium in patients with DED by blocking the expression of proinflammatory cytokines, chemokines, and leukocyte adhesion molecules
[20][21]. Thus, loss of eNOS biological activity increases inflammation and cell proliferation. Indeed, increased expression of inflammatory markers was observed in these patients.
Moreover, AGEs themselves stimulate the expression of cytokines on monocytes and macrophages, while chronic hyperglycaemia leads to inflammation and contributes to the production of reactive oxygen species (ROS)
[21][22][22,23]. In this context, the growing interest in the possible role of drugs that lower blood glucose levels, and thus chronic hyperglycaemia and AGEs, in erectile dysfunction is noteworthy.
The recent review by Cignarelli et al. examined the mechanism of action of antidiabetic drugs in the possible remission of ED. Further studies are needed to define the role of these drugs in the treatment of ED
[23][24].
3. Stem Cell Therapy and Erectile Dysfunction
Stem cells (SCs) are undifferentiated cells capable of unlimited proliferation, multi-differentiation potency, and perpetual self-renewal. The specific mechanisms underlying the effectiveness of SCs in the treatment of ED are not yet understood. Since pre-clinical studies have shown that few stem cells can be detected after transplantation, and almost no direct evidence supports the theory that transplanted stem cells have differentiated into vascular endothelial cells, smooth muscle cells, or nerves, the main mechanisms of action of stem cell transplantation would seem to be related to their paracrine action [24][30].
Moreover, preclinical research has shown that SCs exert their therapeutic effects on the basis of active factors contained in their secretions that can act as messengers. Indeed, the effect of SCs has been shown to persist after their disappearance, and even cell-free treatments have shown benefits [25][26][31,32]. Bioactive factors may represent a future treatment option for ED due to their pro-angiogenic, anti-inflammatory, anti-apoptotic, and anti-fibrotic properties [25][26][31,32].
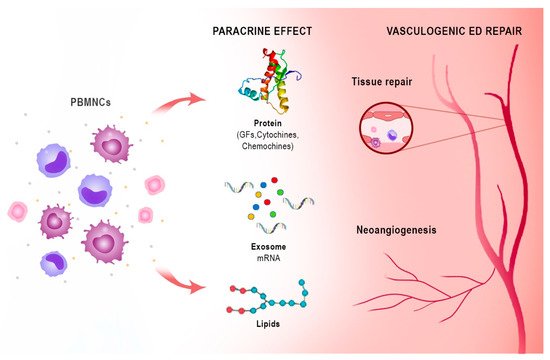
Notably, the peripheral blood mononuclear cell (PBMNC) secretome differs only slightly from the stem cell secretome in its ability to promote cell proliferation
[27][33]. These active paracrine factors, represented by different types of protein molecules, lipid mediators, microRNAs, and exosomes, underlie the regenerative effects of both stem cells and PBMNCs. The proteins are mainly growth factors, cytokines, and chemokines (e.g., CXCL8, CXCL5, CXCL1, CCL5, and VEGF). Lipids and especially oxidised phospholipids (e.g., PLPC-OOH, PAPC-OOH, SGPC, and PGPC) have also shown pleiotropic biological effects, such as neoangiogenesis, but also inflammation modulation by acting on Toll-like receptors (TLRs) and neutrophil granulocytes. Exosomes that arise intracellularly may contain a mixture of proteins, lipids, messenger RNA (mRNA), and micro RNA (miRNA). Due to the complexity of cellular paracrine activity, other factors also play an active role in this process, but these are the biological factors that have been most extensively studied both in vivo and in vitro
[28][34]. Apoptotic PBMNCs have been shown to induce angiogenesis and vasodilation, enhance re-epithelialisation, promote macrophage polarization, and modulate the immune system through their paracrine factors. Since the isolation and cultivation of stem cells is not easy, while the secretome is easier to obtain, the latter could take on a central role in regenerative therapy
[28][34] (
Figure 23).
Figure 23. Immune-centric revolution: vasculogenic repair ED. PBMNCs: peripheral blood mononuclear cells. GFs: growth factors. PBMNCs based on monocytes/macrophages and lymphocytes are a novel autologous cell therapy that has shown efficacy in angiogenesis and tissue regeneration through the action of bioactive substances with paracrine effects represented by different types of protein molecules such as growth factors, chemokines and cytokines, lipid mediators as oxidized phospholipids, microRNAs, and exosomes.
4. Platelet-Rich Plasma Therapy
Although PRP is an emerging treatment option in several fields of medicine, including ED, there are limited studies that support its efficacy. Ding et al.
[29][56] studied the PRP effect on regeneration and restoring the cavernous nerve function after its damage in a rat model. Animals were divided into three groups receiving either sham operation, bilateral cavernous nerve (CN) crush injury with immediate injection of PRP at the site of injury, or bilateral CN crush injury with no further intervention. Intracavernous pressure (ICP) was measured to evaluate erectile function. In the PRP group, the ICP was significantly higher than in the non-treated group but lower than in the placebo group (
p < 0.05).
Furthermore, in the PRP treatment group compared to the other injured group, they found more CN myelinated axons and more NADPH-diaphorase-positive nerve fibres.
In 2012, Wu et al.
[30][57] reported that rats in the experimental group receiving intralesional PRP therapy immediately after the CN damage improved erectile function (
p < 0.05) and improved preservation of myelinated axons of the CN significantly (
p < 0.05) compared with animals that did not receive PRP therapy. PRP administration significantly reduced the level of apoptotic markers, as a reduction in TGF-b1 staining. The absence of the type III collagen and the prevalence of the type I collagen was observed in the histologic study of penile tissue from the treated group.
Wu The
t al. authors believe that PRP accelerated the nerve repair processes through its neuro-regenerative and neuroprotective effects, and it also inhibited the fibrosis process in the corpora cavernosa. The limit
of the study was the small sample of rats; this did not allow
peopleus to consider the use of PRP therapy as an effective treatment method, despite the positive results.
In conclusion, although it seems to be a promising therapy, more extensive studies are needed to understand PRP’s mechanism of action better before PRP therapy enters clinical practice.
5. Harnessing the Immune System for Tissue Repair and Regeneration
Tissue healing and regeneration is a complex, organized, spatiotemporal process involving a plethora of cell subsets, the action of which is strictly regulated to obtain an effective tissue
[31][61]. Heart disease, critical limb ischemia, diabetic foot, and severe musculoskeletal disorders require new therapeutic strategies to repair damaged tissue, especially considering an aging population where diabetes and obesity have reached gigantic proportions.
Clinical trials using adult stem cells to regenerate damaged heart tissue are ongoing, notwithstanding the questions of efficacy and scarcity of understanding of the mechanism of action and of the biological effects
[32][62]. The rationale for adult stem cell therapy clinical trials to repair damaged heart tissue is derived from animal studies that showed a limited but reproducible recovery in cardiac function after ischaemic injury
[33][63]. Vagnozzi
erand al.co-authors proved that after cells implant after ischaemia–reperfusion injury, although heart function was improved, it was not correlated with the production of new cardiomyocytes
[34][64]. On the contrary, two different types of adult stem cell bone marrow mononuclear cells (BM-MNCs), which were the most heavily used stem cell type used in clinical trials, and cardiac mesenchymal cells from the heart that express the receptor tyrosine kinase c-Kit), improved heart function through an acute sterile immune response due to strong recruitment of specific macrophage populations (CCR2+ and CX3CR1+). Moreover, both intracardiac injection of killed stem cell or zymosan, a non-cellular and potent activator of the innate immune response, induced an analogous local macrophage accumulation that provided functional recovery after ischemic damage. Vagnozzi et al. proved that this selective macrophage response acted in multiple fashions altering the activity of the cardiac fibroblasts, reducing in the border zone the extracellular matrix content, and increasing the mechanical asset of the damaged area.
These data showed that cardiac cell therapy’s functional benefit was due to the immune system’s acute inflammatory wound-healing response. Interesting to notice, and surprisingly, a zymosan immune-based response maintained its effect for a longer time as opposed to stem-cell therapies.
6. Harness Peripheral Blood Mononuclear Cells Angiogenic Potency: From Critical Limb Ischemia to ED
PBMNCs based on monocytes/macrophages and lymphocytes are an innovative autologous cell therapy that have shown angiogenesis potency and tissue regeneration in no-option critical limb patients and in diabetic foot patients
[35][36][37][38][80,81,82,83] (
Figure 23).
The detailed mechanism of action of BriePBMNCs is beyond the scope of this review and is adequately described in a review on autologous cell therapy [46]. Briefly, the angiogenic and arteriogenic ability of monocytes/macrophages is well known and extensively described
[39][40][41][42][84,85,86,87].
It has also been observed that monocytes/macrophages are able to repair cerebrovascular ruptures in haemorrhagic strokes due to their ability to physically adhere to rupture sites and generate mechanical traction
[43][88]. Krishnasamy et al. demonstrated that Notch signalling recruits macrophage differentiation and maturation from monocytes, promoting arteriogenesis and tissue repair in ischaemic tissue
[44][89].
A point-of-care (POC) device has recently shown reasonable efficacy in therapeutic angiogenesis both in vivo and in vitro. It is a device based on selective filtration of peripheral blood and is intended for intraoperative use in human cell therapy to produce fresh autologous PBMNCs.
[45][46][97,98]. PBMNCs produced with this device (Hematrate Blood Filtration system—Cook Regentec) have shown promising results in several clinical trials, including studies in diabetic patients
[36][47][48][81,99,100].
7. Conclusions
Recommended ED treatments frequently do not achieve adequate results, particularly in diabetic patients. Regenerative therapies, including platelet-rich plasma (PRP) and stem cell therapy (SCT), are starting to be used for ED treatment as an adjunct or alternative therapy, although on a limited number of patients. PRP delivers an autologous sample rich in growth factors to damaged tissue. PRP studies have shown an increased erectile function recovery and preservation of cavernous nerve axons on animal models; however, studies with PRP in humans are very limited. SCT has been used in diabetic patients and post-prostatectomy ED with mixed results in clinical trials, although SCT treatments improved erectile rigidity and functionality. Still, there is a lack of evidence to support the efficacy of these treatments. The scenario seems similar to the initial enthusiasm for cell therapy in ischemic heart disease, which was dampened by less than brilliant results in the clinic despite promising efficacy data in animal models.