Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 4 by Catherine Yang and Version 3 by Catherine Yang.
Archaea represent a resource of great potential for the identification of new metabolites because of their adaptation to extreme environmental conditions and their original metabolic pathways, allowing the synthesis of unique biomolecules. Archaea also represent a valuable source of novel pigments, including carotenoids (detailed further below) and proteins called bacteriorhodopsins.
- archaeal carotenoids
- carotenoids
- marine pigments
1. Bacteriorhodopsin
Some halophilic Archaea integrate a purple pigment, made of an assembly of opsin with retinal, an apocarotenoid. This complex, denominated as bacteriorhodopsin (BR), crosses the cell membrane and is strongly bound to it through hydrophobic interactions. BR is a seven-α-helical protein containing a chromophore molecule (retinal). It shares structural similarities to the mammalian GPCR protein rhodopsin and is a light-dependent proton pump. Light photons activate the pump to produce ATP by creating a proton gradient across the membrane [1][2]. The word archaerhodopsins is also used to designate this family of proteins and to specify that they are synthesized by Archaea.
Halobacterium salinarum bacteriorhodopsin was the first microbial rhodopsin documented [3] and it has been broadly studied as a model for membrane protein structure and activity [4][5][6]. Halobacterium salinarum is the most studied Archaea for the production of bacteriorhodopsin [7]. This halophilic species grows in high salinity environments such as salt crystallization ponds and often reaches densities of 107 to 108 cells·mL−1 [8]. At this high density, oxygen availability is reduced and aerobic metabolism is almost impossible. In this sense, the synthesis of retinal and bacteriorhodopsin allows the species to be autonomous for its ATP production through photosynthesis.
The biotechnological interest of bacteriorhodopsin lies in its ability to exist in two stable forms at two different wavelengths, allowing its use to be to considered in “biological computer” projects. Bacteriorhodopsin could indeed be used as an extremely miniaturized memory storage unit controlled by light pulses (at a rate of one bit per molecule, a 12 cm-diameter disk could hold 20 to 50 TB). The use of bacteriorhodopsin is one of the first applications of organic molecular electronics, an emerging discipline in nanocomputing [9].
Bacteriorhodopsin is also a likely candidate for integration into nanostructures. It is a light collector that can effectively be used as a solar collector to collect light energy, that could either be consumed directly or stored in batteries. The ability of bacteriorhodopsin to convert light energy into chemical energy or sunlight into electricity has been used in various applications, including optical appliances but also therapeutic/medical applications and research [10][11][12][13].
Various therapeutic uses of this protein have been considered, including the treatment of degenerated retinal blindness and eye disorders, use as adjuvant in vaccination protocols, treatment of malignant tumors, regulation of gene transcription, modulation of drug delivery, transport and release of drugs, cell signaling, apoptosis of neoplastic cells, cell signaling disruption, manufacture of neuro-stimulation devices and pharmaceutical applications [2][14][15][16][17].
2. Archaea Carotenoids
2.1. Overview of the Structure and Function of Carotenoids
Carotenoids are natural pigments that have received particular attention because of their ecophysiological function, biotechnological applications, and their potential beneficial effects on human health [18][19][20][21][22][23][24][25][26][27][28][29]. The color of these molecules can range from colorless to red, through various yellow-orange tones, and they represent the second most abundant natural pigments in nature [30][31]. For example, fucoxanthin, an epoxycarotenoid found in brown microalgae and seaweeds, is the major carotenoid present in marine ecosystems, representing 10% of the total carotenoid production [19]. Apocarotenoids, such as retinal, are defined as derivatives of carotenoids produced by chemical or enzymatic oxidative cleavage.
The most important ecophysiological function of carotenoids is photosynthesis, as they actively participate in the harvesting of photons in the light-harvesting complexes of photosynthetic organisms. They are therefore found in all phototrophic bacteria, cyanobacteria, algae and land plants. Their biological activities have been studied in detail in photosynthetic organisms and include other important ecophysiological functions such as membrane stabilization, photoprotection and antioxidant activities. Animals, including humans, consume phototrophic organisms and utilize carotenoids or apocarotenoids for important physiological functions, including body coloration, sexual attractiveness, vitamin A production, antioxidant activity, transcription factor activation and photoreception. Pharmacological studies have also demonstrated the diverse biological activities of carotenoids and apocarotenoids, including antimicrobial, antifungal, antidiabetic, immunostimulant, anti-obesity, anti-inflammatory, anticarcinogenic and cancer-preventive, antimetastatic, antiangiogenic, radioprotective, anti-atherosclerotic, neuroprotective and chemosensitizing properties of multidrug-resistant cancer cells [22][24][26][28][29][32][33][34][35][36][37][38][39]
Due to their color and health-promoting properties, carotenoids find a wide range of applications in the food, pharmaceutical, nutraceutical and cosmeceutical industries [40].
To date in 2022, 1204 natural carotenoids and apocarotenoids have been described in the three domains of life [41]. These include 324 carotenoids present in bacteria (from which 262 were exclusively found in bacteria), 680 carotenoids in eukaryotes (from which 621 are exclusive to eukaryotes) and 25 carotenoids in Archaea (including 13 exclusively found in Archaea) (Figure 1). Bacterial carotenoids and apocarotenoids are synthesized by photosynthetic bacteria, cyanobacteria and non-photosynthetic bacteria. All or most Archaea synthesize carotenoids. Eukaryotic carotenoids and apocarotenoids are present in photosynthetic carotenogenic species (microalgae, seaweeds, land and marine plants), non-photosynthetic carotenogenic species (fungi) and non-photosynthetic non-carotenogenic species (animals and humans) that obtain them by the consumption of producing species. Given this biodiversity, the number of species containing and/or synthesizing carotenoids and apocarotenoids is immense and impossible to determine exactly.
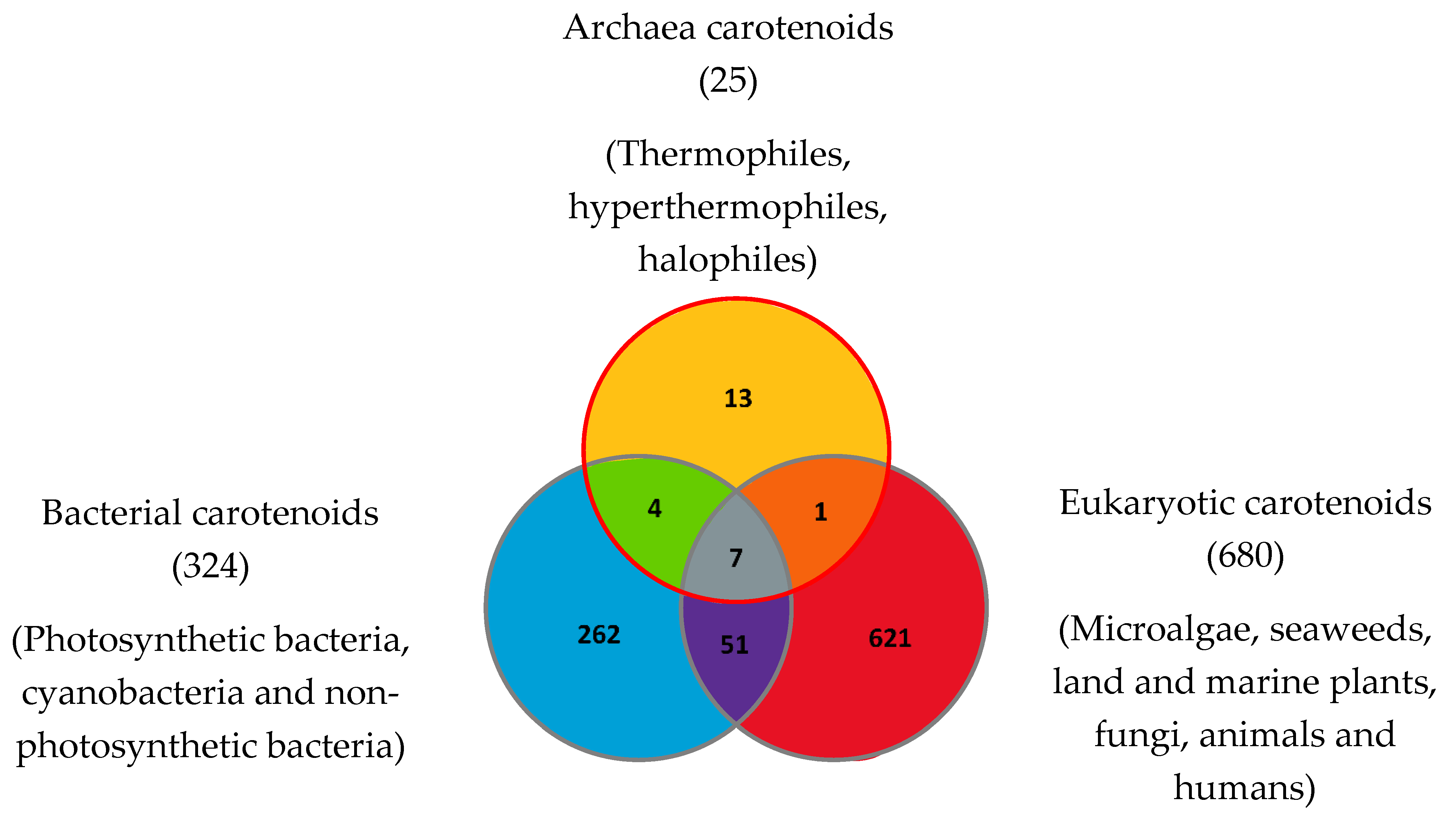
Figure 1. Carotenoids and apocarotenoids in the three domains of life. A total of 1204 carotenoids and apocarotenoids have been identified, some of them being exclusive to one of the three domains of life and some of them being shared [32]. For 245 carotenoids and apocarotenoids, the Japanese carotenoids database gives no indication of the producer organisms [32].
The carotenoid family mostly consist of C40 hydrocarbon backbone molecules (1121 molecules) but also includes a smaller number of molecules containing 30 (37 carotenoids), 35 (5 carotenoids), 45 (13 carotenoids) and 50 carbon atoms (33 molecules). The C40 carotenoids are formed by the polymerization of 8 isoprene units, which are often modified by various oxygen-containing functional groups to obtain cyclic or acyclic xanthophylls. Thus, their polarity can vary from highly hydrophobic to amphiphilic or relatively polar. All carotenoids possess a long chain of conjugated double bonds and a near bilateral symmetry around the central double bond, as common chemical features [42]. According to the absence or presence of oxygen atoms, carotenoids are basically classified into carotenes or carotenoid hydrocarbons, composed of carbon and hydrogen only (e.g., lycopene and β,β-carotene) and xanthophylls or oxygenated carotenoids that can contain epoxy, carbonyl, hydroxyl, methoxy or carboxylic acid functional groups (e.g., lutein, canthaxanthin, zeaxanthin, violaxanthin, capsorubin, fucoxanthin and astaxanthin) [43][44]. Some xanthophylls exist as fatty acid esters, glycosides, sulfates, and can be found associated to protein complexes such as rhodopsin. Figure 2 presents the 2D structures of two model carotenoids, one carotene and one xanthophyll.
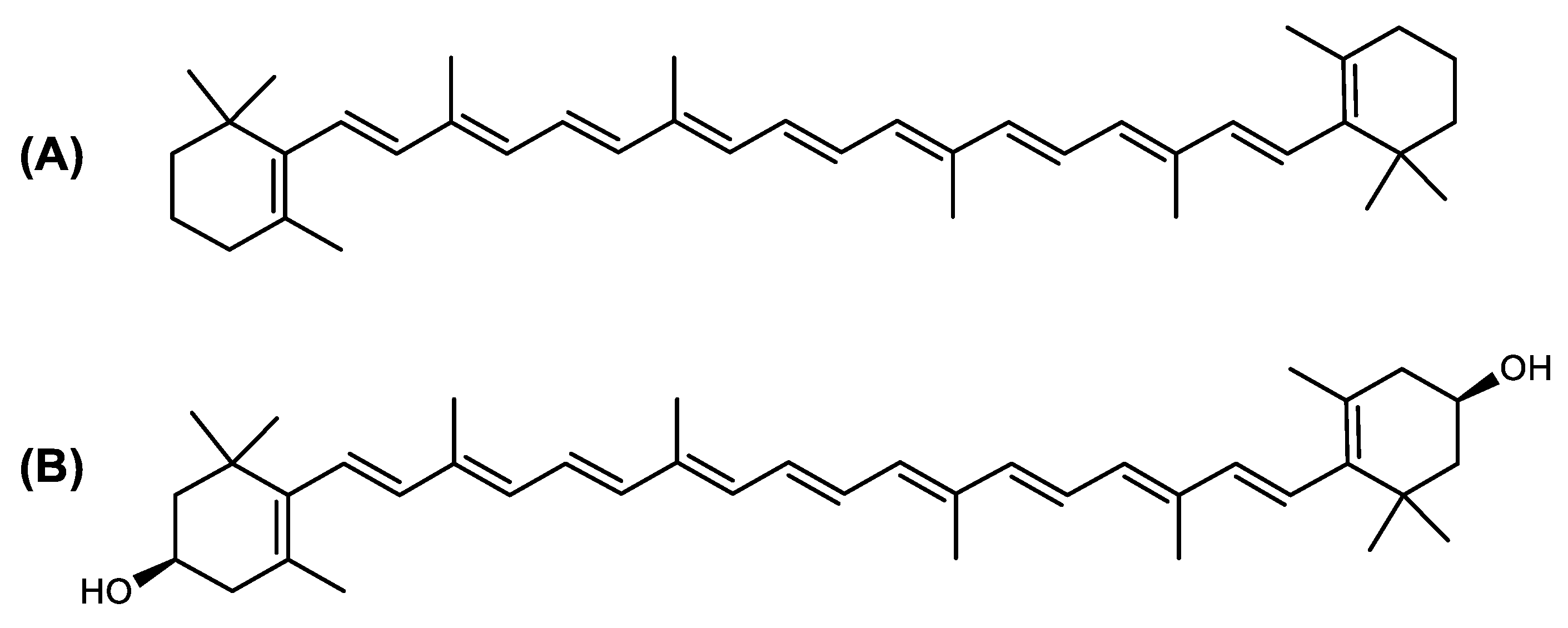
Figure 2. 2D structures of model carotenoids. (A) β,β-carotene (CID: 5280489) and (B) zeaxanthin (CID: 5280899). Chemical 2D were obtained from PubChem (NIH).
The absorption properties of each carotenoid are determined by the level of conjugation and the isomerization state of the backbone polyene chromophore. Carotenoids occur as diverse stereoisomers with various chemical and physical properties, due to the numerous conjugated double bonds and cyclic end groups. The most important are geometric isomers (E-/Z- or trans-/cis-). A double bond links the two residual parts of the molecule either in an E-configuration with both parts on opposite sides of the plane, or a Z-configuration with both parts on the same side of the plane. UV-Vis spectrophotometry, NMR or Raman spectroscopy can be used to differentiate the carotenoids’ isomers [45]. Although some Z-(cis) isomers of carotenoids could be isolated from marine microorganisms, including Archaea, it is important to note that these molecules might be produced by photoisomerization during the extraction, which does not allow us to affirm their presence in the living cell before extraction.
In microorganisms, carotenoids play a key role in the processes of photoprotection, photosynthesis, phototropism and resistance to oxidative stress. Some carotenoids have been described as virulence factors in bacteria, permitting the cells to counter the oxidative stress caused by phagocytes (e.g., staphyloxanthin produced in Staphylococcus aureus). Importantly, some carotenoids, such as salinixanthin or thermozeaxanthin, are only produced by extremophilic microorganisms [46][47][48], suggesting a potential function in thermal, pH or salinity adaptation. Carotenoids are widely known for their remarkable antioxidant properties [49] which are attributed to their double bond structure and ability to delocalize unpaired electrons [50]. Therefore, carotenoids are able to quench free radicals, such as superoxide (O2•−), hydroxyl (OH•) and peroxyl (ROO•) radicals [51]. Reactive nitrogen species (RNS) and reactive oxygen species (ROS) are metabolic by-products generated by almost all biological systems. When there is an imbalance in favor of ROS or RNS production, most biomolecules and cellular structures are negatively affected by excessive oxidation or nitration of lipids, nucleic acids and proteins. Over the years, it has been much detailed that oxidative stress is one of the reasons for the beginning and progression of many diseases, including cancer, heart disease or diabetes. From this perspective, the high antioxidant activity of dietary carotenoids likely explains their cancer-preventive effect, as they can protect cellular macromolecules from oxidation and thus limit inflammation [52]. Carotenoids also modulate gene expression and have anti-inflammatory and immunomodulatory activities [53] that limit the emergence of cancer cells. Additionally, extremophilic microorganisms that live in solar salt flats (halophilic microorganisms) are exposed to high amounts of oxidative stress due to intense solar radiation or high temperatures (up to 50 °C in summer). In response to this stress, they have evolved the synthesis of carotenoids, which are very active in neutralizing ROS [54].
A number of in vivo studies have also demonstrated that carotenoids slow down tumor growth and metastasis and have antiangiogenic activity. The ability of carotenoids to prevent cancer or limit its spread needs to be examined in relation to their behavior in various cellular and tissue environments. In normal tissues, where inflammation and ROS concentration are very low, carotenoids prevent cancer because of their antioxidant activity. However, this ability to neutralize ROS has a downside, as carotenoids oxidize themselves instead of biological macromolecules, leading to the production of oxidized derivatives such as carotenals and apocarotenals. This is especially the case in high inflammatory environments. For example, β-carotene supplementation in smokers increases the risk of lung cancer, as pro-carcinogenic oxidized apocarotenal derivatives are produced by chemical reaction with the pro-oxidative polyaromatic hydrocarbons contained in cigarette smoke [55]. Carotenoid supplementation for the prevention or treatment of cancer should thus be considered with caution, depending on the inflammatory and pro-oxidative status of tissues. The contribution of pro-oxidative metabolites to the initiation or prevention of cancer is still a matter of discussion among researchers. Recent studies have provided evidence on the pro-oxidative activity of some carotenoids (especially epoxycarotenoids), which could partly explain their cytotoxic and pro-apoptotic activity in cancer cells [56]. The pro-apoptotic activity of carotenoids in cancer cells may be associated with their ability to incorporate the cell membrane, sensitize lipids to oxidation, alter membrane fluidity, and interfere with cell signaling pathways. Tumor cells can have different redox statuses, from highly sensitive to very resistant to ROS, which may also partly explain the variable cytotoxicity of carotenoids depending on the cell line considered. Carotenoids have been shown to promote the PI3K/Akt and nuclear factor erythroid 2 (Nrf2) signaling pathways [57], inhibit NF-kB, p38 MAPK, and JAK-2/STAT-3 signaling pathways, which are also linked to inflammation and tumorigenesis. Furthermore, carotenoids can increase gap junction formation, a cellular process that may contribute to their anti-carcinogenic properties [58]. At last, an anti-adiposity activity has also been documented for some carotenoids such as cantaxanthin or fucoxanthin, through the stimulation of lipolysis in adipocytes [59].
Given their many attractive biological and pharmacological activities, societal demand for carotenoids has increased dramatically in recent years, especially for the food, pharma and cosmetics markets. In this view, the biosynthesis and purification of carotenoids from large-scale cultures of microorganisms are broadly studied, and a specific interest exists for the production of novel molecules produced by bacteria, marine living organisms and Archaea.
2.2. Ecophysiological Function of Carotenoids in Archaea
Archaea, especially Haloarchaea, mostly synthesize an uncommon C50 carotenoid called bacterioruberin (BR) and its intermediates: bisanhydrobacterioruberin (BABR), monoanhydrobacterioruberin (MABR), and 2-isopentenyl-3,4-dehydrorhodopin (IDR) [60][61][62][63]. These 50-carbon carotenoids function to increase membrane rigidity [64] and provide protection against UV light [65]. The presence of carotenoids in the membrane of archaeal cells might assist cells to adapt to hypersaline conditions by acting as a water barrier, allowing ions and oxygen molecules to pass through the cell membrane. Therefore, these carotenoids can stabilize archaeal cells under high osmotic and oxidative stresses [66][67]. Despite the fact that β-carotene, lycopene, and phytoene have been identified in haloarchaeal extracts, they are present at low concentrations [61][68]. Those carotenoids are located in the cell membrane and are responsible for the color shown by the red colonies when haloarchaea cells develop on solid media or the red color that can be observed in the close environment of salted lakes such as Torrevieja lake in Spain, for example. Carotenoids also contribute to the modulation of the physicochemical properties of membranes [69]. For example, bacterioruberin is a fundamental component of specific transmembrane proteins [70] and controls membrane organization through the modulation of membrane dynamics and physics [64]. It is also assumed that carotenoids exert antioxidant activities and regulate membrane functions in Archaea, especially in extremophile species.
2.3. Focus on Archaea Carotenoids Structures, Biosynthesis Pathways and Archaea Producing Species
Structure of Archaea Carotenoids
Archaea produce 25 different carotenoids and apocarotenoids, 13 of which are specific to this domain of life [41]. In total, 4 of the 25 molecules are produced by both bacterial and archaeal domains and only one carotenoid is produced by both eukaryotes and Archaea.
Biosynthesis of Carotenoids in Archaea
All carotenoids are derived from isopentenyl pyrophosphate (IPP) and its isomer, dimethylallyl pyrophosphate (DMAPP) [71]. The biosynthetic pathway from isopentenyl pyrophosphate (IPP) to lycopene is common to prokaryotes. The first reaction performed by a farnesyl diphosphate synthase FDPS (2.5.1.10) converts IPP to farnesyl pyrophosphate (FPP). Next, the enzyme CrtE (2.5.1.29), a geranylgeranyl diphosphate synthase, synthesizes geranylgeranyl diphosphate (GGPP). From this molecule, phytoene is obtained by the action of a 15-cis-phytoene synthase called CrtB (2.5.1.32). Lycopene is generated from phytoene by a series of desaturations catalyzed by phytoene desaturase CrtI. From lycopene, some Archaea have specific enzymes and pathways that lead to the synthesis of Archaea-specific carotenoids. These enzymes are described in the Kyoto Encyclopedia of Genes and Genomes (KEGG) and identified by their identification number in parenthesis in the metabolic pathways [72].
Carotenoids Biosynthesis in Haloarcula japonica
Haloarcula japonica is a predominantly triangular, disc-shaped, extremely halophilic Archaea that requires high concentrations of NaCl for growth [73]. In this species, lycopene is generated from phytoene via a series of desaturation reactions that are catalyzed by phytoene desaturase (CrtI) (Figure 3). Subsequently, a bifunctional lycopene elongase and 1,2-hydratase (LyeJ) perform the next reactions, resulting in dihydroisopentenyldehydrorhodopin (DH-IDR). In the next step, CrtD forms double bonds at C-3,4 of the lycopene derivative which leads to the 2-isopentenyl-3,4-dehydrorhodopin (IDR). CrtD was characterized as a carotenoid 3,4-desaturase. The next two steps are the same as the previous ones and result in the formation of dihydrobisanhydrobacterioruberin (DH-BABR) with the LyeJ enzyme and then bisanhydrobacterioruberin (BABR) through the activity of the CrtD enzyme.
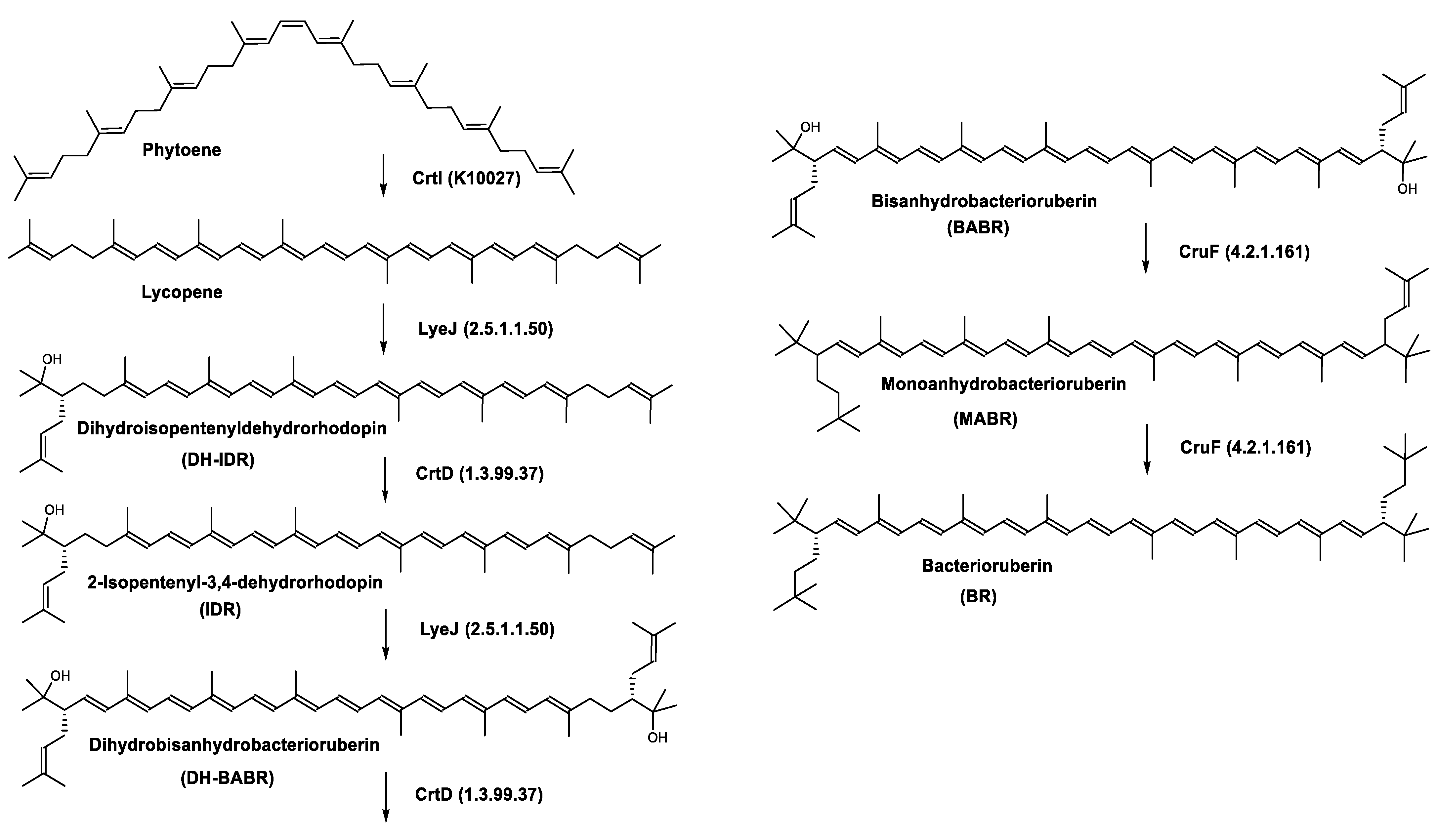
Figure 3. Carotenoids biosynthesis pathway in Haloarcula japonica [74]. Numbers in parenthesis indicate the enzyme number or EC number.
The last two steps are catalyzed by a C50 carotenoid 2″,3″-hydratase (CruF) which generates bacterioruberin (BR). It has been suggested that the antioxidant capacity of carotenoids is linked to the number of conjugated double bonds and hydroxyl groups [75][76]. In this view, bacterioruberin, which contains 13 conjugated double bonds and 4 hydroxyl groups, is a highly effective free-radical scavenger.
Retinal is also synthesized by Haloarcula japonica. In the same method as in the biosynthetic pathway of bacterioruberin, lycopene is produced from phytoene through a series of desaturation reactions catalyzed by phytoene desaturase (CrtI). Retinal is synthesized through the cyclization of lycopene to β-carotene by lycopene β-cyclase (CrtYcd) [77] and the subsequent cleavage of β-carotene to retinal by β-carotene dioxygenase (Brp) [78] (Figure 4).
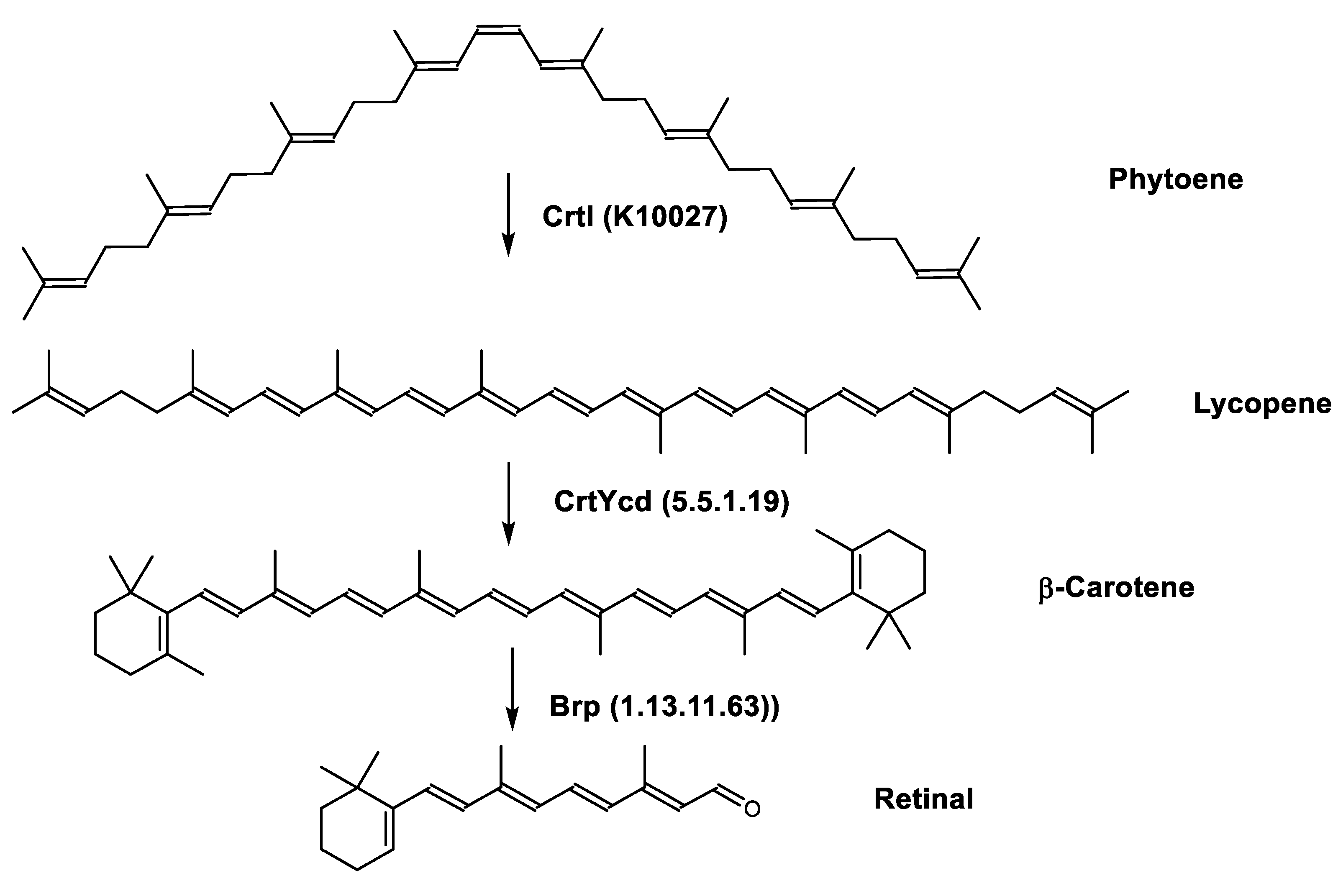
Figure 4. Biosynthetic pathways of retinal (apocarotenoid) in Haloarcula japonica [74]. Numbers in parenthesis indicate the enzyme number or EC number.
Regulation of the Bacterioruberin Synthesis
Lycopene is the last common intermediate for retinal and bacterioruberin biosynthesis [79]. CrtYcd, the lycopene cyclase, catalyzes the conversion of lycopene to β-carotene that is subsequently cleaved to form retinal. LyeJ, the lycopene elongase catalyzes the prenylation of lycopene to the first bacterioruberin intermediate. Therefore, lycopene is a key intermediate that may be used either for retinal or bacterioruberin biosynthesis and the LyeJ enzyme is a potential target for regulation of these pathways.
In the haloarchaea H. salinarum, when bacterioopsin (BO) is not linked to the retinal, LyeJ activity is repressed, so that bacterioruberin production is reduced and lycopene is available for the synthesis of retinal. The regulation of retinal and BO synthesis is coordinated in Archaea (H. salinarum for example) through the control of gene transcription. Low oxygen pressure results in the induction of the bop gene, which encodes bacterioopsin (BO) [80][81]. This increased transcription of the bop gene is mediated by the bacterioopsin gene activator (Bat), a transcription factor that also activates the transcription of genes encoding the retinal biosynthetic enzymes [82]. A study suggested that another transcription factor, brz, is also required for the induction of BO and related enzymes [83]. The most plausible explanation for these findings is that BO, in the absence of its retinal cofactor, directly binds LyeJ, and that when sufficient retinal is available to convert BO to bacteriorhodopsin, LyeJ is released and catalyzes the conversion of lycopene to bacterioruberin [79] (Figure 5).
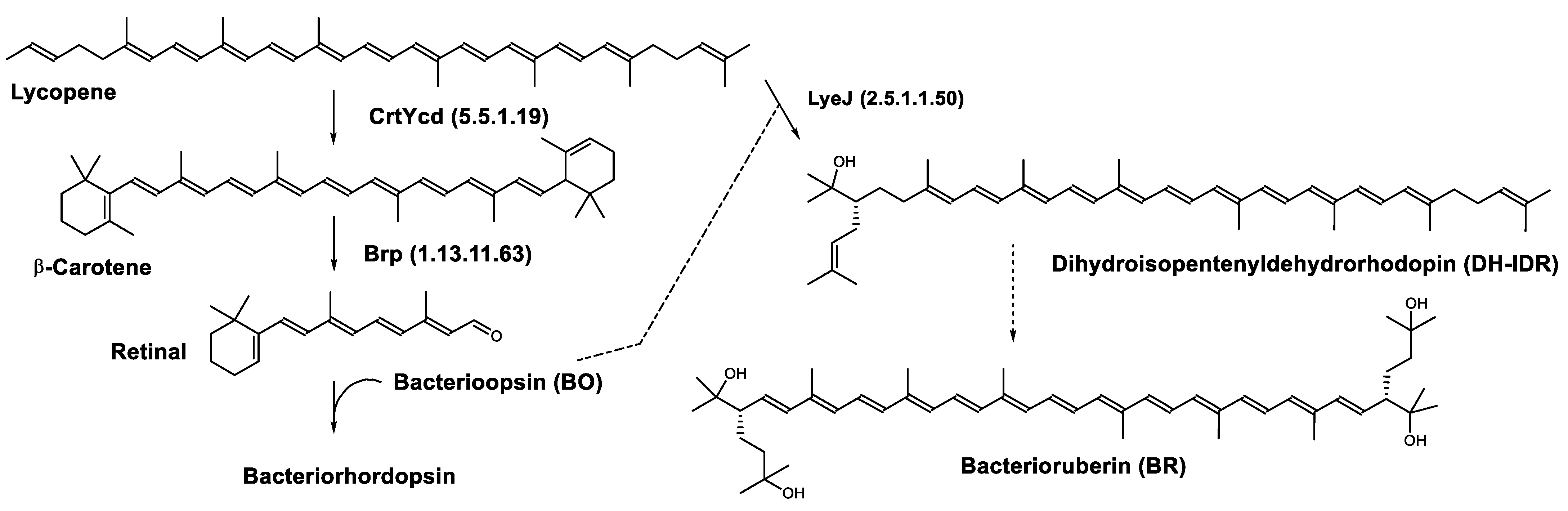
Figure 5. Biosynthesis of bacteriorhodopsin and bacterioruberin in Halobacterium salinarum [79]. It is hypothesized that bacterioruberin synthesis is inhibited by bacterioopsin as indicated by the dashed line. Numbers in parenthesis indicate the enzyme number or EC number.
This regulatory mechanism plays a major role in the response of H. salinarum and possibly haloarchaea in general to environmental changes. Opsin-based inhibition of bacterioruberin biosynthesis is a widely distributed mechanism in Archaea and Bacteria. In addition, interactions between opsins and other proteins are numerous and raise the possibility that this regulatory mechanism confers a selective advantage on organisms that express ion-pumping rhodopsins.
Carotenoids Biosynthesis in Sulfolobus shibatae
Zeaxanthin glycosides are the major carotenoids present in Sulfolobus shibatae. The (all-E)-isomers 1, 2, and 4 (Figure 6) had been found before in bacteria, algae, and other organism, but never in an archaeal species [84]. Naturally occurring (Z)-isomers of zeaxanthin glycosides were first reported in 1989. It can be speculated that these glycosides act in Sulfolobus as membrane reinforcers, as previously proposed for bacteria [84]. Although shorter than C50-carotenoids, their short length may be compensated by the presence of the carbohydrate moieties to act as membrane reinforces.
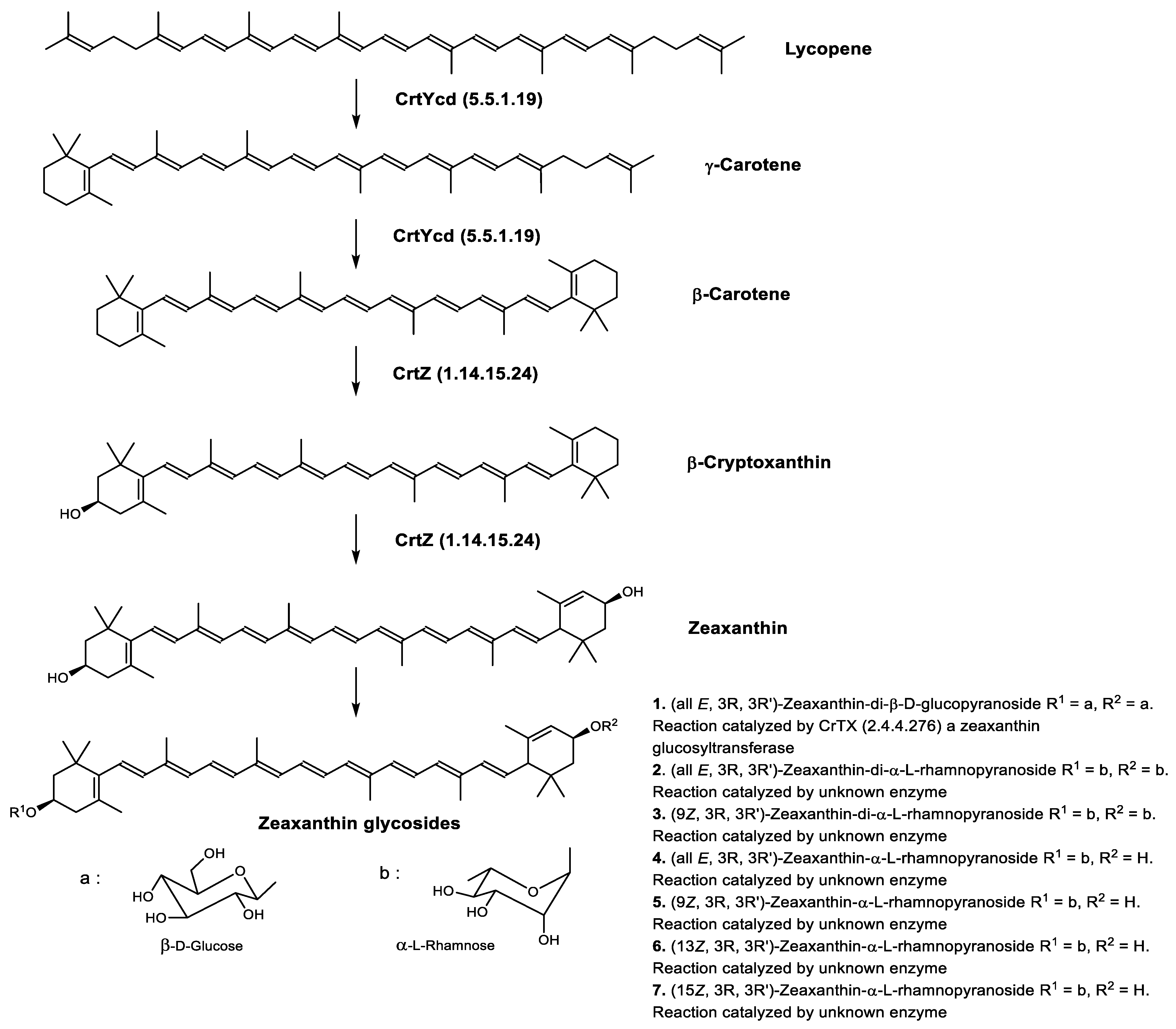
Figure 6. Sulfolobus shibatae carotenoids reconstructed pathways [85]. The bold numbers 1. to 7. correspond to all identified zeaxanthin glycosides. Numbers in parenthesis indicate the enzyme number or EC number.
2.2.4. Biological Activities of Archaea Carotenoids
2.4. Biological Activities of Archaea Carotenoids
Table 1 summarizes the biological activities described in the literature for carotenoids and apocarotenoids produced by Archaea.
Table 1. Review of Archaea carotenoids biological and pharmacological activities according to the literature.
| Common Name | Biological Activities and Properties |
|---|---|
| C40 Hydrocarbons | |
| β-Carotene | Photosynthetic pigment present in all organisms making oxygenic photosynthesis from cyanobacteria to higher plants [86][87] Photoprotective agent [88] Present in the reaction-center complexes (RC) and the light-harvesting complexes (LHC) of photosystem I (PSI) as well as the RC and the core LHC of photosystem II (PSII) [89][90][91] Provitamin A [92][93] Antioxidant—free radical scavenger/singlet oxygen quencher, 101 times stronger than that of α-tocopherol (SOAC value: 101) [88][89][94][95][96][97] Anti-apoptotic agent (mouse model of traumatic brain injury) preventing loss of Bcl2, preventing accumulation of Bax, and preventing accumulation or activation of Caspase 3 [98] β-carotene-derived retinoid acids bind with retinoid acid receptor (PAR) and retinoid X receptor. Receptors dimerization leads to a functional transcription factor regulating gene expression during neurogenesis. Neuroprotective activity against apoptosis [98] Anticarcinogenic activity [99][100] Cell differentiation and proliferation promoter by upregulating Connexin 43 gene [101] Immune response enhancement in animals and humans [102] Found in human skin throughout the epidermis, dermis and also the subcutaneous [103] |
| (13Z)-β-Carotene | Provitamin A activity (10% of that of all-trans-β-carotene) [104][105] |
| Lycopene or (all-E)-Lycopene | Photoprotection [92] Radioprotection against gamma-radiation-induced cellular damages [106] Strong antioxidant —strong singlet-quenching ability—141 times stronger than that of α-tocopherol (SOAC value: 141) [89][92][94][95][96][97] Protecting mitochondria and mitochondrial DNA by antioxidant properties and treatment with lycopene prevents loss of mitochondrial inner membrane potential during ROS challenge [98] Antiradical activity [107] Found in human skin throughout the epidermis, dermis and also the subcutaneous [103] Anticarcinogenic activity by reducing insulin growth factor 1 (IGF-1) stimulation with an increase in membrane-associated IGF-binding proteins; also slows down IGF-1-stimulated cell cycle progression [92][94][99][100][108] Inhibits the proliferation of androgen-dependent human prostate tumor cells through activation of PPARγ-LXRα-ABCA1 [109] Anti-apoptotic agent—preventing loss of Bcl2 and Bcl-xL, preventing accumulation of Bax, preventing accumulation or release of Cytochrome C, and preventing accumulation or activation of Caspase 3 [98] Anti-inflammation [92] Anti-inflammatory effects of lycopene may help alleviate neuropsychiatric diseases such as post-traumatic stress disorder and depression [98] Antimicrobial activity against S. aureus, and E. coli O-157 [110] Antifungal activity against C. albicans by arresting their cell cycle [110] Cell differentiation and proliferation promoter by upregulating Connexin 43 gene [101] Non-Provitamin A [92] |
| Phytoene | Anticarcinogenic activity [99] |
| Phytofluene | Anticarcinogenic activity—more active than β-carotene [100] |
| (all-E)-Phytofluene | No biological activity reported |
| Zeaxanthin diglucoside | No biological activity reported |
| (9Z)-Zeaxanthin-3′-rhamnoside | No biological activity reported |
| (13Z)-Zeaxanthin-3′-rhamnoside | No biological activity reported |
| (15Z)-Zeaxanthin-3′-rhamnoside | No biological activity reported |
| Zeaxanthin dirhamnoside | No biological activity reported |
| (9Z)-Zeaxanthin dirhamnoside | No biological activity reported |
| Zeaxanthin monorhamnoside | No biological activity reported |
| C45 Hydroxycarotenoids | |
| Dihydroisopentenyldehydrorhodopin (DH-IDR) | No biological activity reported |
| 2-Isopentenyl-3,4-dehydrorhodopin (IPR) |
No biological activity reported |
| C50 Hydroxycarotenoids | |
| Bacterioruberin (BR) | Antioxidant activity—much better radical scavenger than that of β-carotene as it contains 13 pairs of conjugated double bonds [30][111] limits oxidation due to H2O2 exposure [65][112] Photoprotective activity—limits oxidative DNA damage from UV irradiation [65][112] Radio protective activity—limits oxidative DNA damage from gamma irradiation [65][112] |
| Bisanhydrobacterioruberin (BABR) |
No biological activity reported |
| Dihydrobisanhydrobacterioruberin (DH-BABR) | No biological activity reported |
| 3′,4′-dihydromonoanhydrobacterioruberin | No biological activity reported |
| 1′,2′-epoxy-2′-(2,3-epoxy-3-methylbutyl)-2-(3-hydroxy-3-methylbutyl)-3′,4′-didehydro-1,2,1′,2′-tetrahydro-ψ,ψ-caroten-1-ol | No biological activity reported |
| Monoanhydrobacterioruberin (MABR) |
No biological activity reported |
| 3,4,3′,4′-tetrahydrobisanhydrobacterioruberin | No biological activity reported |
| Trisanhydrobacterioruberin | No biological activity reported |
| Apocarotenoids | |
| Retinal or Vitamin A aldehyde |
Photoreception in human retina Abolishes the function of the toxin suberitine at a stoichiometric ratio of 1:1 [113] Isomerized to 13Z-retinal under light, and isomerized back to all-trans retinal in the dark |
2.5. Biotechnological Considerations for the Production of Archaea Carotenoids
Because of the extreme growth conditions of the majority of Archaea species, the development of laboratory culture conditions has been a challenge, and only a small amount of studies discussing the growth and production of Archaea have been published to date. Archaea have historically been eclipsed by bacteria and eukaryotes in terms of public attention, industrial uses and scientific studies, although their biochemical and physiological characteristics offer possibilities for a wide range of biotechnological applications, including the production of polysaccharides, biomaterials, thermophilic enzymes, and novel pigments [114].
Carotenoids represent a significant fraction of lipids, such as 0.2% (w/w) in Haloferax alexandrinus, an extreme halophile, most of them being bacterioruberin and canthaxanthin with small quantities of 3-hydroxyechinenone and β-carotene [115]. Likewise, important quantities of bacterioruberin were found in another extremely halophilic Archaea, Haloarcula japonica [30]. In contrast, only zeaxanthin was identified in Sulfolobus shibatae, a thermoacidophilic Archaea [85].
Although the high concentration of salt in the medium corrodes stainless steel fermenters and piping systems, inexpensive materials such as plastic, carbon steel and ceramics can be used to build fermenters and piping systems to avoid corrosion, since high-pressure sterilisation is not required. Once the pigments have accumulated inside the haloArchaea cells, the next step to complete the production process is the extraction from the biomass. When carotenoid production is carried out in microalgae cells, which are well-known natural producers of carotenoids, extraction can become a key limitation in terms of process costs. The cells of many microalgae species are difficult to break down due to their cell wall composition which is highly resistant to standard cell-breaking tools, including the freezing and thawing of algal pellets in liquid nitrogen or the use of sonication. One of the main advantages of haloarchaeal species for carotenoid extraction is that low concentrations of salt cause cell lysis, thus avoiding the energy investment required to enable efficient cell disruption [115] and making carotenoids readily available for solvent extraction compared to direct extraction from unbroken cells. This means that haloarchaeal cells may be suitable for maximizing pigment recovery at lower costs compared to other microorganisms.
The massic yield of carotenoids in haloarchaea is mainly dependent on the strain and culture conditions used. The total carotenoid content in Haloarcula japonica was 335 μg·g−1 dry mass [30], and the contents in Halobacterium salinarum and Halococcus morrhuae were 89 and 45 μg·g−1, respectively [46]. A major limitation to the industrial application of Archaea enzymes and metabolites is the low productivity of the fermentation processes as a result of low growth rates and low biomass production yields. To overcome these limitations, attention has been focused on studying the physiology of Archaea of biotechnological interest and on designing bioreactors and bioprocesses that increase productivity.
In physiological studies, continuous cultures have often been reported as effective systems, especially for demonstrating the correlation between substrates and biosynthesis of enzymes and other metabolites. In fact, the non-conventional environmental conditions necessary for the culture of Archaea allow long-term experiments without contamination problems. Optimization of the growth medium is also of key importance in the production of extremophilic biomasses for subsequent exploitation in industry. Continuous culture experiments are fundamental in clarifying the metabolism of particular substrates and the significance of specific enzymes in unconventional biochemical pathways. This operational model has also helped in delineating the key growth requirements for a variety of extremophilic microorganisms, which often were demonstrated to require special minerals, amino acids or vitamins to grow at reasonable rates.
For the production of extremozymes, gene cloning in a mesophilic host easy to cultivate [116] is of crucial interest, especially for the simplification of downstream processing. Indeed, specific procedures, based on the difference in stability under extreme conditions, can be easily developed to purify the host protein product. Genes coding for several extremozymes have been cloned into heterologous hosts, with the aim of overproducing the enzyme. The best results could be obtained by applying recent molecular biology techniques and new fermentation strategies. Concerning lipids, archaeal molecules are unique in term of structure and relation to their topology and function in membranes. This singularity is of interest for the biotechnological applications of these compounds as they can be used for the formation of liposomes with remarkable thermostability and tightness against solute leakage [117]. In vitro and in vivo studies have shown that archaeosomes (liposomes from archaeal lipids) are safe and that their stability offers superior alternatives for several biotechnological applications, including drug delivery systems, gene delivery systems or cancer imaging agents [118]. Halophilic microorganisms, in particular Halomonas spp., are also interesting for the production of various products used in different industrial domains. Many halophiles are capable of accumulating polyhydroxyalkanoates (PHAs) [119], a family of biodegradable and biocompatible polyesters that are developed in an industrial value chain ranging from bioplastics, biofuels, fine chemicals to medicine [120][121]. PHA accumulation by halophiles was first documented in 1972 [122] and other halophiles were further exploited and found to synthesize high amounts of PHAs [123][124][125]. The use of halophiles for PHA production can reduce the costs of fermentation and recovery processes because of the high salt concentration that prevents contamination by non-halophiles, and the limits energy consumption and complexity of the sterilization process. In addition, the cell membranes of haloarchaea are easy to lyse in the absence of salt, especially when using distilled water. This osmotic shock makes it much easier and cheaper to recover PHAs from crude extracts. In this view, halophiles provide an inexpensive platform for the biotechnological production of PHAs at industrial scale.
3. Focus on Bacterioruberin Biological Activities
The ecophysiological function of bacterioruberin is to serve as a photon sensor in Archaerhodopsin-2 (aR2), a retinal protein–carotenoid complex present in the membrane of Halorubrum sp. This complex functions as a light-driven proton pump and ensures ATP production for the cell [70][126][127][128]. In addition, it was shown that Haloferax mediterranei (Order: Halobacteriales) is able to counteract oxidative stress generated by high concentrations of hydrogen peroxide through the production of bacterioruberin. Bacterioruberin successfully neutralizes hydrogen peroxide, confirming that cells use this carotenoid to maintain oxidative balance and that this compound is indeed very effective against ROS [68]. Studies with Halobacterium halobium carotenoid extracts have also demonstrated the antiproliferative activity of bacterioruberin in human cancer cell lines HepG2 (hepatocarcinoma) and MCF-7 (breast) [129], associated to the activation of caspases and induction of apoptosis. Half of current anticancer drugs are derived from natural compounds or their mimetics [130] and carotenoids are of great interest due to their lack of oral toxicity. A dose-dependent antiproliferative effect in HepG2 cells has also been reported for carotenoid extracts from Haloplanus vescus (62.5 nM–1 μM) and Halogeometricum limi (approximately 1 μM). An anti-haemolytic activity of bacterioruberin was also demonstrated in vitro [131] and bacterioruberin is a potent inhibitor of matrix metalloproteinase 9 (MMP-9) [132]. MMP-9 is one of the key proteases involved in cancer metastasis and angiogenesis [133]. The mechanisms involved in the anticancer activity of bacterioruberin are currently unclear and the fact that the same molecule can have both antioxidant and pro-oxidant activity has been debated within the scientific community. Therefore, its value in treating tumors in humans remains to be clearly established [134]. To date, no studies have been performed to evaluate whether bacterioruberin is resorbed in animal or human models and no information exists regarding its toxicity. However, given its photoprotective and antioxidant properties, the possibility of using it for cosmetic applications and in particular for sunscreen products seems realistic.
References
- Danon, A.; Stoeckenius, W. Photophosphorylation in Halobacterium halobium. Proc. Natl. Acad. Sci. USA 1974, 71, 1234–1238.
- Koch, M.K.; Oesterhelt, D. MpcT Is the Transducer for Membrane Potential Changes in Halobacterium Salinarum: MpcT Is the Transducer for ΔΨ Changes in Halobacterium salinarum. Mol. Microbiol. 2005, 55, 1681–1694.
- Oesterhelt, D.; Stoeckenius, W. Rhodopsin-like Protein from the Purple Membrane of Halobacterium Halobium. Nat. New Biol. 1971, 233, 149–152.
- Kandori, H. Ion-Pumping Microbial Rhodopsins. Front. Mol. Biosci. 2015, 2, 52.
- Ernst, O.P.; Lodowski, D.T.; Elstner, M.; Hegemann, P.; Brown, L.S.; Kandori, H. Microbial and Animal Rhodopsins: Structures, Functions, and Molecular Mechanisms. Chem. Rev. 2014, 114, 126–163.
- Spudich, J.L.; Yang, C.-S.; Jung, K.-H.; Spudich, E.N. Retinylidene Proteins: Structures and Functions from Archaea to Humans. Annu. Rev. Cell Dev. Biol. 2000, 16, 365–392.
- Lee, S.Y.; Chang, H.N.; Um, Y.S.; Hong, S.H. Bacteriorhodopsin Production by Cell Recycle Culture of Halobacterium Halobium. Biotechnol. Lett. 1998, 20, 763–765.
- Oren, A. Molecular Ecology of Extremely Halophilic Archaea and Bacteria. FEMS Microbiol. Ecol. 2002, 39, 1–7.
- Wagner, N.L.; Greco, J.A.; Ranaghan, M.J.; Birge, R.R. Directed Evolution of Bacteriorhodopsin for Applications in Bioelectronics. J. R. Soc. Interface 2013, 10, 20130197.
- Kawasaki, K.; Yin, J.-J.; Subczynski, W.K.; Hyde, J.S.; Kusumi, A. Pulse EPR Detection of Lipid Exchange between Protein-Rich Raft and Bulk Domains in the Membrane: Methodology Development and Its Application to Studies of Influenza Viral Membrane. Biophys. J. 2001, 80, 738–748.
- Hampp, N.; Oesterhelt, D. Bacteriorhodopsin and Its Potential in Technical Applications. In Nanobiotechnology; Niemeyer, C.M., Mirkin, C.A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 146–167. ISBN 978-3-527-60245-2.
- Puthenveetil, R.; Vinogradova, O. Optimization of the Design and Preparation of Nanoscale Phospholipid Bilayers for Its Application to Solution NMR: Nanodiscs and NMR. Proteins 2013, 81, 1222–1231.
- Knoblauch, C.; Griep, M.; Friedrich, C. Recent Advances in the Field of Bionanotechnology: An Insight into Optoelectric Bacteriorhodopsin, Quantum Dots, and Noble Metal Nanoclusters. Sensors 2014, 14, 19731–19766.
- Grout, M.J. Application of Bacteriorhodopsin for Optical Limiting Eye Protection Filters. Opt. Mater. 2000, 14, 155–160.
- Kahya, N.; Brown, D.A.; Schwille, P. Raft Partitioning and Dynamic Behavior of Human Placental Alkaline Phosphatase in Giant Unilamellar Vesicles. Biochemistry 2005, 44, 7479–7489.
- Dummer, A.M.; Bonsall, J.C.; Cihla, J.B.; Lawry, S.M.; Johnson, G.C.; Peck, R.F. Bacterioopsin-Mediated Regulation of Bacterioruberin Biosynthesis in Halobacterium Salinarum. J. Bacteriol. 2011, 193, 5658–5667.
- Patil, A.V.; Premaraban, T.; Berthoumieu, O.; Watts, A.; Davis, J.J. Engineered Bacteriorhodopsin: A Molecular Scale Potential Switch. Chem. Eur. J. 2012, 18, 5632–5636.
- Gagez, A.-L.; Thiery, V.; Pasquet, V.; Cadoret, J.-P.; Picot, L. Epoxycarotenoids and Cancer. Review. Bioact. Compd. 2012, 8, 109–141.
- Pajot, A.; Hao Huynh, G.; Picot, L.; Marchal, L.; Nicolau, E. Fucoxanthin from Algae to Human, an Extraordinary Bioresource: Insights and Advances in up and Downstream Processes. Mar. Drugs 2022, 20, 222.
- de Oliveira-Júnior, R.G.; Grougnet, R.; Bodet, P.-E.; Bonnet, A.; Nicolau, E.; Jebali, A.; Rumin, J.; Picot, L. Updated Pigment Composition of Tisochrysis Lutea and Purification of Fucoxanthin Using Centrifugal Partition Chromatography Coupled to Flash Chromatography for the Chemosensitization of Melanoma Cells. Algal Res. 2020, 51, 102035.
- De Oliveira, R.G., Jr.; Bonnet, A.; Braconnier, E.; Groult, H.; Prunier, G.; Beaugeard, L.; Grougnet, R.; da Silva Almeida, J.R.G.; Ferraz, C.A.A.; Picot, L. Bixin, an Apocarotenoid Isolated from Bixa Orellana, L., Sensitizes Human Melanoma Cells to Dacarbazine-Induced Apoptosis through ROS-Mediated Cytotoxicity. Food Chem. Toxicol. 2019, 125, 549–561.
- De Oliveira, R.G., Jr.; Adrielly, A.F.C.; da Silva Almeida, J.R.G.; Grougnet, R.; Thiéry, V.; Picot, L. Sensitization of Tumor Cells to Chemotherapy by Natural Products: A Systematic Review of Preclinical Data and Molecular Mechanisms. Fitoterapia 2018, 129, 383–400.
- Juin, C.; Oliveira, R.G.D., Jr.; Fleury, A.; Oudinet, C.; Pytowski, L.; Bérard, J.-B.; Nicolau, E.; Thiéry, V.; Lanneluc, I.; Beaugeard, L.; et al. Zeaxanthin from Porphyridium Purpureum Induces Apoptosis in Human Melanoma Cells Expressing the Oncogenic BRAF V600E Mutation and Sensitizes Them to the BRAF Inhibitor Vemurafenib. Rev. Bras. Farmacogn. 2018, 28, 457–467.
- Pasquet, V.; Morisset, P.; Ihammouine, S.; Chepied, A.; Aumailley, L.; Berard, J.-B.; Serive, B.; Kaas, R.; Lanneluc, I.; Thiery, V.; et al. Antiproliferative Activity of Violaxanthin Isolated from Bioguided Fractionation of Dunaliella Tertiolecta Extracts. Mar. Drugs 2011, 9, 819–831.
- Haguet, Q.; Bonnet, A.; Bérard, J.-B.; Goldberg, J.; Joguet, N.; Fleury, A.; Thiéry, V.; Picot, L. Antimelanoma Activity of Heterocapsa Triquetra Pigments. Algal Res. 2017, 25, 207–215.
- Ávila-Román, J.; García-Gil, S.; Rodríguez-Luna, A.; Motilva, V.; Talero, E. Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids. Mar. Drugs 2021, 19, 531.
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188.
- Lau, T.-Y.; Kwan, H.-Y. Fucoxanthin Is a Potential Therapeutic Agent for the Treatment of Breast Cancer. Mar. Drugs 2022, 20, 370.
- Maeda, H.; Kanno, S.; Kodate, M.; Hosokawa, M.; Miyashita, K. Fucoxanthinol, Metabolite of Fucoxanthin, Improves Obesity-Induced Inflammation in Adipocyte Cells. Mar. Drugs 2015, 13, 4799–4813.
- Yatsunami, R.; Ando, A.; Yang, Y.; Takaichi, S.; Kohno, M.; Matsumura, Y.; Ikeda, H.; Fukui, T.; Nakasone, K.; Fujita, N.; et al. Identification of Carotenoids from the Extremely Halophilic Archaeon Haloarcula japonica. Front. Microbiol. 2014, 5, 100.
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82.
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant Compounds from Microalgae: A Review. Mar. Drugs 2021, 19, 549.
- D’Orazio, N.; Gemello, E.; Gammone, M.; de Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A Treasure from the Sea. Mar. Drugs 2012, 10, 604–616.
- Gammone, M.; D’Orazio, N. Anti-Obesity Activity of the Marine Carotenoid Fucoxanthin. Mar. Drugs 2015, 13, 2196–2214.
- Gammone, M.; Riccioni, G.; D’Orazio, N. Marine Carotenoids against Oxidative Stress: Effects on Human Health. Mar. Drugs 2015, 13, 6226–6246.
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential Anti-Atherosclerotic Properties of Astaxanthin. Mar. Drugs 2016, 14, 35.
- Martin, L. Fucoxanthin and Its Metabolite Fucoxanthinol in Cancer Prevention and Treatment. Mar. Drugs 2015, 13, 4784–4798.
- Sathasivam, R.; Ki, J.-S. A Review of the Biological Activities of Microalgal Carotenoids and Their Potential Use in Healthcare and Cosmetic Industries. Mar. Drugs 2018, 16, 26.
- Wu, H.; Niu, H.; Shao, A.; Wu, C.; Dixon, B.; Zhang, J.; Yang, S.; Wang, Y. Astaxanthin as a Potential Neuroprotective Agent for Neurological Diseases. Mar. Drugs 2015, 13, 5750–5766.
- Sandmann, G. Carotenoids of Biotechnological Importance. In Biotechnology of Isoprenoids; Schrader, J., Bohlmann, J., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer International Publishing: Cham, Germany, 2014; Volume 148, pp. 449–467. ISBN 978-3-319-20106-1.
- Yabuzaki, J. Carotenoids Database: Structures, Chemical Fingerprints and Distribution among Organisms. Database 2017, 2017, bax004.
- Rao, A.; Rao, L. Carotenoids and Human Health. Pharmacol. Res. 2007, 55, 207–216.
- Rivera, S.M.; Canela-Garayoa, R. Analytical Tools for the Analysis of Carotenoids in Diverse Materials. J. Chromatogr. A 2012, 1224, 1–10.
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16.
- Jehlička, J.; Oren, A. Raman Spectroscopy in Halophile Research. Front. Microbiol. 2013, 4, 380.
- Mandelli, F.; Miranda, V.S.; Rodrigues, E.; Mercadante, A.Z. Identification of Carotenoids with High Antioxidant Capacity Produced by Extremophile Microorganisms. World J. Microbiol. Biotechnol. 2012, 28, 1781–1790.
- Lutnaes, B.F.; Oren, A.; Liaaen-Jensen, S. New C(40)-Carotenoid Acyl Glycoside as Principal Carotenoid in Salinibacter Ruber, an Extremely Halophilic Eubacterium. J. Nat. Prod. 2002, 65, 1340–1343.
- Jehlička, J.; Edwards, H.G.M.; Oren, A. Bacterioruberin and Salinixanthin Carotenoids of Extremely Halophilic Archaea and Bacteria: A Raman Spectroscopic Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 106, 99–103.
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, Pharmacology and Treatment: Carotenoids: Pharmacology and Treatment. Br. J. Pharmacol. 2017, 174, 1290–1324.
- Mortensen, A.; Skibsted, L.H.; Truscott, T.G. The Interaction of Dietary Carotenoids with Radical Species. Arch. Biochem. Biophys. 2001, 385, 13–19.
- Bayr, H. Reactive Oxygen Species. Crit. Care Med. 2005, 33, S498–S501.
- Jomova, K.; Valko, M. Health Protective Effects of Carotenoids and Their Interactions with Other Biological Antioxidants. Eur. J. Med. Chem. 2013, 70, 102–110.
- Koklesova, L.; Liskova, A.; Samec, M.; Buhrmann, C.; Samuel, S.M.; Varghese, E.; Ashrafizadeh, M.; Najafi, M.; Shakibaei, M.; Büsselberg, D.; et al. Carotenoids in Cancer Apoptosis—The Road from Bench to Bedside and Back. Cancers 2020, 12, 2425.
- Oren, A. The Microbiology of Red Brines. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 113, pp. 57–110. ISBN 978-0-12-820709-3.
- Wang, X.-D.; Russell, R.M. Procarcinogenic and Anticarcinogenic Effects of β-Carotene. Nutr. Rev. 2009, 57, 263–272.
- Ribeiro, D.; Freitas, M.; Silva, A.M.S.; Carvalho, F.; Fernandes, E. Antioxidant and Pro-Oxidant Activities of Carotenoids and Their Oxidation Products. Food Chem. Toxicol. 2018, 120, 681–699.
- Chang, M.X.; Xiong, F. Astaxanthin and Its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules 2020, 25, 5342.
- Esatbeyoglu, T.; Rimbach, G. Canthaxanthin: From Molecule to Function. Mol. Nutr. Food Res. 2017, 61, 1600469.
- Bonet, M.L.; Ribot, J.; Galmés, S.; Serra, F.; Palou, A. Carotenoids and Carotenoid Conversion Products in Adipose Tissue Biology and Obesity: Pre-Clinical and Human Studies. Biochim. Biophys. Acta Mol. Cell Biol Lipids 2020, 1865, 158676.
- Rodrigo-Baños, M.; Montero, Z.; Torregrosa-Crespo, J.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Haloarchaea: A Promising Biosource for Carotenoid Production. In Carotenoids: Biosynthetic and Biofunctional Approaches; Misawa, N., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2021; Volume 1261, pp. 165–174. ISBN 9789811573590.
- Rodrigo-Baños, M.; Garbayo, I.; Vílchez, C.; Bonete, M.; Martínez-Espinosa, R. Carotenoids from Haloarchaea and Their Potential in Biotechnology. Mar. Drugs 2015, 13, 5508–5532.
- Montero-Lobato, Z.; Ramos-Merchante, A.; Fuentes, J.; Sayago, A.; Fernández-Recamales, Á.; Martínez-Espinosa, R.; Vega, J.; Vílchez, C.; Garbayo, I. Optimization of Growth and Carotenoid Production by Haloferax Mediterranei Using Response Surface Methodology. Mar. Drugs 2018, 16, 372.
- Kelly, M.; Jensen, S.L.; Theander, O.; Cyvin, S.J.; Hagen, G. Bacterial Carotenoids. XXVI. C50-Carotenoids. 2. Bacterioruberin. Acta Chem. Scand. 1967, 21, 2578–2580.
- Lazrak, T.; Wolff, G.; Albrecht, A.-M.; Nakatani, Y.; Ourisson, G.; Kates, M. Bacterioruberins Reinforce Reconstituted Halobacterium Lipid Membranes. Biochim. Biophys. Acta Biomembr. 1988, 939, 160–162.
- Shahmohammadi, H.R.; Asgarani, E.; Terato, H.; Saito, T.; Ohyama, Y.; Gekko, K.; Yamamoto, O.; Ide, H. Protective Roles of Bacterioruberin and Intracellular KCl in the Resistance of Halobacterium Salinarium against DNA-Damaging Agents. J. Radiat. Res. 1998, 39, 251–262.
- Fang, C.-J.; Ku, K.-L.; Lee, M.-H.; Su, N.-W. Influence of Nutritive Factors on C50 Carotenoids Production by Haloferax Mediterranei ATCC 33500 with Two-Stage Cultivation. Bioresour. Technol. 2010, 101, 6487–6493.
- Will Chen, C.; Hsu, S.; Lin, M.-T.; Hsu, Y. Mass Production of C50 Carotenoids by Haloferax Mediterranei in Using Extruded Rice Bran and Starch under Optimal Conductivity of Brined Medium. Bioprocess. Biosyst. Eng. 2015, 38, 2361–2367.
- Giani, M.; Martínez-Espinosa, R.M. Carotenoids as a Protection Mechanism against Oxidative Stress in Haloferax Mediterranei. Antioxidants 2020, 9, 1060.
- Gruszecki, W.I.; Strzałka, K. Carotenoids as Modulators of Lipid Membrane Physical Properties. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 108–115.
- Yoshimura, K.; Kouyama, T. Structural Role of Bacterioruberin in the Trimeric Structure of Archaerhodopsin-2. J. Mol. Biol. 2008, 375, 1267–1281.
- Naziri, D.; Hamidi, M.; Hassanzadeh, S.; Tarhriz, V.; Maleki Zanjani, B.; Nazemyieh, H.; Hejazi, M.A.; Hejazi, M.S. Analysis of Carotenoid Production by Halorubrum sp. TBZ126; an Extremely Halophilic Archeon from Urmia Lake. Adv. Pharm. Bull. 2014, 4, 61–67.
- Squillaci, G.; Parrella, R.; Carbone, V.; Minasi, P.; La Cara, F.; Morana, A. Carotenoids from the Extreme Halophilic Archaeon Haloterrigena Turkmenica: Identification and Antioxidant Activity. Extremophiles 2017, 21, 933–945.
- Horikoshi, K.; Aono, R.; Nakamura, S. The Triangular Halophilic ArchaebacteriumHaloarcula Japonica Strain TR-1. Experientia 1993, 49, 497–502.
- Yang, Y.; Yatsunami, R.; Ando, A.; Miyoko, N.; Fukui, T.; Takaichi, S.; Nakamura, S. Complete Biosynthetic Pathway of the C50 Carotenoid Bacterioruberin from Lycopene in the Extremely Halophilic Archaeon Haloarcula japonica. J. Bacteriol. 2015, 197, 1614–1623.
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant Activities of Carotenes and Xanthophylls. FEBS Lett. 1996, 384, 240–242.
- Naguib, Y.M.A. Antioxidant Activities of Astaxanthin and Related Carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154.
- Peck, R.F.; Johnson, E.A.; Krebs, M.P. Identification of a Lycopene β-Cyclase Required for Bacteriorhodopsin Biogenesis in the Archaeon Halobacterium salinarum. J. Bacteriol. 2002, 184, 2889–2897.
- Peck, R.F.; Echavarri-Erasun, C.; Johnson, E.A.; Ng, W.V.; Kennedy, S.P.; Hood, L.; DasSarma, S.; Krebs, M.P. Brp and Blh Are Required for Synthesis of the Retinal Cofactor of Bacteriorhodopsin in Halobacterium Salinarum. J. Biol. Chem. 2001, 276, 5739–5744.
- Peck, R.F.; Pleşa, A.M.; Graham, S.M.; Angelini, D.R.; Shaw, E.L. Opsin-Mediated Inhibition of Bacterioruberin Synthesis in Halophilic Archaea. J. Bacteriol. 2017, 199, e00303-17.
- Shand, R.F.; Betlach, M.C. Expression of the Bop Gene Cluster of Halobacterium Halobium Is Induced by Low Oxygen Tension and by Light. J. Bacteriol. 1991, 173, 4692–4699.
- Yang, C.F.; DasSarma, S. Transcriptional Induction of Purple Membrane and Gas Vesicle Synthesis in the Archaebacterium Halobacterium Halobium Is Blocked by a DNA Gyrase Inhibitor. J. Bacteriol 1990, 172, 4118–4121.
- Baliga, N.S.; Kennedy, S.P.; Ng, W.V.; Hood, L.; DasSarma, S. Genomic and Genetic Dissection of an Archaeal Regulon. Proc. Natl. Acad. Sci. USA 2001, 98, 2521–2525.
- Tarasov, V.Y.; Besir, H.; Schwaiger, R.; Klee, K.; Furtwängler, K.; Pfeiffer, F.; Oesterhelt, D. A Small Protein from the Bop-Brp Intergenic Region of Halobacterium Salinarum Contains a Zinc Finger Motif and Regulates Bop and CrtB1 Transcription: Zinc Finger Regulator of Bop and CrtB1 Expression. Mol. Microbiol. 2008, 67, 772–780.
- Ourisson, G.; Nakatani, Y. Bacterial Carotenoids as Membrane Reinforcers: A General Role for Polyterpenoids: Membrane Stabilization. In Carotenoids Chemistry and Biology; Krinsky, N.I., Mathews-Roth, M.M., Taylor, R.F., Eds.; Springer: Boston, MA, USA, 1989; pp. 237–246.
- Kull, D.R.; Pfander, H. Isolation and Structure Elucidation of Carotenoid Glycosides from the Thermoacidophilic Archaea Sulfolobus shibatae. J. Nat. Prod. 1997, 60, 371–374.
- Yasushi, K. New Trends in Photobiology. J. Photochem. Photobiol. B Biol. 1991, 9, 265–280.
- de Las Rivas, J.; Abadía, A.; Abadía, J. A New Reversed Phase-HPLC Method Resolving All Major Higher Plant Photosynthetic Pigments. Plant. Physiol. 1989, 91, 190–192.
- Moshell, A.N.; Bjornson, L. Photoprotection in Erythropoietic Protoporphyria: Mechanism of Photoprotection by Beta Carotene. J. Investig. Dermatol. 1977, 68, 157–160.
- Takahashi, S.; Iwasaki-Kino, Y.; Aizawa, K.; Terao, J.; Mukai, K. Development of Singlet Oxygen Absorption Capacity (SOAC) Assay Method Using a Microplate Reader. J. AOAC Int. 2016, 99, 193–197.
- Sugawara, T.; Ganesan, P.; Li, Z.; Manabe, Y.; Hirata, T. Siphonaxanthin, a Green Algal Carotenoid, as a Novel Functional Compound. Mar. Drugs 2014, 12, 3660–3668.
- Stamatakis, K.; Tsimilli-Michael, M.; Papageorgiou, G.C. On the Question of the Light-Harvesting Role of β-Carotene in Photosystem II and Photosystem I Core Complexes. Plant. Physiol. Biochem. 2014, 81, 121–127.
- Mein, J.R.; Lian, F.; Wang, X.-D. Biological Activity of Lycopene Metabolites: Implications for Cancer Prevention. Nutr. Rev. 2008, 66, 667–683.
- Vílchez, C.; Forján, E.; Cuaresma, M.; Bédmar, F.; Garbayo, I.; Vega, J.M. Marine Carotenoids: Biological Functions and Commercial Applications. Mar. Drugs 2011, 9, 319–333.
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer Chemoprevention by Carotenoids. Molecules 2012, 17, 3202–3242.
- Mukai, K. Antioxidant Activity of Foods: Development of Singlet Oxygen Absorption Capacity (SOAC) Assay Method. J. Nutr. Sci. Vitam. 2019, 65, 285–302.
- Ouchi, A.; Aizawa, K.; Iwasaki, Y.; Inakuma, T.; Terao, J.; Nagaoka, S.; Mukai, K. Kinetic Study of the Quenching Reaction of Singlet Oxygen by Carotenoids and Food Extracts in Solution. Development of a Singlet Oxygen Absorption Capacity (SOAC) Assay Method. J. Agric. Food Chem. 2010, 58, 9967–9978.
- Aizawa, K.; Iwasaki, Y.; Ouchi, A.; Inakuma, T.; Nagaoka, S.; Terao, J.; Mukai, K. Development of Singlet Oxygen Absorption Capacity (SOAC) Assay Method. 2. Measurements of the SOAC Values for Carotenoids and Food Extracts. J. Agric. Food Chem. 2011, 59, 3717–3729.
- Park, H.-A.; Hayden, M.M.; Bannerman, S.; Jansen, J.; Crowe-White, K.M. Anti-Apoptotic Effects of Carotenoids in Neurodegeneration. Molecules 2020, 25, 3453.
- Nishino, H.; Murakosh, M.; Ii, T.; Takemura, M.; Kuchide, M.; Kanazawa, M.; Mou, X.Y.; Wada, S.; Masuda, M.; Ohsaka, Y.; et al. Carotenoids in Cancer Chemoprevention. Cancer Metastasis Rev. 2002, 21, 257–264.
- Tsushima, M.; Maoka, T.; Katsuyama, M.; Kozuka, M.; Matsuno, T.; Tokuda, H.; Nishino, H.; Iwashima, A. Inhibitory Effect of Natural Carotenoids on Epstein-Barr Virus Activation Activity of a Tumor Promoter in Raji Cells. A Screening Study for Anti-Tumor Promoters. Biol. Pharm. Bull. 1995, 18, 227–233.
- Zhang, L.X.; Cooney, R.V.; Bertram, J.S. Carotenoids Up-Regulate Connexin43 Gene Expression Independent of Their Provitamin A or Antioxidant Properties. Cancer Res. 1992, 52, 5707–5712.
- Chew, B.P.; Park, J.S. Carotenoid Action on the Immune Response. J. Nutr. 2004, 134, 257S–261S.
- Obana, A.; Gohto, Y.; Gellermann, W.; Ermakov, I.V.; Sasano, H.; Seto, T.; Bernstein, P.S. Skin Carotenoid Index in a Large Japanese Population Sample. Sci. Rep. 2019, 9, 9318.
- Nagao, A.; Olson, J.A. Enzymatic Formation of 9-Cis, 13-Cis, and All-Trans Retinals from Isomers of Beta-Carotene. FASEB J. 1994, 8, 968–973.
- Eroglu, A.; Harrison, E.H. Carotenoid Metabolism in Mammals, Including Man: Formation, Occurrence, and Function of Apocarotenoids. J. Lipid Res. 2013, 54, 1719–1730.
- Srinivasan, M.; Devipriya, N.; Kalpana, K.B.; Menon, V.P. Lycopene: An Antioxidant and Radioprotector against γ-Radiation-Induced Cellular Damages in Cultured Human Lymphocytes. Toxicology 2009, 262, 43–49.
- Méndez-Robles, M.D.; Permady, H.H.; Jaramillo-Flores, M.E.; Lugo-Cervantes, E.C.; Cardador-Martínez, A.; Canales-Aguirre, A.A.; López-Dellamary, F.; Cerda-García-Rojas, C.M.; Tamariz, J. C-26 and C-30 Apocarotenoids from Seeds of Ditaxis Heterantha with Antioxidant Activity and Protection against DNA Oxidative Damage. J. Nat. Prod. 2006, 69, 1140–1144.
- Ford, N.; Elsen, A.; Zuniga, K.; Lindshield, B.; Erdman, J. Lycopene and Apo-12′-Lycopenal Reduce Cell Proliferation and Alter Cell Cycle Progression in Human Prostate Cancer Cells. Nutr. Cancer 2011, 63, 256–263.
- Yang, C.-M.; Lu, I.-H.; Chen, H.-Y.; Hu, M.-L. Lycopene Inhibits the Proliferation of Androgen-Dependent Human Prostate Tumor Cells through Activation of PPARγ-LXRα-ABCA1 Pathway. J. Nutr. Biochem. 2012, 23, 8–17.
- Sung, W.S.; Lee, I.-S.; Lee, D.G. Damage to the Cytoplasmic Membrane and Cell Death Caused by Lycopene in Candida Albicans. J. Microbiol. Biotechnol. 2007, 17, 1797–1804.
- Saito, T.; Miyabe, Y.; Ide, H.; Yamamoto, O. Hydroxyl Radical Scavenging Ability of Bacterioruberin. Radiat. Phys. Chem. 1997, 50, 267–269.
- Kottemann, M.; Kish, A.; Iloanusi, C.; Bjork, S.; DiRuggiero, J. Physiological Responses of the Halophilic Archaeon Halobacterium sp. Strain NRC1 to Desiccation and Gamma Irradiation. Extremophiles 2005, 9, 219–227.
- Müller, W.E.G.; Wang, X.; Binder, M.; von Lintig, J.; Wiens, M.; Schröder, H.C. Differential Expression of the Demosponge (Suberites Domuncula) Carotenoid Oxygenases in Response to Light: Protection Mechanism Against the Self-Produced Toxic Protein (Suberitine). Mar. Drugs 2012, 10, 177–199.
- Giani, M.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Haloarchaeal Carotenoids: Healthy Novel Compounds from Extreme Environments. Mar. Drugs 2019, 17, 524.
- Asker, D.; Awad, T.; Ohta, Y. Lipids of Haloferax Alexandrinus Strain TMT: An Extremely Halophilic Canthaxanthin-Producing Archaeon. J. Biosci. Bioeng. 2002, 93, 37–43.
- Van der Oost, J.; Ciaramella, M.; Moracci, M.; Pisani, F.M.; Rossi, M.; de Vos, W.M. Molecular Biology of Hyperthermophilic Archaea. In Biotechnology of Extremophiles; Antranikian, G., Ed.; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 1998; Volume 61, pp. 87–115. ISBN 978-3-540-63817-9.
- Gambacorta, A.; Gliozzi, A.; De Rosa, M. Archaeal Lipids and Their Biotechnological Applications. World J. Microbiol. Biotechnol. 1995, 11, 115–131.
- Patel, G.B.; Sprott, G.D. Archaeobacterial Ether Lipid Liposomes (Archaeosomes) as Novel Vaccine and Drug Delivery Systems. Crit. Rev. Biotechnol. 1999, 19, 317–357.
- Quillaguamán, J.; Guzmán, H.; Van-Thuoc, D.; Hatti-Kaul, R. Synthesis and Production of Polyhydroxyalkanoates by Halophiles: Current Potential and Future Prospects. Appl. Microbiol. Biotechnol. 2010, 85, 1687–1696.
- Chen, G.-Q. A Microbial Polyhydroxyalkanoates (PHA) Based Bio- and Materials Industry. Chem. Soc. Rev. 2009, 38, 2434.
- Chen, G.-Q.; Patel, M.K. Plastics Derived from Biological Sources: Present and Future: A Technical and Environmental Review. Chem. Rev. 2012, 112, 2082–2099.
- Kirk, R.G.; Ginzburg, M. Ultrastructure of Two Species of Halobacterium. J. Ultrastruct. Res. 1972, 41, 80–94.
- Legault, B.A.; Lopez-Lopez, A.; Alba-Casado, J.C.; Doolittle, W.F.; Bolhuis, H.; Rodriguez-Valera, F.; Papke, R.T. Environmental Genomics of “Haloquadratum Walsbyi” in a Saltern Crystallizer Indicates a Large Pool of Accessory Genes in an Otherwise Coherent Species. BMC Genom. 2006, 7, 171.
- Koller, M.; Hesse, P.; Bona, R.; Kutschera, C.; Atlić, A.; Braunegg, G. Potential of Various Archae- and Eubacterial Strains as Industrial Polyhydroxyalkanoate Producers from Whey. Macromol. Biosci. 2007, 7, 218–226.
- Han, J.; Lu, Q.; Zhou, L.; Zhou, J.; Xiang, H. Molecular Characterization of the PhaEC Hm Genes, Required for Biosynthesis of Poly(3-Hydroxybutyrate) in the Extremely Halophilic Archaeon Haloarcula marismortui. Appl. Environ. Microbiol. 2007, 73, 6058–6065.
- Li, Q.; Sun, Q.; Zhao, W.; Wang, H.; Xu, D. Newly Isolated Archaerhodopsin from a Strain of Chinese Halobacteria and Its Proton Pumping Behavior. Biochim. Biophys. Acta Biomembr. 2000, 1466, 260–266.
- Feng, J.; Liu, H.-C.; Chu, J.-F.; Zhou, P.-J.; Tang, J.-A.; Liu, S.-J. Genetic Cloning and Functional Expression in Escherichia Coli of an Archaerhodopsin Gene from Halorubrum Xinjiangense. Extremophiles 2006, 10, 29–33.
- Cao, Z.; Ding, X.; Peng, B.; Zhao, Y.; Ding, J.; Watts, A.; Zhao, X. Novel Expression and Characterization of a Light Driven Proton Pump Archaerhodopsin 4 in a Halobacterium Salinarum Strain. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 390–398.
- Abbes, M.; Baati, H.; Guermazi, S.; Messina, C.; Santulli, A.; Gharsallah, N.; Ammar, E. Biological Properties of Carotenoids Extracted from Halobacterium Halobium Isolated from a Tunisian Solar Saltern. BMC Complement. Altern. Med. 2013, 13, 255.
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803.
- Hou, J.; Cui, H.-L. In Vitro Antioxidant, Antihemolytic, and Anticancer Activity of the Carotenoids from Halophilic Archaea. Curr. Microbiol. 2018, 75, 266–271.
- Hegazy, G.E.; Abu-Serie, M.M.; Abo-Elela, G.M.; Ghozlan, H.; Sabry, S.A.; Soliman, N.A.; Abdel-Fattah, Y.R. In Vitro Dual (Anticancer and Antiviral) Activity of the Carotenoids Produced by Haloalkaliphilic Archaeon Natrialba sp. M6. Sci. Rep. 2020, 10, 5986.
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249.
- Singh, K.; Bhori, M.; Kasu, Y.A.; Bhat, G.; Marar, T. Antioxidants as Precision Weapons in War against Cancer Chemotherapy Induced Toxicity—Exploring the Armoury of Obscurity. Saudi Pharm. J. 2018, 26, 177–190.
More
