The extensive use of pesticides may negatively affect human health. Additionally, it is one of the main reasons for the decline of pollinators and is thus a hazard for most crops and biodiversity as a whole. Good candidates for the replacement of pesticides with ones less toxic to humans and pollinators are natural products (bioactive compounds extracted from plants), even though it should be kept in mind that some of them can be toxic too. Ailanthus altissima (Mill.), swingle, known also as tree of heaven, (Simaroubaceae) is one of the most aggressive alien invasive plants. It demonstrates a high tolerance to various habitat conditions and a potent propagation ability. This plant has a prominent ability to suppress the seed development of local vegetation.
- biopesticides
- essential oils
- quassinoids
- invasive plants’ management
1. Ethnobotanical Data about Ailanthus altissima (Mill.) Swingle
2. Chemical Constituents of Ailanthus altissima and Extraction Methods
3. Essential Oil of Ailanthus altissima: Composition and Extraction Overview
4. Quassinoids Extraction, Fractionation, and Isolation Overview
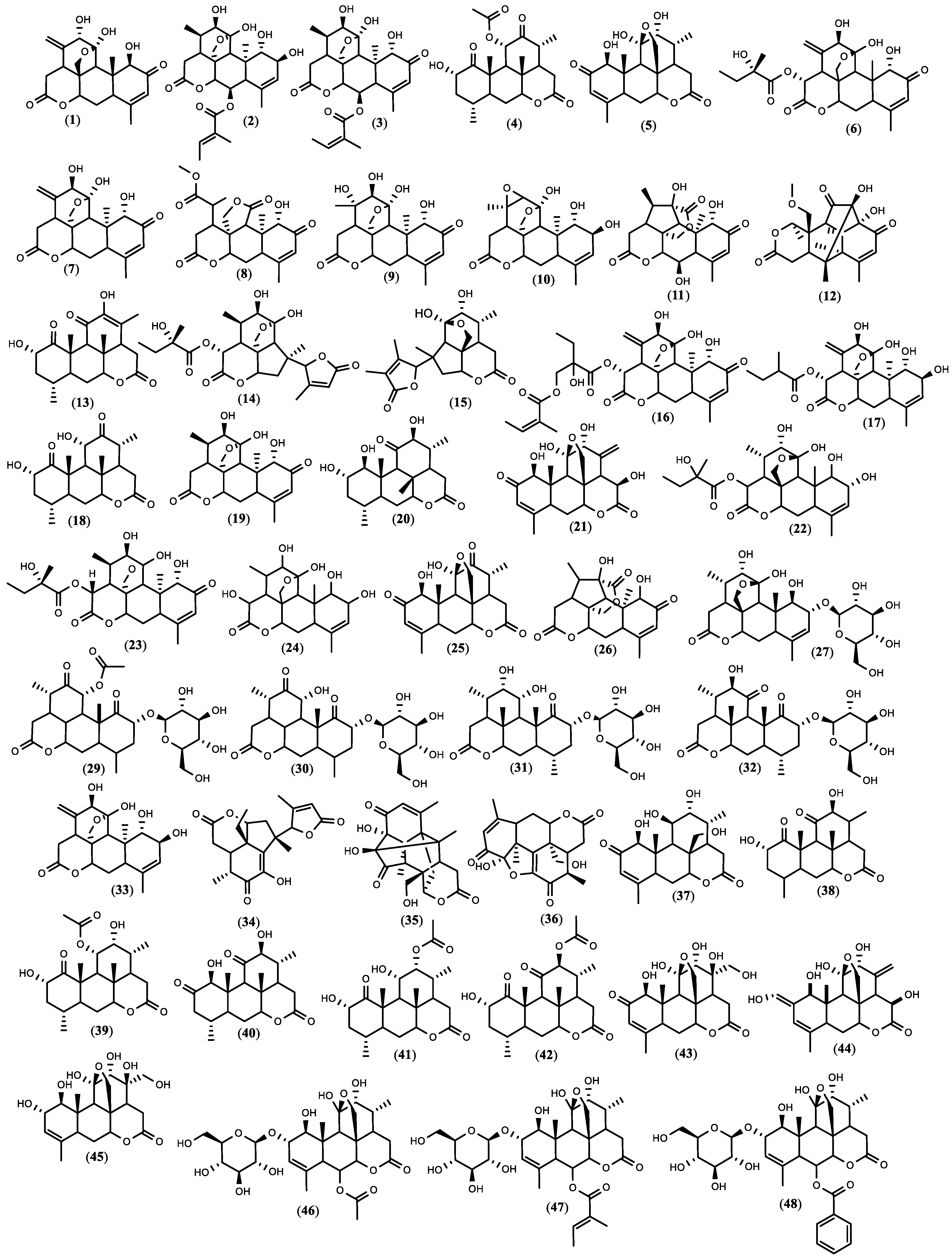
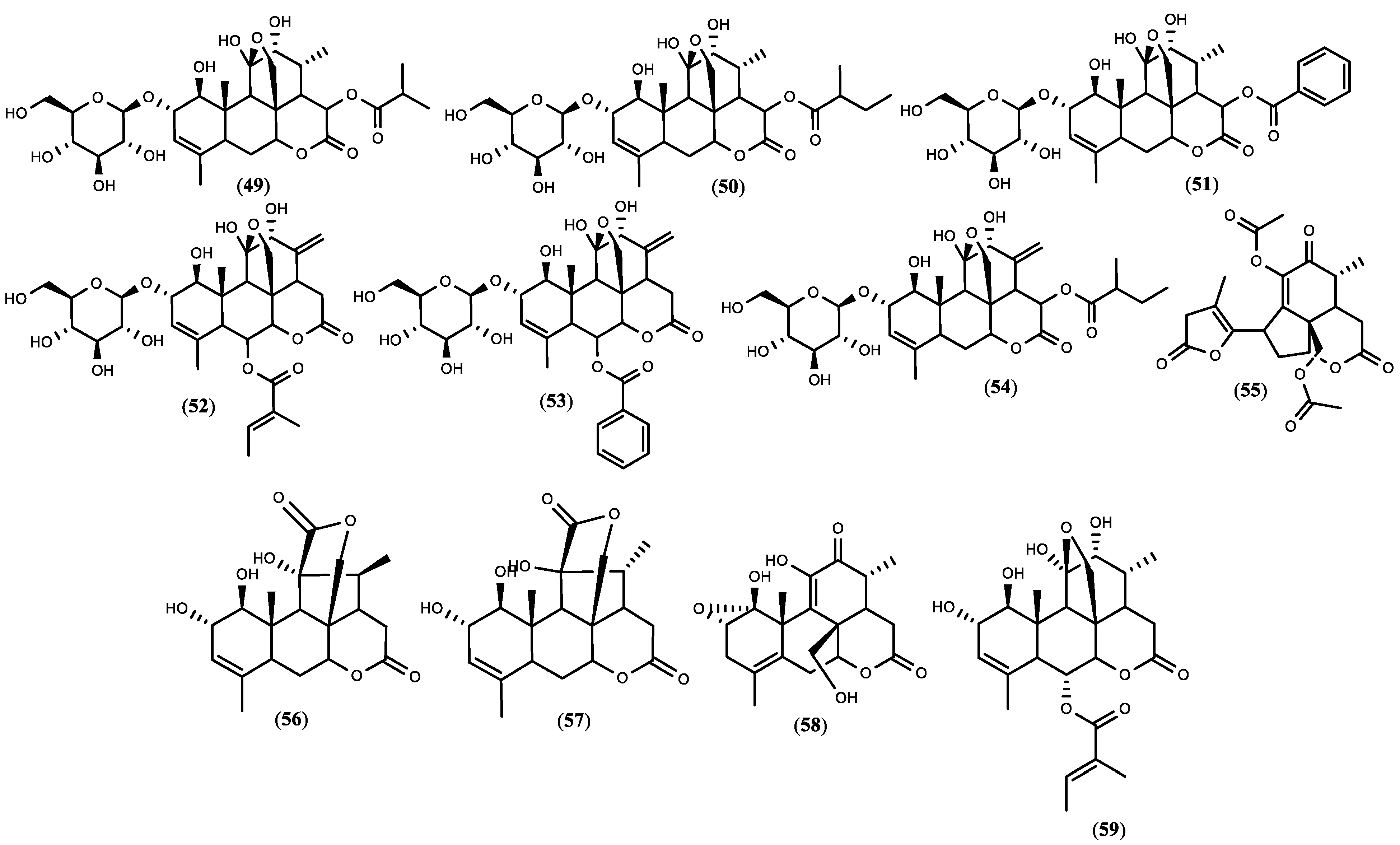 Figure 1. The structure of quassinoids isolated from A. altissima. The compound numbers correspond to the list in Table 1.
Figure 1. The structure of quassinoids isolated from A. altissima. The compound numbers correspond to the list in Table 1.5. Biopesticide Potential of Ailanthus altissima and Tests’ Design
5.1. Phytotoxicity Assay of Ailanthus altissima
Essential Oil Phytotoxicity
Phytotoxicity of Polar Ailanthus altissima Extracts
5.2. Antifungal Activity
5.3. Fumigant and Insect Repellent Activity
Essential Oil Fumigant and Insect Repellent Activity
Polar Extracts’ Fumigant and Insect-Repellent Activity
References
- Li, X.; Li, Y.; Ma, S.; Zhao, Q.; Wu, J.; Duan, L.; Wang, S. Traditional uses, phytochemistry, and pharmacology of Ailanthus altissima (Mill.) Swingle bark: A comprehensive review. J. Ethnopharmacol. 2021, 275, 114121.
- Lü, J.H.; He, Y.Q. Fumigant toxicity of Ailanthus altissima Swingle, Atractylodes lancea (Thunb.) DC. and Elsholtzia stauntonii Benth extracts on three major stored-grain insects. Ind. Crops Prod. 2010, 32, 681–683.
- Ohmoto, T.; Koike, K.; Sakamoto, Y. Studies on the constituents of A. altissima Swingle II. The alkaloid constituent. Chem. Pharm. Bull. 1981, 29, 390–395.
- Ohmoto, T.; Koike, K. Studies on the constituents of A. altissima Swingle III. The alkaloid constituents. Chem. Pharm. Bull. 1984, 32, 170–173.
- Mastelić, J.; Jerković, I. Volatile Constituents from the Leaves of Young and Old Ailanthus altissima (Mili.) Swingle Tree. Croat. Chem. Acta 2002, 75, 189–197.
- Kozuharova, E.; Lebanova, H.; Getov, I.; Benbassat, N.; Kochmarov, V. Ailanthus altissima (Mill.) Swingle—A terrible invasive pest in Bulgaria or potential useful medicinal plant? Bothalia 2014, 44, 213–230.
- Zhelev, I.; Georgiev, K.; Dimitrova-Dyulgerova, I. Carotenoid profile of Ailanthus altissima stem bark, in-vitro antioxidant and antineoplastic activities. World J. Pharm. Res. 2016, 5, 1816.
- Cho, S.K.; Jeong, M.; Jang, D.S.; Choi, J.H. Anti-inflammatory Effects of Canthin-6-one Alkaloids from Ailanthus altissima. Planta Med. 2018, 50, 527–535.
- Poljuha, D.; Sladonja, B.; Šola, I.; Dudaš, S.; Bilić, J.; Rusak, G.; Eloff, J.N. Phenolic composition of leaf extracts of Ailanthus altissima (Simaroubaceae) with antibacterial and antifungal activity equivalent to standard antibiotics. Nat. Prod. Commun. 2017, 12, 1934578X1701201021.
- Du, Y.Q.; Yan, Z.Y.; Shi, S.C.; Hou, Z.L.; Huang, X.X.; Song, S.J. Benzoic acid derivatives from the root barks of Ailanthus altissima. J. Asian Nat. Prod. Res. 2021, 23, 103–109.
- Du, Y.Q.; Yan, Z.Y.; Chen, J.J.; Wang, X.B.; Huang, X.X.; Song, S.J. The identification of phenylpropanoids isolated from the root bark of Ailanthus altissima (Mill.) Swingle. Nat. Prod. Res. 2021, 35, 1139–1146.
- Du, Y.Q.; Bai, M.; Yu, X.Q.; Lv, T.M.; Lin, B.; Huang, X.X.; Song, S.J. Quassinoids from the Root Barks of Ailanthus altissima: Isolation, Configurational Assignment, and Cytotoxic Activities. Chin. J. Chem. 2021, 39, 879–886.
- Wang, C.M.; Li, H.F.; Wang, X.K.; Li, W.G.; Su, Q.; Xiao, X.; Zhang, C.H. Ailanthus altissima-derived ailanthone enhances gastric cancer cell apoptosis by inducing the repression of base excision repair by downregulating p23 Expression. Int. J. Biol. Sci. 2021, 17, 2811.
- Duan, Z.K.; Lin, B.; Du, Y.Q.; Li, C.; Yu, X.Q.; Xue, X.B.; Huang, X.X. Monoterpenoid coumarins and monoterpenoid phenylpropanoids from the root bark of Ailanthus altissima. New J. Chem. 2021, 45, 1100–1108.
- Caramelo, D.; Pedro, S.I.; Marques, H.; Simão, A.Y.; Rosado, T.; Barroca, C.; Gallardo, E. Insights into the Bioactivities and Chemical Analysis of Ailanthus altissima (Mill.) Swingle. Appl. Sci. 2021, 11, 11331.
- Bray, D.H.; Boardman, P.; ONeill, M.J.; Chan, K.L.; Phillipson, J.D.; Warhurst, D.C.; Suffness, M. Plants as a source of antimalarial drugs 5. Activities of Ailanthus altissima stem constituents and of some related quassinoids. Phytother. Res. 1987, 1, 22–24.
- Okunade, A.L.; Bikoff, R.E.; Casper, S.J.; Oksman, A.; Goldberg, D.E.; Lewis, W.H. Antiplasmodial activity of extracts and quassinoids isolated from seedlings of Ailanthus altissima (Simaroubaceae). Phytother. Res. 2003, 17, 675–677.
- Li, Y.; Zhao, M.; Zhang, Z. Quantitative proteomics reveals the antifungal effect of canthin-6-one isolated from Ailanthus altissima against Fusarium oxysporum f. sp. cucumerinum in vitro. PLoS ONE 2021, 16, e0250712.
- Landrigan, P.J. Pesticides and Human Reproduction. JAMA Intern. Med. 2018, 178, 26–27.
- Adeyemi, J.A.; Ukwenya, V.O.; Arowolo, O.K.; Olise, C.C. Pesticides-induced Cardiovascular Dysfunctions: Prevalence and Associated Mechanisms. Curr. Hypertens. Rev. 2021, 17, 27–34.
- Needleman, H.L.; Gunnoe, C.; Leviton, A.; Reed, R.; Peresie, H.; Maher, C.; Barrett, P. Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N. Engl. J. Med. 1979, 300, 689–695.
- FAO. Pollinators Vital to Our Food Supply under Threat. 2021. Available online: http://www.fao.org/news/story/en/item/384726/icode/ (accessed on 25 July 2021).
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 2006, 313, 351–354.
- Brown, M.J.; Paxton, R.J. The conservation of bees: A global perspective. Apidologie 2009, 40, 410–416.
- Potts, S.; Biesmeijer, K.; Bommarco, R.; Breeze, T.; Carvalheiro, L.; Franzén, M.; González-Varo, J.P.; Holzschuh, A.; Kleijn, D.; Klein, A.-M.; et al. Status and Trends of European Pollinators. Key Findings of the STEP Project; Pensoft Publishers: Sofia, Bulgaria, 2015; p. 72.
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353.
- Carvalheiro, L.G.; Kunin, W.E.; Keil, P.; Aguirre-Gutiérrez, J.; Ellis, W.N.; Fox, R.; Biesmeijer, J.C. Species richness declines and biotic homogenisation have slowed down for NW-European pollinators and plants. Ecol. Lett. 2013, 16, 870–878.
- Ollerton, J.; Erenler, H.; Edwards, M.; Crockett, R. Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 2014, 346, 1360–1362.
- Albouchi, F.; Hassen, I.; Casabianca, H.; Hosni, K. Phytochemicals, antioxidant, antimicrobial and phytotoxic activities of Ailanthus altissima (Mill.) Swingle leaves. S. Afr. J. Bot. 2013, 87, 164–174.
- El Ayeb-Zakhama, A.; Ben Salem, S.; Sakka-Rouis, L.; Flamini, G.; Ben Jannet, H.; Harzallah-Skhiri, F. Chemical Composition and phytotoxic effects of essential oils obtained from Ailanthus altissima (Mill.) Swingle cultivated in Tunisia. Chem. Biodivers. 2014, 11, 1216–1227.
- Kozuharova, E.; Benbassat, N.; Berkov, S.; Ionkova, I. Ailanthus altissima and Amorpha fruticosa—Invasive arboreal alien plants as cheap sources of valuable essential oils. Pharmacia 2020, 67, 71.
- Lü, J.; Wu, S. Bioactivity of essential oil from Ailanthus altissima bark against 4 major stored-grain insects. Afr. J. Microbiol. Res. 2010, 4, 154–157.
- Zhou, L.; Wang, J.; Wang, K.; Xu, J.; Zhao, J.; Shan, T.; Luo, C. Secondary metabolites with antinematodal activity from higher plants. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2012; Volume 37, pp. 67–114.
- He, Q.; Xiao, H.; Li, J.; Liu, Y.; Jia, M.; Wang, F.; Zhang, Y.; Wang, W.; Wang, S. Fingerprint analysis and pharmacological evaluation of Ailanthus altissima. Int. J. Mol. Med. 2018, 41, 3024–3032.
- Quintana, N.; El Kassis, E.G.; Stermitz, F.R.; Vivanco, J.M. Phytotoxic compounds from roots of Centaurea diffusa Lam. Plant Signal. Behav. 2009, 4, 9–14.
- De Martino, L.; Formisano, C.; Mancini, E.; Feo, V.D.; Piozzi, F.; Rigano, D.; Senatore, F. Chemical composition and phytotoxic effects of essential oils from four Teucrium species. Nat. Prod. Commun. 2010, 5, 1969–1976.
- Szabó, L. Juglone index—A possibility for expressing allelopathic potential of plant taxa with various life strategies. Acta Bot. Hung. 1999, 42, 295–305.
- Csiszár, Á. Allelopathic effects of invasive woody plant species in Hungary. Acta Silv. Lignaria Hung. 2009, 5, 9–17. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.1066.4899&rep=rep1&type=pdf (accessed on 25 July 2022).
- Csiszár, Á.; Korda, M.; Schmidt, D.; Šporčić, D.; Süle, P.; Teleki, B.; Tiborcz, V.; Zagyvai, G.; Bartha, D. Allelopathic potential of some invasive plant species occurring in Hungary. Allelopath. J. 2013, 31, 309–318.
- Novak, N.; Novak, M.; Barić, K.; Šćepanović, M.; Ivić, D. Allelopathic potential of segetal and ruderal invasive alien plants. J. Cent. Eur. Agric. 2018, 19, 408–422.
- Heisy, R. Allelopathic and herbicidal effects of extracts from tree of heaven (Ailanthus altissima). Am. J. Bot. 1990, 77, 662–670.
- Tsao, R.; Romanchuk, F.E.; Peterson, C.J.; Coats, J.R. Plant growth regulatory effect and insecticidal activity of extracts of tree of Heaven (Ailanthus altissima L.). BMC Ecol. 2002, 2, 1. Available online: https://bmcecol.biomedcentral.com/articles/10.1186/1472-6785-2-1 (accessed on 25 July 2022).
- Bostan, C.; Borlea, F.; Mihoc, C.; Selesan, M. Ailanthus altissima species invasion on biodiversity caused by potential allelopathy. J. Agric. Sci. 2014, 46, 95–103. Available online: http://cormoran.portiledefier.ro/wp-content/uploads/2013/02/bostan_cristian_1.pdf (accessed on 25 July 2022).
- Sladonja, B.; Pohulja, D.; Sušek, M.; Dudaš, S. Herbicidal effect of Ailanthus altissima leaves water extracts on Medicago sativa seeds germination. In Book of Abstracts of the 3rd Conference with International Participation Conference VIVUS; Biotechnical Centre Naklo: Naklo, Slovenia, 2014; pp. 476–481. Available online: http://civ.iptpo.hr/wp-content/uploads/publikacije/Znanstveni%20rad%20u%20zborniku%20skupa_VIVUS_2014.pdf (accessed on 25 July 2022).
- Heisey, R.M. Identification of an allelopathic compound from Ailanthus altissima (Simaroubaceae) and characterization of its herbicidal activity. Am. J. Bot. 1996, 83, 192–200.
- De Feo, V.; Mancini, E.; Voto, E.; Curini, M.; Digilio, M.C. Bioassay-oriented isolation of an insecticide from Ailanthus altissima. J. Plant Interact. 2009, 4, 119–123.
- Casinovi, C.G.; Ceccherelli, P.; Fardella, G.; Grandolini, G. Isolation and structure of a quassinoid from Ailanthus glandulosa. Phytochemistry 1983, 22, 2871–2873.
- Lin, L.-J.; Peiser, G.; Ying, B.-P.; Mathias, K.; Karasina, F.; Wang, Z.; Itatani, J.; Green, L.; Hwang, Y.-S. Identification of plant growth inhibitory principles in Ailanthus altissima and Castela tortuosa. J. Agric. Food Chem. 1995, 43, 1706–1711.
- De Feo, V.; De Martino, L.; Quaranta, E.; Pizza, C. Isolation of phytotoxic compounds from tree-of-heaven (Ailanthus altissima Swingle). J. Agric. Food Chem. 2003, 51, 1177–1180.
- De Feo, V.; Martino, L.D.; Santoro, A.; Leone, A.; Pizza, C.; Franceschelli, S.; Pascale, M. Antiproliferative effects of tree-of-heaven (Ailanthus altissima Swingle). Phytother. Res. 2005, 19, 226–230.
- Lebedev, V.G.; Krutovsky, K.V.; Shestibratov, K.A. Fell Upas Sits, the Hydra-Tree of Death†, or the Phytotoxicity of Trees. Molecules 2019, 24, 1636.
- Borchardt, J.R.; Wyse, D.L.; Sheaffer, C.C.; Kauppi, K.L.; Fulcher, R.G.; Ehlke, N.J.; Biesboer, D.D.; Bey, R.F. Antimicrobial activity of native and naturalized plants of Minnesota and Wisconsin. J. Med. Plant Res. 2008, 2, 98–110.
- Heisey, R.M.; Heisey, T.K. Herbicidal effects under field conditions of Ailanthus altissima bark extract, which contains ailanthone. Plant Soil 2003, 256, 85–99.
- Anonymous. National Center for Biotechnology Information. PubChem Database. Ailanthone, CID=72965; 2019. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ailanthone (accessed on 1 January 2022).
- Balkan, B.; Balkan, S.; Aydoğdu, H.; Özcan, Ö. Antifungal activities of Ailanthus altissima Swingle and Juglans regia L. leaves against some cereal fungi. J. Appl. Environ. Biol. Sci. 2014, 8, 76–79.
- Jabeen, K.; Asad, S.; Zakria, M. Antifungal Evaluation and Phytochemical Identification of Selected Botanicals against Ceratocystis manginecans Causing Mango Sudden Death. J. Plant Pathol. Microbiol. 2018, 9, 465.
- Joshi, B.C.; Pandey, A.; Chaurasia, L.; Pal, M.; Sharma, R.P.; Khare, A. Antifungal activity of the stem bark of Ailanthus excelsa. Fitoterapia 2003, 74, 689–691.
- Chen, J.J.; Bai, W.; Lu, Y.B.; Feng, Z.Y.; Gao, K.; Yue, J.M. Quassinoids with Inhibitory Activities against Plant Fungal Pathogens from Picrasma javanica. J. Nat. Prod. 2021, 84, 2111–2120.
- Lü, J. The insecticidal activities of Ailanthus altissima extracts on several kinds of important stored-grain insects. Grain Storage 2007, 36, 17–20.
- Lü, J.H.; Lu, Y.J.; Hu, Y.Y. Controlling effects of three plant essential oils on Liposcelis paeta. J. Henan Agric. Sci. 2006, 5, 18.
- Lü, J.H.; Shi, Y.L. The bioactivitiy of essential oil from Ailanthus altissima Swingle (Sapindales: Simaroubaceae) bark on Lasioderma serricorne (Fabricius) (Coleoptera: Anobiidae). Adv. Mater. Res. 2012, 365, 428–432.
- Wei, J.; Kang, L. Roles of (Z)-3-hexenol in plant-insect interactions. Plant Signal. Behav. 2011, 6, 369–371.
- Flint, H.M.; Salter, S.S.; Walters, S. Caryophyllene: An attractant for the green lacewing. Environ. Entomol. 1979, 8, 1123–1125.
- Goulson, D. The Garden Jungle: Or Gardening to Save the Planet; Random House: New York, NY, USA, 2019; p. 261.
- Gu, X.; Fang, C.; Yang, G.; Xie, Y.; Nong, X.; Zhu, J.; Wang, S.; Peng, X.; Yan, Q. Acaricidal properties of an Ailanthus altissima bark extract against Psoroptes cuniculi and Sarcoptes scabiei var. cuniculi in vitro. Exp. Appl. Acarol. 2014, 62, 225–232.
- Caboni, P.; Ntalli, N.G.; Aissani, N.; Cavoski, I.; Angioni, A. Nematicidal activity of (E, E)-2, 4-decadienal and (E)-2-decenal from Ailanthus altissima against Meloidogyne javanica. J. Agric. Food Chem. 2012, 60, 1146–1151.
- Lucchetti, L.; Zitti, S.; Taffetani, F. Ethnobotanical uses in the Ancona district (Marche region, Central Italy). J. Ethnobiol. Ethnomed. 2019, 15, 9.
- Wagner, R.L.; Card, J.A. Ailanthus altissima aqueous extract deters Spodoptera frugiperda oviposition. Gt. Lakes Entomol. 2020, 53, 11. Available online: https://scholar.valpo.edu/tgle/vol53/iss1/11 (accessed on 25 July 2022).
- Wagner, L.R.; Leach, E.M.; Wallace, J.R. Leaf Extract from Ailanthus altissima negatively impacts life history aspects in Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Kansas Entomol. Soc. 2021, 93, 140–152.
- Souza, J.R.; Carvalho, G.A.; Moura, A.P.; Couto, M.H.; Maia, J.B. Impact of insecticides used to control Spodoptera frugiperda (JE Smith) in corn on survival, sex ratio, and reproduction of Trichogramma pretiosum Riley offspring. Chil. J. Agric. Res. 2013, 73, 122–127.
- Lu, J.-H.L.; Lu, Y.J.; Tan, Y.B.; Liu, J.J.; Zhong, J.F. The controlling effects of plant extracts on Oryzaephilus surinamensis (Linnaeus). J. Henan Uni. Tech. 2006, 3, 17–20.
- Chermenskaya, T.D.; Stepanycheva, E.A.; Shchenikova, A.V.; Chakaeva, A.S. Insectoacaricidal and deterrent activities of extracts of Kyrgyzstan plants against three agricultural pests. Ind. Crops Prod. 2010, 32, 157–163.
- Stepanycheva, E.A.; Chermenskya, T.D.; Chakaeva, A.S. Effect of biologically active substances of Ailanthus altissima Mill. Swingle)(Simarubaceae) on spider mite Tetranychus urticae Koch (Akari: Tetranychidae). Agric. Chem. 2011, 4, 52–59. (In Russian)
- Polonsky, J.; Bhatnagar, S.C.; Griffiths, D.C.; Pickett, J.A.; Woodcock, C.M. Activity of quassinoids as antifeedants against aphids. J. Chem. Ecol. 1989, 15, 993–998.
- Pavela, R.; Zabka, M.; Tylova, T.; Kresinova, Z. Insecticidal activity of compounds from Ailanthus altissima against Spodoptera littoralis larvae. Pak. J. Agric. Sci. 2014, 51, 101–112. Available online: https://pakjas.com.pk/papers/2248.pdf (accessed on 25 July 2022).
- Fokt, H.; Pereira, A.; Ferreira, A.M.; Cunha, A.; Aguiar, C. How do bees prevent hive infections? The antimicrobial properties of propolis. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 1, 481–493.
- Connolly, J.D.; Hill, R.A. Triterpenoids. Nat. Prod. Rep. 2011, 28, 1087–1117.
- Slave, J. Effects of Calcium hydroxide and Quassia extract on Honey bees (Apis mellifera). In Proceedings of the 18th International Conference on Organic Fruit-Growing, Hohenheim, Germany, 19–21 February 2018; Foerdergemeinschaft Oekologischer Obstbau e.V. (FOEKO): Weinsberg, Germany, 2018; pp. 247–248.
- Yang, K.; Wen, X.; Ren, Y.; Wen, J. Control of Eucryptorrhynchus scrobiculatus (Coleoptera: Cuculionidae), a major pest of Ailanthus altissima (Sapindales: Simaroubaceae), using a modified square trap net. J. Econ. Entomol. 2018, 111, 1760–1767.
- Todorova, T.; Boyadzhiev, K.; Shkondrov, A.; Parvanova, P.; Dimitrova, M.; Ionkova, I.; Kozuharova, E.; Chankova, S. Screening of Amorpha fruticosa and Ailanthus altissima extracts for genotoxicity/antigenotoxicity, mutagenicity/antimutagenicity and carcinogenicity/anticarcinogenicity. BioRisk 2022, 17, 201–212.
