Chromatography was born approximately one century ago and has undergone outstanding technological improvements in innovation, research, and development since then that has made it fundamental to advances in knowledge at different levels, with a relevant impact on the well-being and health of individuals. Chromatography boosted a comprehensive and deeper understanding of the complexity and diversity of human–environment interactions and systems, how these interactions affect our life, and the several societal challenges currently facing, namely those related to the sustainability of our planet and the future generations. From the life sciences, which allowed to identify endogenous metabolites relevant to disease mechanisms, to the OMICS field, nanotechnology, clinical and forensic analysis, drug discovery, environment, and “foodprint”, among others, the wide range of applications of today’s chromatographic techniques is impressive. This is fueled by a great variability of powerful chromatographic instruments currently available, with very high sensitivity, resolution, and identification capacity, that provide a strong basis for an analytical platform able to support the challenging demands of the postgenomic and post COVID-19 eras.
- gas chromatography
- liquid chromatography
- science advances
- societal challenges
1. Introduction
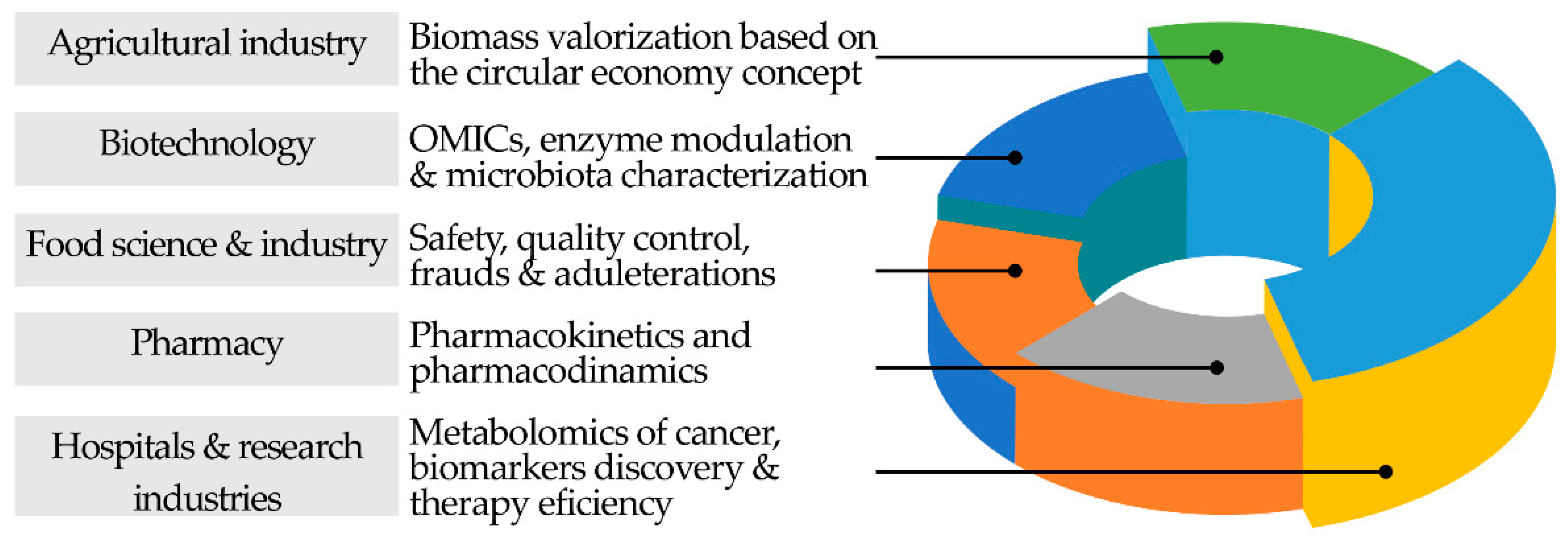
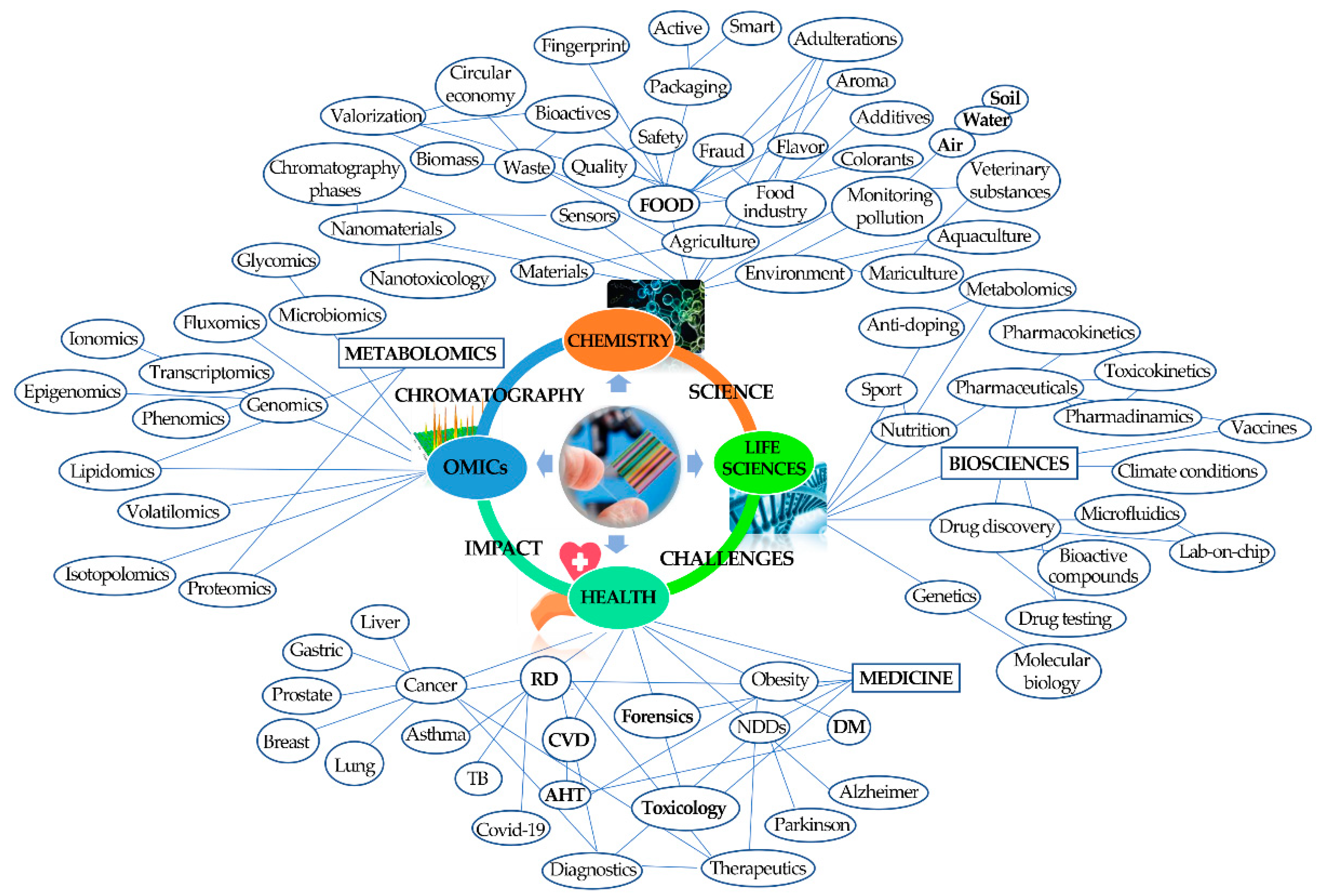
2. Chromatography Helping in Societal Challenges
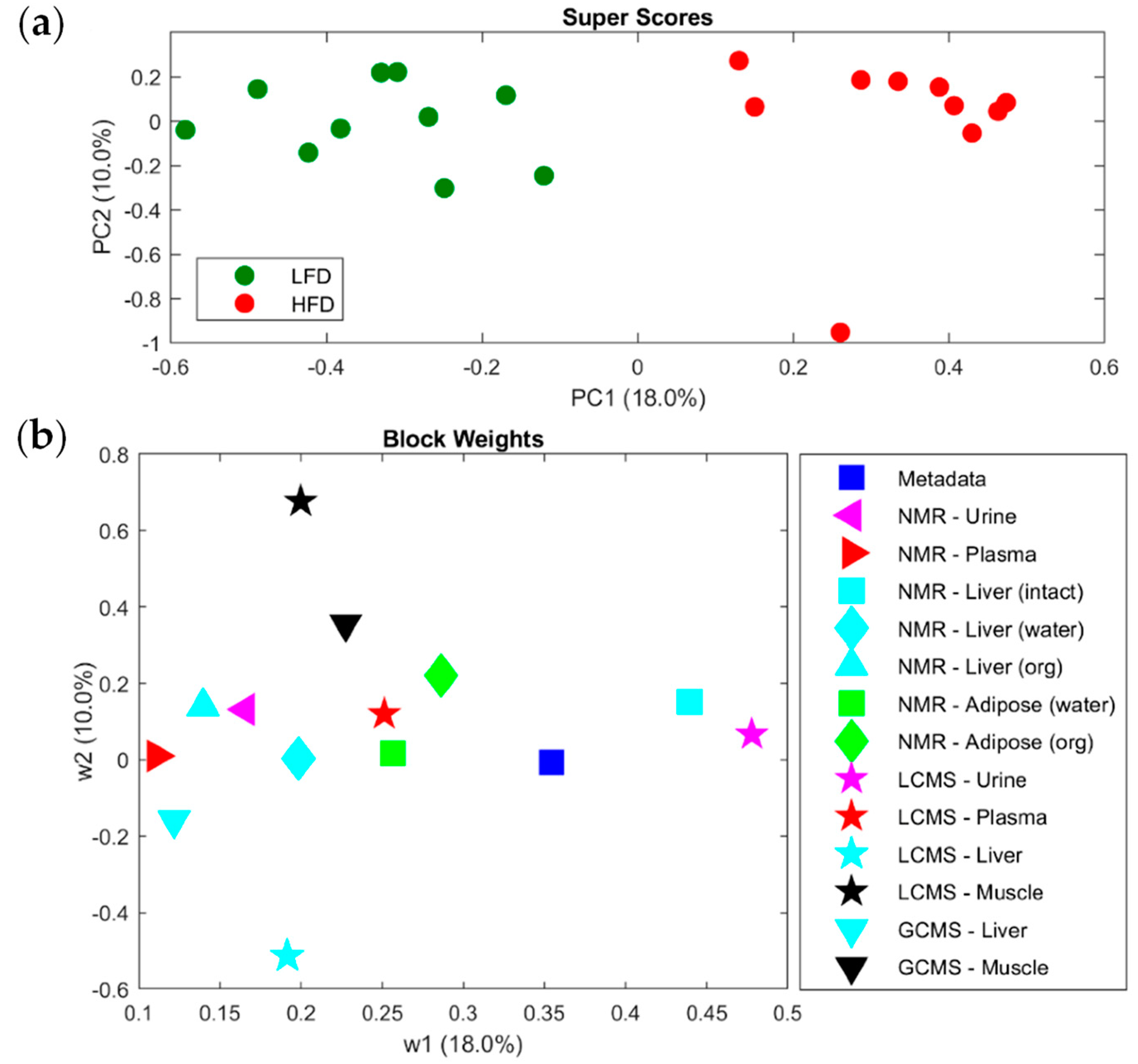
2.1. Chromatography-Based Metabolomics Utility to Unveil Disease and/or Physiological Status
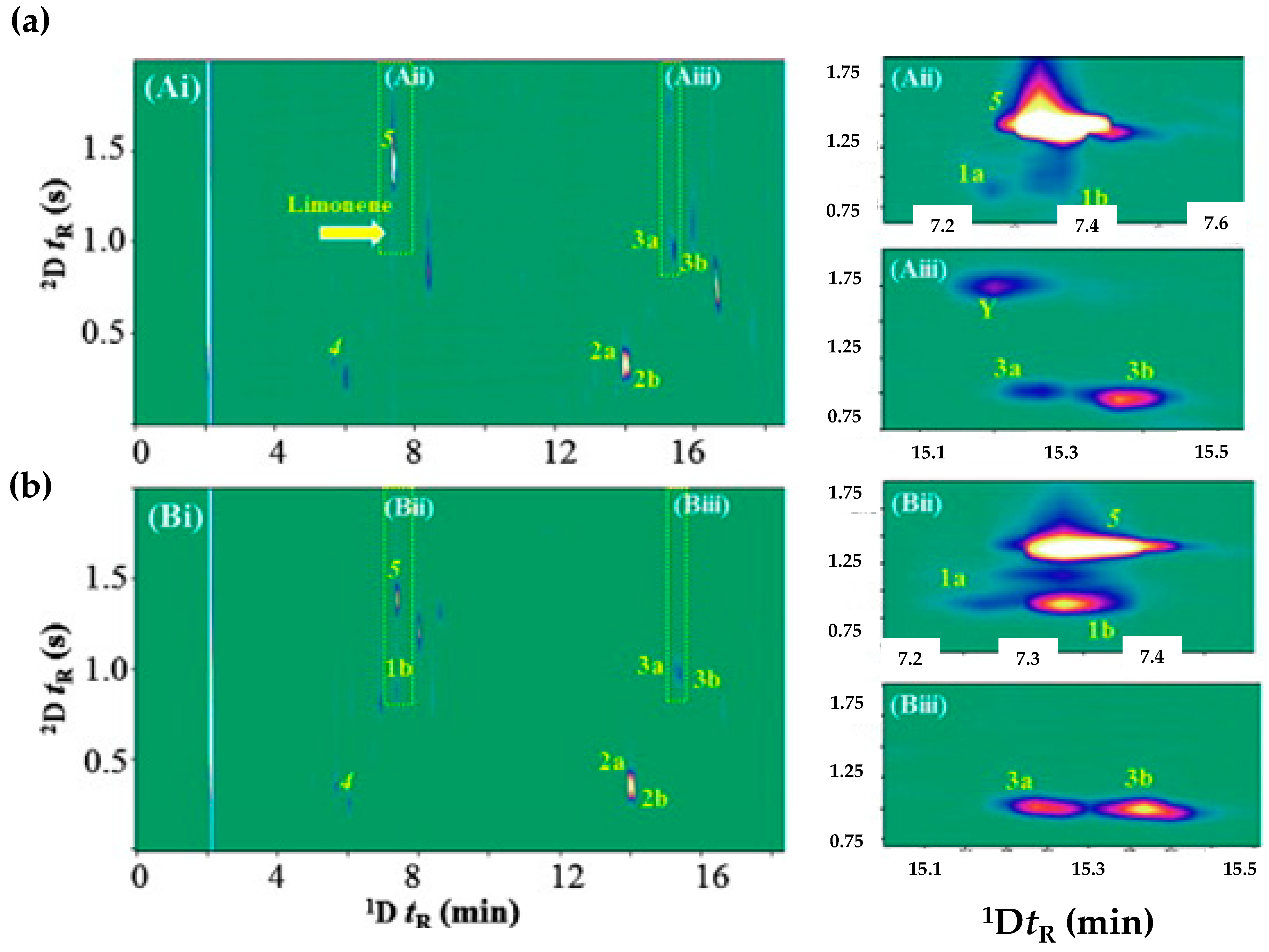
2.2. The Role of Chromatography to Respond Current Agri-Food Industries Challenges and Sustainability
2.3. Using Chromatography to Monitor Environmental Contaminants
References
- Salter, T.L.; Magee, B.A.; Waite, J.H.; Sephton, M.A. Mass Spectrometric Fingerprints of Bacteria and Archaea for Life Detection on Icy Moons. Astrobiology 2022, 22, 143–157.
- Leseigneur, G.; Bredehöft, J.H.; Gautier, T.; Giri, C.; Krüger, H.; MacDermott, A.J.; Meierhenrich, U.J.; Caro, G.M.M.; Raulin, F.; Steele, A.; et al. COSAC’s Only Gas Chromatogram Taken on Comet 67P/Churyumov-Gerasimenko. ChemPlusChem 2022, 87, e202200116.
- Cassaro, A.; Pacelli, C.; Baqué, M.; Cavalazzi, B.; Gasparotto, G.; Saladino, R.; Botta, L.; Böttger, U.; Rabbow, E.; de Vera, J.; et al. Investigation of fungal biomolecules after Low Earth Orbit exposure: A testbed for the next Moon missions. Environ. Microbiol. 2022, 24, 2938–2950.
- Silva, C.L.; Perestrelo, R.; Capelinha, F.; Tomás, H.; Câmara, J.S. An integrative approach based on GC–qMS and NMR metabolomics data as a comprehensive strategy to search potential breast cancer biomarkers. Metabolomics 2021, 17, 72.
- Lee, H.; Kang, S.J.; Lee, J.; Park, K.H.; Rhee, W.J. Isolation and Characterization of Urinary Extracellular Vesicles from Healthy Donors and Patients with Castration-Resistant Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 7134.
- Telleria, O.; Alboniga, O.E.; Clos-Garcia, M.; Nafría-Jimenez, B.; Cubiella, J.; Bujanda, L.; Falcón-Pérez, J.M. A Comprehensive Metabolomics Analysis of Fecal Samples from Advanced Adenoma and Colorectal Cancer Patients. Metabolites 2022, 12, 550.
- Avataneo, V.; De Nicolò, A.; Cusato, J.; Antonucci, M.; Manca, A.; Palermiti, A.; Waitt, C.; Walimbwa, S.; Lamorde, M.; Di Perri, G.; et al. Development and validation of a UHPLC-MS/MS method for quantification of the prodrug remdesivir and its metabolite GS-441524: A tool for clinical pharmacokinetics of SARS-CoV-2/COVID-19 and Ebola virus disease. J. Antimicrob. Chemother. 2020, 75, 1772–1777.
- Viode, A.; Smolen, K.K.; Fatou, B.; Wurie, Z.; Van Zalm, P.; Konde, M.K.; Keita, B.M.; Ablam, R.A.; Fish, E.N.; Steen, H. Plasma Proteomic Analysis Distinguishes Severity Outcomes of Human Ebola Virus Disease. mBio 2022, 13, e0056722.
- Ahmed, N.; Kidane, B.; Wang, L.; Nugent, Z.; Moldovan, N.; McElrea, A.; Shariati-Ievari, S.; Qing, G.; Tan, L.; Buduhan, G.; et al. Metabolic Alterations in Sputum and Exhaled Breath Condensate of Early Stage Non-Small Cell Lung Cancer Patients After Surgical Resection: A Pilot Study. Front. Oncol. 2022, 12, 874964.
- Bargaje, M.; Bharaswadkar, S.; Lohidasan, S.; Panda, B.K. Plasma drug concentrations of 4-drug fixed-dose combination regimen and its efficacy for treatment of pulmonary tuberculosis under National Tuberculosis Elimination Programme: A prospective pilot study. Indian J. Tuberc. 2021, 69, 311–319.
- Cozzolino, R.; Stocchero, M.; Perestrelo, R.; Câmara, J.S. Comprehensive Evaluation of the Volatomic Fingerprint of Saffron from Campania towards Its Authenticity and Quality. Foods 2022, 11, 366.
- Yang, J.; Wilson, I.; Rainville, P. Evaluation of hybrid surface technology for the analysis of the B-group vitamins by LC-ESI-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2022, 1204, 123336.
- Liu, X.; Tian, X.; Qinghong, S.; Sun, H.; Jing, L.; Tang, X.; Guo, Z.; Liu, Y.; Wang, Y.; Ma, J.; et al. Characterization of LC-MS based urine metabolomics in healthy children and adults. PeerJ 2022, 10, e13545.
- Muresan, A.A.; Rusu, A.; Roman, G.; Bala, C. Metabolomic Analysis of Normal Weight, Healthy and Unhealthy Obesity: Amino Acid Change Across the Spectrum of Metabolic Wellbeing in Women. Acta Endocrinol. 2021, 17, 427–431.
- Kheiri, A.; Aliakbarlu, J.; Tahmasebi, R. Antioxidant potential and fatty acid profile of fish fillet: Effects of season and fish species. Vet. Res. Forum 2022, 13, 91–99.
- Luo, B.; Lv, J.; Li, K.; Liao, P.; Chen, P. Structural Characterization and Anti-inflammatory Activity of a Galactorhamnan Polysaccharide from Citrus medica L. var. sarcodactylis. Front. Nutr. 2022, 9, 916976.
- Dhanshetty, M.; Shinde, R.; Goon, A.; Oulkar, D.; Elliott, C.T.; Banerjee, K. Analysis of aflatoxins and ochratoxin a in chilli powder using ultrahigh performance liquid chromatography with fluorescence detection and tandem mass spectrometry. Mycotoxin Res. 2022, 38, 193–203.
- Medina, S.; Perestrelo, R.; Silva, P.; Pereira, J.A.; Câmara, J.S. Current trends and recent advances on food authenticity technologies and chemometric approaches. Trends Food Sci. Technol. 2019, 85, 163–176.
- Focker, M.; Borne, B.H.P.; Fischer, M.; Schuh, E.; Mader, A.; Andersson, M.G.; Ali, B.M.; Fels-Klerx, H.J. Interactions between risk assessors and risk managers during three major food incidents in Europe. J. Food Sci. 2021, 86, 3611–3627.
- Ádám, A.L.; Kátay, G.; Künstler, A.; Király, L. Detection of Lipid Peroxidation-Derived Free Azelaic Acid, a Biotic Stress Marker and Other Dicarboxylic Acids in Tobacco by Reversed-Phase HPLC-MS Under Non-derivatized Conditions. Methods Mol. Biol. 2022, 2526, 191–200.
- Niimi, J.; Deveau, A.; Splivallo, R. Geographical-based variations in white truffle Tuber magnatum aroma is explained by quantitative differences in key volatile compounds. New Phytol. 2021, 230, 1623–1638.
- Omeje, J.S.; Asegbeloyin, J.N.; Ihedioha, J.N.; Ekere, N.R.; Ochonogor, A.E.; Abugu, H.O.; Alum, O.L. Monitoring of pesticide residues in fresh fruits and vegetables available in Nigerian markets and assessment of their associated health risks. Environ. Monit. Assess. 2022, 194, 516.
- Rawat, S.; Singh, J. Synthesis of nZnO from waste batteries by hydrometallurgical method for photocatalytic degradation of organic pollutants under visible light irradiation. J. Environ. Manag. 2022, 318, 115518.
- Yang, J.; Zhang, G.; Wang, Z.; Meng, J.; Wen, H. Metabolic Study of Stable Isotope Labeled Indolinone Derivative in Hepatocyte Cell by UPLC/Q TOF MS. J. Am. Soc. Mass Spectrom. 2021, 32, 1538–1544.
- Zeng, W.; Lao, S.; Guo, Y.; Wu, Y.; Huang, M.; Tomlinson, B.; Zhong, G. The Influence of EGCG on the Pharmacokinetics and Pharmacodynamics of Bisoprolol and a New Method for Simultaneous Determination of EGCG and Bisoprolol in Rat Plasma. Front. Nutr. 2022, 9, 907986.
- Amaral, M.S.S.; Nolvachai, Y.; Marriott, P.J. Comprehensive Two-Dimensional Gas Chromatography Advances in Technology and Applications: Biennial Update. Anal. Chem. 2020, 92, 85–104.
- Stoll, D.R.; Carr, P.W. Two-Dimensional Liquid Chromatography: A State of the Art Tutorial. Anal. Chem. 2017, 89, 519–531.
- Erjavec, G.N.; Konjevod, M.; Perkovic, M.N.; Strac, D.S.; Tudor, L.; Barbas, C.; Grune, T.; Zarkovic, N.; Pivac, N. Short overview on metabolomic approach and redox changes in psychiatric disorders. Redox Biol. 2018, 14, 178–186.
- Sundekilde, U.K.; Yde, C.C.; Honore, A.H.; Rae, J.M.C.; Burns, F.R.; Mukerji, P.; Mawn, M.P.; Stenman, L.; Dragan, Y.; Glover, K.; et al. An Integrated Multi-Omics Analysis Defines Key Pathway Alterations in a Diet-Induced Obesity Mouse Model. Metabolites 2020, 10, 80.
- Alonso, A.; Marsal, S.; Juliã, A. Analytical Methods in Untargeted Metabolomics: State of the Art in 2015. Front. Bioeng. Biotechnol. 2015, 3, 23.
- Ahmed, Z. Practicing precision medicine with intelligently integrative clinical and multi-omics data analysis. Hum. Genom. 2020, 14, 35.
- Branco, I.; Choupina, A. Bioinformatics: New tools and applications in life science and personalized medicine. Appl. Microbiol. Biotechnol. 2021, 105, 937–951.
- Mattrey, F.T.; Makarov, A.A.; Regalado, E.L.; Bernardoni, F.; Figus, M.; Hicks, M.B.; Zheng, J.; Wang, L.; Schafer, W.; Antonucci, V.; et al. Current challenges and future prospects in chromatographic method development for pharmaceutical research. TrAC Trends Anal. Chem. 2017, 95, 36–46.
- Fu, W.; Xu, L.; Yu, Q.; Fang, J.; Zhao, G.; Li, Y.; Pan, C.; Dong, H.; Wang, D.; Ren, H.; et al. Artificial Intelligent Olfactory System for the Diagnosis of Parkinson’s Disease. ACS Omega 2022, 7, 4001–4010.
- Costa, C.P.; Marques, J.; Silva, D.; Barbosa, C.; Oliveira, A.S.; Santos, M.; Rocha, S.M. Metabolomics Profiling of Human Exhaled Breath Condensate by SPME/GC×GC-ToFMS: Exploratory study on the use of face masks at the level of lipid peroxidation volatile markers. Microchem. J. 2021, 171, 106830.
- Caldeira, M.; Perestrelo, R.; Barros, A.; Bilelo, M.; Morête, A.; Câmara, J.; Rocha, S. Allergic asthma exhaled breath metabolome: A challenge for comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2012, 1254, 87–97.
- Bosona, T.; Gebresenbet, G. Swedish Consumers’ Perception of Food Quality and Sustainability in Relation to Organic Food Production. Foods 2018, 7, 54.
- Lauterbach, J.; Bantle, C. “For More Diversity, Better Taste and My Own Health” Exploring Organic Consumers’ Purchasing Motives for Heirloom Vegetable Varieties. Sustainability 2022, 14, 4068.
- Saadat, S.; Pandya, H.; Dey, A.; Rawtani, D. Food forensics: Techniques for authenticity determination of food products. Forensic Sci. Int. 2022, 333, 111243.
- Stenmarck, Å.; Jensen, C.; Quested, T.; Moates, G.; Cseh, B.; Juul, S.; Parry, A.; Politano, A.; Redlingshofer, B.; Scherhaufer, S.; et al. Estimates of European Food Waste Levels—Fusions EU Project; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2014; pp. 1–80.
- Difonzo, G.; de Gennaro, G.; Pasqualone, A.; Caponio, F. Potential use of plant-based by-products and waste to improve the quality of gluten-free foods. J. Sci. Food Agric. 2022, 102, 2199–2211.
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918.
- Patra, A.; Abdullah, S.; Pradhan, R.C. Review on the extraction of bioactive compounds and characterization of fruit industry by-products. Bioresour. Bioprocess. 2022, 9, 14.
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods 2022, 11, 362.
- Rocha, S.M.; Costa, C.P.; Martins, C. Aroma Clouds of Foods: A Step Forward to Unveil Food Aroma Complexity Using GC × GC. Front. Chem. 2022, 10, 820749.
- Medina, S.; Perestrelo, R.; Pereira, R.; Câmara, J.S. Evaluation of Volatilomic Fingerprint from Apple Fruits to Ciders: A Useful Tool to Find Putative Biomarkers for Each Apple Variety. Foods 2020, 9, 1830.
- Bento-Silva, A.; Duarte, N.; Santos, M.; Costa, C.P.; Patto, M.C.V.; Rocha, S.M.; Bronze, M.R. Comprehensive Two-Dimensional Gas Chromatography as a Powerful Strategy for the Exploration of Broas Volatile Composition. Molecules 2022, 27, 2728.
- Perestrelo, R.; Barros, A.S.; Rocha, S.M.; Câmara, J.S. Establishment of the varietal profile of Vitis vinifera L. grape varieties from different geographical regions based on HS-SPME/GC–qMS combined with chemometric tools. Microchem. J. 2014, 116, 107–117.
- Petronilho, S.; Rudnitskaya, A.; Coimbra, M.; Rocha, S. Comprehensive Study of Variety Oenological Potential Using Statistic Tools for the Efficient Use of Non-Renewable Resources. Appl. Sci. 2021, 11, 4003.
- Fernandes, S.; Gois, A.; Mendes, F.; Perestrelo, R.; Medina, S.; Câmara, J.S. Typicality Assessment of Onions (Allium cepa) from Different Geographical Regions Based on the Volatile Signature and Chemometric Tools. Foods 2020, 9, 375.
- Quintanilla-Casas, B.; Torres-Cobos, B.; Guardiola, F.; Servili, M.; Alonso-Salces, R.M.; Valli, E.; Bendini, A.; Toschi, T.G.; Vichi, S.; Tres, A. Geographical authentication of virgin olive oil by GC–MS sesquiterpene hydrocarbon fingerprint: Verifying EU and single country label-declaration. Food Chem. 2022, 378, 132104.
- Silva, I.; Coimbra, M.A.; Barros, A.; Marriott, P.; Rocha, S.M. Can volatile organic compounds be markers of sea salt? Food Chem. 2015, 169, 102–113.
- Asimi, S.; Ren, X.; Zhang, M.; Li, S.; Guan, L.; Wang, Z.; Liang, S.; Wang, Z. Fingerprinting of Volatile Organic Compounds for the Geographical Discrimination of Rice Samples from Northeast China. Foods 2022, 11, 1695.
- Zhao, Q.; Xi, J.; Xu, D.; Jin, Y.; Wu, F.; Tong, Q.; Yin, Y.; Xu, X. A comparative HS-SPME/GC-MS-based metabolomics approach for discriminating selected japonica rice varieties from different regions of China in raw and cooked form. Food Chem. 2022, 385, 132701.
- Kalogiouri, N.P.; Manousi, N.; Paraskevopoulou, A.; Mourtzinos, I.; Zachariadis, G.A.; Rosenberg, E. Headspace Solid-Phase Microextraction Followed by Gas Chromatography-Mass Spectrometry as a Powerful Analytical Tool for the Discrimination of Truffle Species According to Their Volatiles. Front. Nutr. 2022, 9, 856250.
- Wang, C.; Zhang, W.; Li, H.; Mao, J.; Guo, C.; Ding, R.; Wang, Y.; Fang, L.; Chen, Z.; Yang, G. Analysis of Volatile Compounds in Pears by HS-SPME-GC×GC-TOFMS. Molecules 2019, 24, 1795.
- Capetti, F.; Marengo, A.; Cagliero, C.; Liberto, E.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Adulteration of Essential Oils: A Multitask Issue for Quality Control. Three Case Studies: Lavandula angustifolia Mill., Citrus limon (L.) Osbeck and Melaleuca alternifolia (Maiden & Betche) Cheel. Molecules 2021, 26, 5610.
- Ojha, P.K.; Poudel, D.K.; Dangol, S.; Rokaya, A.; Timsina, S.; Satyal, P.; Setzer, W.N. Volatile Constituent Analysis of Wintergreen Essential Oil and Comparison with Synthetic Methyl Salicylate for Authentication. Plants 2022, 11, 1090.
- Ma, W.; Zhu, Y.; Shi, J.; Wang, J.; Wang, M.; Shao, C.; Yan, H.; Lin, Z.; Lv, H. Insight into the volatile profiles of four types of dark teas obtained from the same dark raw tea material. Food Chem. 2021, 346, 128906.
- Zhu, Y.; Yan, H.; Zhang, Z.-F.; Zeng, J.-M.; Zhang, Y.; Wang, J.-T.; Ma, W.-J.; Wang, M.-Q.; Peng, Q.-H.; Lv, H.-P.; et al. Assessment of the contribution of chiral odorants to aroma property of baked green teas using an efficient sequential stir bar sorptive extraction approach. Food Chem. 2021, 365, 130615.
- Khvalbota, L.; Machyňáková, A.; Čuchorová, J.; Furdíková, K.; Špánik, I. Enantiomer composition of chiral compounds present in traditional Slovak Tokaj wines. J. Food Compos. Anal. 2020, 96, 103719.
- Langen, J.; Wegmann-Herr, P.; Schmarr, H.-G. Quantitative determination of α-ionone, β-ionone, and β-damascenone and enantiodifferentiation of α-ionone in wine for authenticity control using multidimensional gas chromatography with tandem mass spectrometric detection. Anal. Bioanal. Chem. 2016, 408, 6483–6496.
- Elbashir, A.A.; Aboul-Enein, H.Y. Multidimensional Gas Chromatography for Chiral Analysis. Crit. Rev. Anal. Chem. 2018, 48, 416–427.
- Bejar, E. Adulteration of tea tree oil (Melaleuca alternifolia and M. linariifolia). Bot. Adulterants Bull. 2017, 1–8. Available online: https://www.melaleuca-alternifolia.com/kaufen/BAP-BABs-TTO-CC-v5.pdf (accessed on 28 July 2022).
- Wong, Y.F.; West, R.N.; Chin, S.-T.; Marriott, P.J. Evaluation of fast enantioselective multidimensional gas chromatography methods for monoterpenic compounds: Authenticity control of Australian tea tree oil. J. Chromatogr. A 2015, 1406, 307–315.
- ISO 4730:2017/Amd 1:2018; Essential oil of Melaleuca, Terpinen-4-Ol Type (Tea Tree Oil). ISO: Geneva, Switzerland, 2018.
- Campmajó, G.; Núñez, N.; Núñez, O. The role of liquid chromatography-Mass spectrometry in food integrity and authenticity. In Mass Spectrometry—Future Perceptions and Applications Substances; Kamble, G.S., Ed.; IntechOpen: London, UK, 2019; pp. 3–20. ISBN 978-953-51-7845-3.
- Guijarro-Díez, M.; Castro-Puyana, M.; Crego, A.L.; Marina, M.L. A novel method for the quality control of saffron through the simultaneous analysis of authenticity and adulteration markers by liquid chromatography-(quadrupole-time of flight)-mass spectrometry. Food Chem. 2017, 228, 403–410.
- Bressanello, D.; Marengo, A.; Cordero, C.; Strocchi, G.; Rubiolo, P.; Pellegrino, G.; Ruosi, M.R.; Bicchi, C.; Liberto, E. Chromatographic Fingerprinting Strategy to Delineate Chemical Patterns Correlated to Coffee Odor and Taste Attributes. J. Agric. Food Chem. 2021, 69, 4550–4560.
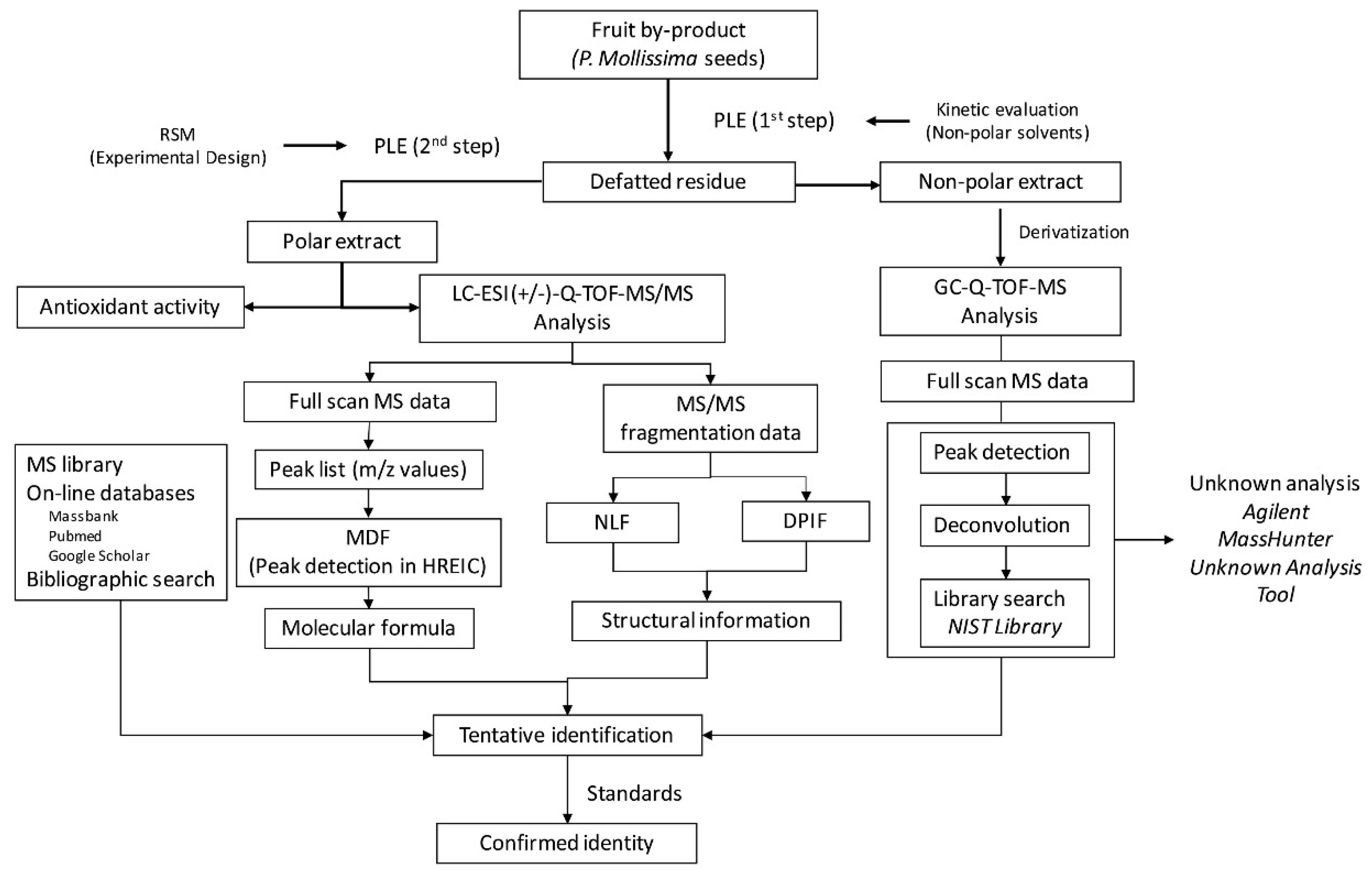
- Vivas, D.B.; Alvarez-Rivera, G.; Ibánez, E.; Parada-Alfonso, F.; Cifuentes, A. Integrated strategy for the extraction and profiling of bioactive metabolites from Passiflora mollissima seeds combining pressurized-liquid extraction and gas/liquid chromatography–high resolution mass spectrometry. J. Chromatogr. A 2019, 1595, 144–157.
- Cuco, R.P.; Cardozo-Filho, L.; da Silva, C. Simultaneous extraction of seed oil and active compounds from peel of pumpkin (Cucurbita maxima) using pressurized carbon dioxide as solvent. J. Supercrit. Fluids 2019, 143, 8–15.
- Patinha, S.; Murteira, J.V.; Costa, C.P.; Salvador, A.C.; Santos, S.A.O.; Silvestre, A.J.D.; Rocha, S.M. Elderberry Stalks as a Source of High-Value Phytochemical: Essential Minerals and Lipophilic Compounds. Appl. Sci. 2022, 12, 382.
- Salvador, C.; Simões, M.M.Q.; Silva, A.M.S.; Santos, S.A.O.; Rocha, S.M.; Silvestre, A.J.D. Vine Waste Valorisation: Integrated Approach for the Prospection of Bioactive Lipophilic Phytochemicals. Int. J. Mol. Sci. 2019, 20, 4239.
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crops Prod. 2015, 75, 141–149.
- Peralbo-Molina, Á.; Priego-Capote, F.; de Castro, M.D.L. Tentative Identification of Phenolic Compounds in Olive Pomace Extracts Using Liquid Chromatography–Tandem Mass Spectrometry with a Quadrupole–Quadrupole-Time-of-Flight Mass Detector. J. Agric. Food Chem. 2012, 60, 11542–11550.
- Jurčević, I.L.; Dora, M.; Guberović, I.; Petras, M.; Brnčić, S.R.; Đikić, D.; Landeka, I.; Rimac, S. Wine Lees Polyphenols as a Novel Functional Bioactive Compound in the Protection against Oxidative Stress and Hyperlipidemia. Food Technol. Biotechnol. 2017, 55, 109–116.
- European Comission. Our Oceans, Seas and Coasts, Descriptor D8 Documents: Contaminants. Available online: https://ec.europa.eu/environment/marine/good-environmental-status/descriptor-8/index_en.htm (accessed on 28 July 2022).
- Fries, E.; Dekiff, J.H.; Willmeyer, J.; Nuelle, M.-T.; Ebert, M.; Remy, D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci. Process. Impacts 2013, 15, 1949–1956.
- Dümichen, E.; Barthel, A.-K.; Braun, U.; Bannick, C.G.; Brand, K.; Jekel, M.; Senz, R. Analysis of polyethylene microplastics in environmental samples, using a thermal decomposition method. Water Res. 2015, 85, 451–457.
- Ivleva, N.P.; Wiesheu, A.C.; Niessner, R. Microplastic in Aquatic Ecosystems. Angew. Chem. Int. Ed. Engl. 2017, 56, 1720–1739.
- Liu, C.; Li, J.; Zhang, Y.; Wang, L.; Deng, J.; Gao, Y.; Yu, L.; Zhang, J.; Sun, H. Widespread distribution of PET and PC microplastics in dust in urban China and their estimated human exposure. Environ. Int. 2019, 128, 116–124.
- Luo, X.; Wang, Z.; Yang, L.; Gao, T.; Zhang, Y. A review of analytical methods and models used in atmospheric microplastic research. Sci. Total Environ. 2022, 828, 154487.
- Zhang, J.; Wang, L.; Kannan, K. Microplastics in house dust from 12 countries and associated human exposure. Environ. Int. 2020, 134, 105314.
- Tian, L.; Skoczynska, E.; Siddhanti, D.; van Putten, R.-J.; Leslie, H.A.; Gruter, G.-J.M. Quantification of polyethylene terephthalate microplastics and nanoplastics in sands, indoor dust and sludge using a simplified in-matrix depolymerization method. Mar. Pollut. Bull. 2022, 175, 113403.
- Neng, N.R.; Nogueira, J.M.F. Determination of Phenol Compounds in Surface Water Matrices by Bar Adsorptive Microextraction-High Performance Liquid Chromatography-Diode Array Detection. Molecules 2014, 19, 9369–9379.
- Ahmad, S.M.; Calado, B.B.; Oliveira, M.N.; Neng, N.R.; Nogueira, J. Bar Adsorptive Microextraction Coated with Carbon-Based Phase Mixtures for Performance-Enhancement to Monitor Selected Benzotriazoles, Benzothiazoles, and Benzenesulfonamides in Environmental Water Matrices. Molecules 2020, 25, 2133.
- Silva, A.R.M.; Neng, N.R.; Nogueira, J.M.F. Multi-Spheres Adsorptive Microextraction (MSAμE)—Application of a Novel Analytical Approach for Monitoring Chemical Anthropogenic Markers in Environmental Water Matrices. Molecules 2019, 24, 931.
- U.S. Environmental Protection Agency. Bisphenol A Action Plan (CASRN 80-05-7); U.S. Environmental Protection Agency: Washington, DC, USA, 2010; pp. 1–22.
- Le Coadou, L.; Le Ménach, K.; Labadie, P.; Dévier, M.-H.; Pardon, P.; Augagneur, S.; Budzinski, H. Quality survey of natural mineral water and spring water sold in France: Monitoring of hormones, pharmaceuticals, pesticides, perfluoroalkyl substances, phthalates, and alkylphenols at the ultra-trace level. Sci. Total Environ. 2017, 603–604, 651–662.
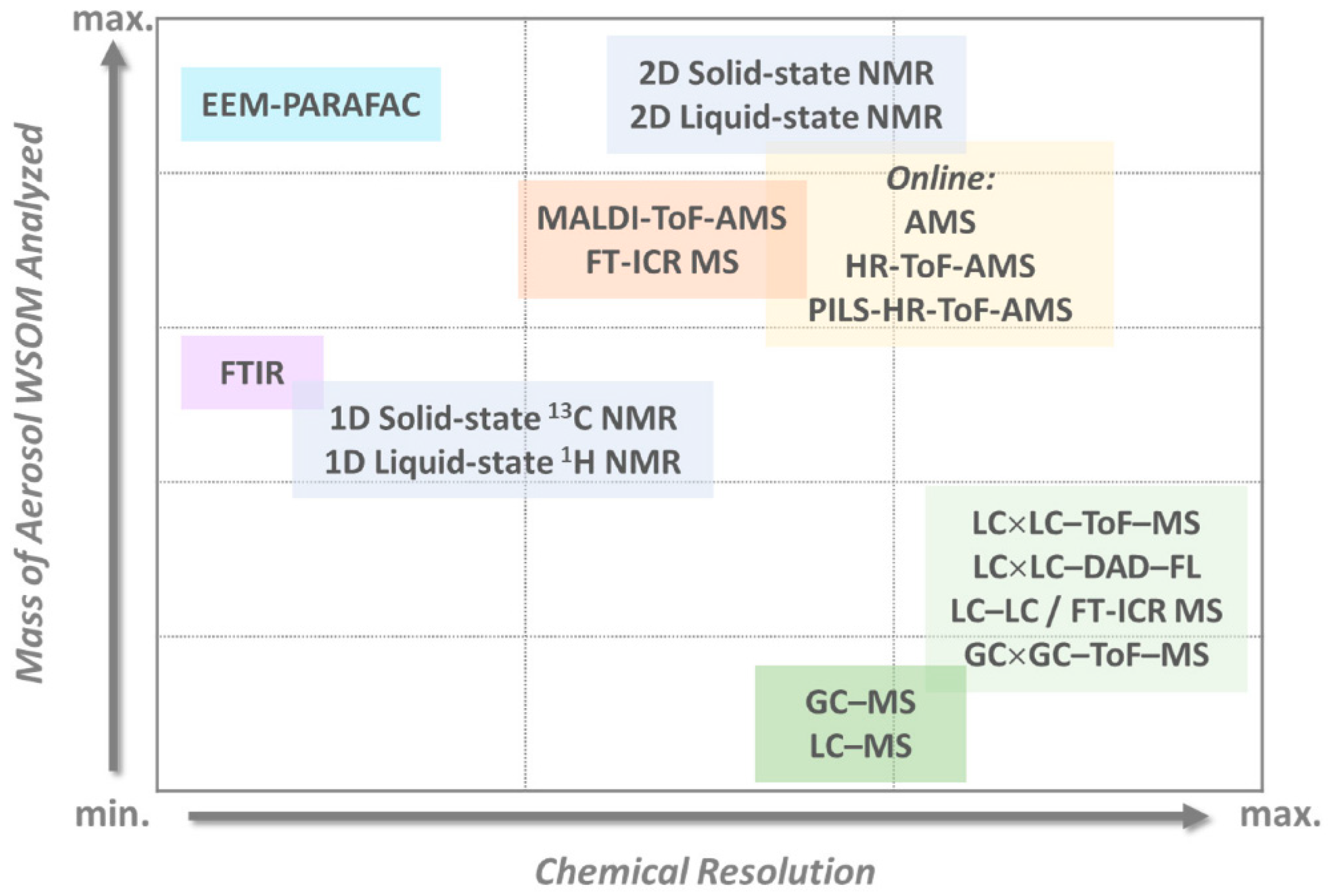
- Duarte, R.; Matos, J.; Duarte, A. Multidimensional Analytical Characterization of Water-Soluble Organic Aerosols: Challenges and New Perspectives. Appl. Sci. 2021, 11, 2539.
