Atypical atrial flutters (AAFL) are difficult-to-manage atrial arrhythmias, yet potentially amenable to effective radiofrequency catheter ablation (CA). However, data on CA feasibility are only sparingly reported in the literature in different clinical settings, such as AAFL related to surgical correction of congenital heart disease. The aim of this review was to provide an overview of the clinical settings in which AAFL may occur to help the cardiac electrophysiologist in the prediction of the tachycardia circuit location before CA. Moreover, the role and proper implementation of cutting-edge technologies in this setting were investigated as well as which procedural and clinical factors are associated with long-term failure to maintain sinus rhythm (SR) to find out which patients may, or may not, benefit from this procedure. Not only different surgical and non-surgical scenarios are associated with peculiar anatomical location of AAFL, but we also found that CA of AAFL is generally feasible. The success rate may be as low as 50% in surgically corrected congenital heart disease (CHD) patients but up to about 90% on average after pulmonary vein isolation (PVI) or in patients without structural heart disease. Over the years, the progressive implementation of three-dimensional mapping systems and high-density mapping tools has also proved helpful for ablation of these macro-reentrant circuits. However, the long-term maintenance of SR may still be suboptimal due to the progressive electroanatomic atrial remodeling occurring after cardiac surgery or other interventional procedures, thus limiting the likelihood of successful ablation in specific clinical settings.
- atypical atrial flutter
- atrial fibrillation
- catheter ablation
1. Introduction
2. Clinical Settings Associated with Atypical Atrial Flutters
2.1. Surgical Correction for Congenital Heart Disease
Macro-rentrant atrial arrhythmias or post-incisional IART represent common complications after surgical correction for congenital heart disease [8]. IART generally develops in adulthood several years after surgery and is often poorly tolerated in these patients [2]. Cavo-tricuspid isthmus-dependent AFL is seen in at least 58% of patients after cardiac surgery [2[2][9],9], whereas IART occurs in up to 25% of cases [2]. On the one hand, anatomical position of surgical scars deeply influences IART location. In patients with a history of atrial septal defect (ASD) and Tetralogy of Fallot repair, the observed macro-rentrant circuits revolving around areas of dense scar or through electrical gaps along double potential lines are generally consistent with the right-sided location of surgical atriotomies [2]. Re-entry around septal patch and left-sided IART have been also observed in rarer cases after ASD correction [2,10][2][10]. The electrophysiology substrate is even more complex when Fontan procedure for univentricular hearts is considered [11]. Due to the major hemodynamic abnormalities in these patients, the anatomical location of IART is difficult to predict and depends on the combination of iatrogenic areas of conduction block in heavily remodeled right atrial chambers [11]. However, the classic Fontan (i.e., right atrial to pulmonary artery anastomosis) and the intracardial lateral tunnel were more recently replaced by the so-called extracardiac Fontan where completely external conduits are used. Thanks to a total cavopulmonary connection created through right atrial bypass, the extracardiac Fontan operation has progressively led to a significant reduction in IART occurrence in these patients [12]. In this complex scenario, the implementation of three-dimensional electroanatomic mapping systems proved invaluable in the better understanding of the pathophysiological substrates of these cardiac arrhythmias [11]. F2.2. Cardiac Surgery for Acquired Heart Disease
Cardiac surgery for the correction of mitral valve (MV) disease is common and associated with the development of complex, macro-reentrant arrhythmias revolving around iatrogenic scars [13,14][13][14]. In this setting, AAFL is observed in up to 55% of cases [3] and their anatomical location is greatly influenced by atriotomies and cannulation sites performed at the time of surgery [13]. Three major atriotomies have been described for surgical correction of MV disease, as follows: (1) left atrial atriotomy as an incision between the right pulmonary veins and the interatrial septum (Waterston’s groove) (Figure 1A); (2) Guiraudon’s approach or superior trans-septal access involving a vertical right atriotomy extended over the superior right atrium, the septum, and the dome of the left atrium (Figure 1B); and, finally, (3) combined trans-septal approach consistent of a vertical right atriotomy parallel to the atrio-ventricular sulcus (Figure 1C) followed by a separate incision in the interatrial septum (Figure 1D) [15].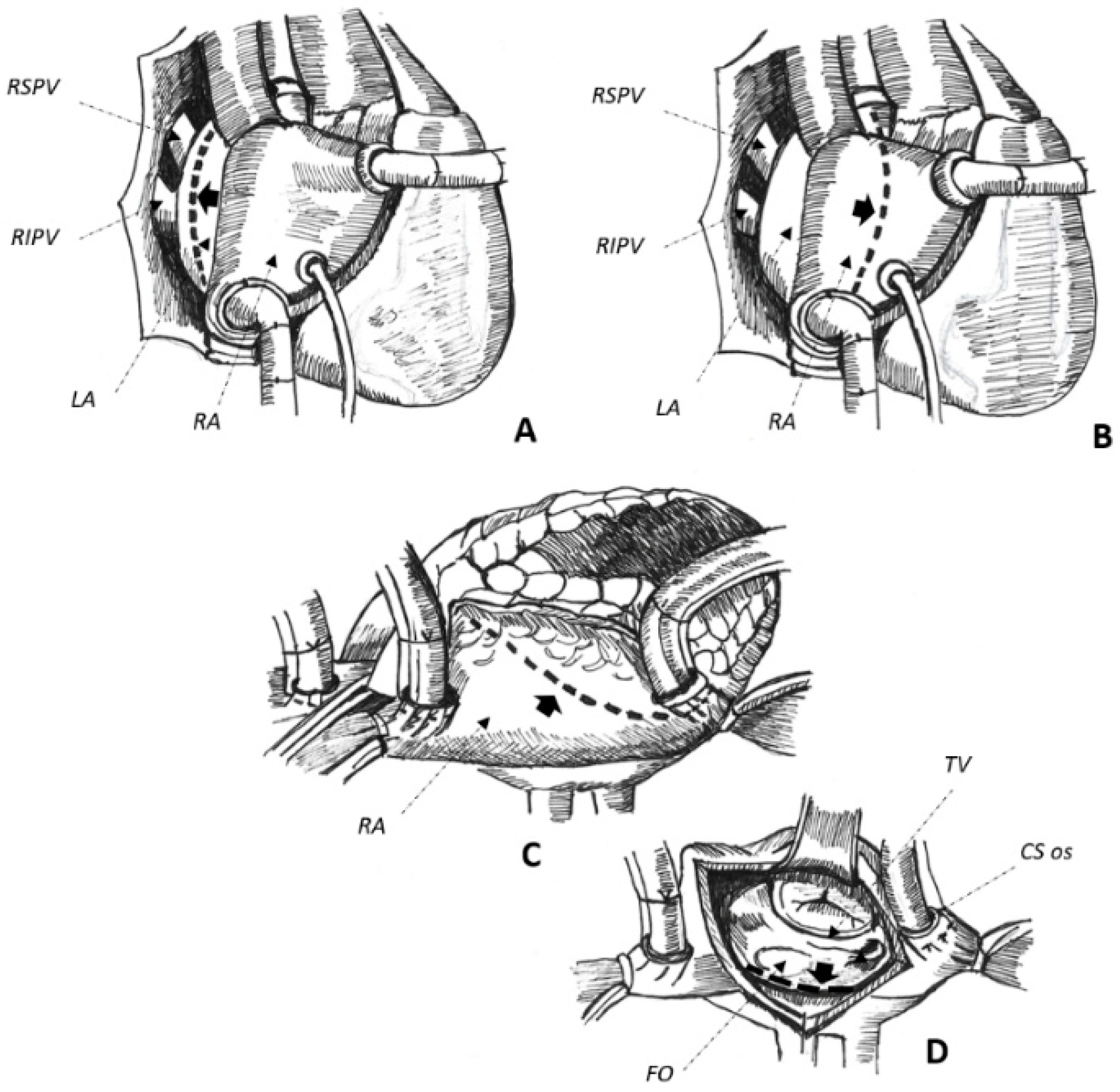
2.3. Non-Surgical Pulmonary Vein Isolation
2.4. Absence of Manifest Structural Heart Disease
In up to 6% of cases, AAFL occurs in patients with no evidence of structural heart disease [5]. Reasons for spontaneous atrial scarring are not clear. However, chronically increased atrial pressure overload in hypertension, occlusion of small coronary artery branches, isolated inflammation, and finally amyloid infiltration may explain an arrhythmogenic substrate in otherwise apparently healthy individuals [5,30][5][27]. Most of these circuits are right-sided and usually involve electrical silent areas located at the posterior or the lateral free wall of the right atrium, which can be effectively treated by radiofrequency energy applications delivered from these scars to the inferior vena cava ostium [5,31][5][28]. However, narrow and slow-conducting channels may also be found in left-sided, antero-septal circuits, as the result of the complex interweaving of epicardial fibers promoting AAFL [5]. Finally, even in normal hearts, transverse conduction across the crista terminalis [30][27] and the complex anatomy of the interatrial septum [32][29] may lead to macro-reentrant arrhythmias due to mechanisms of non-uniform anisotropy [30,33][27][30].3. Overall Peri-Procedure Feasibility
Although pioneering works based their CA strategy on conventional mapping through the systematic evaluation of transient concealed entrainment and post-pacing intervals at different pacing sites [10[10][31][32],34,35], the feasibility of entrainment is known to be limited due to pacing-mediated arrhythmia termination, degeneration into AF, or increased pacing thresholds in patients on antiarrhythmic medications [31,36,44,46][28][33][34][35]. Moreover, AAFL are complex arrhythmogenic circuits sustained by double or multiple loops in up to 60% of cases, which would make an ablation strategy based on conventional mapping particularly challenging [10]. To overcome these issues, three-dimensional electroanatomic mapping systems have been progressively implemented in cardiac electrophysiology to guide mapping [36,46,58][33][35][36] and to achieve effective radiofrequency ablation of these complex circuits [7,9,44,46,49,55,57][7][9][34][35][37][38][39]. In fact, when these systems are used, the peri-procedure success rate spans from 65% [37][40] to 100% [9,49,53,57][9][37][39][41], with better results observed in patients with a history of non-surgical PVI or in case of no structural heart disease [48,53,58][36][41][42]. However, despite the implementation of the latest technologic developments in experienced hands, such as high-density mapping tools [54,56][43][44] or contact-force sensing catheters [56][44], peri-procedure failure is observed in up to 15–20% of cases [54[43][44],56], with a greater chance of acute failure in patients with history of surgical correction for CHD [37][40]. Difficult-to-ablate anatomical substrates [10], peculiar features of the targeted isthmi [51[45][46],52], and their anatomical locations [46,59][35][47], may explain failures. Further, a CA procedure may also be prematurely interrupted for safety issues to avoid right hemidiaphragm palsy [35][32], inadvertent block of the atrioventricular node [46][35], or atrial wall perforation with possible cardiac tamponade. Finally, the inherent complexity of CA of AAFL is proved by the reported long procedure [39][48] and fluoroscopy times [36][33]. As for the overall peri-procedure safety, local complications may occur in up to 7% of cases, including groin hematoma (up to 7%) [39][48], arteriovenous fistula (3–4%) [46[35][45],51], and femoral pseudoaneurysm (1.4%) [34][31] in generally anticoagulated patients. On the other hand, regarding systemic complications, cerebral [4,36][4][33] and peripheral [35][32] embolism could be as high as 4–6% with potentially life-threatening major bleedings only sparingly described, including retroperitoneal hemorrhage (2.2%) reported in one study only [3]. Finally, patients with mechanical valve prostheses may portend even a greater risk of peri-procedure thromboembolic or hemorrhagic complications. Therefore, particular attention should be paid to periprocedural antithrombotic regimens in this patient population to avoid potentially life-threatening events [14].4. Maintenance of Sinus Rhythm after a Successful Procedure
As displayed in Table 1, AAFL recurrence is observed in up to 62% of cases after a single CA procedure with an overall SR maintenance as low as 38% on/off AAD after a variable follow-up duration, spanning from 7 ± 3 [54][43] to 37 ± 15 [5] months. Data on whether patients were on AAD before the procedure and at follow-up was not available in most of the studies, and the effect of AAD is therefore unclear in this setting.
The older the publication date, the greater the incidence of arrhythmia recurrence. This would suggest that the recent implementation of dedicated mapping tools [57][39] and irrigated-tip catheters [42,49][37][49] could help the cardiac electrophysiologist to achieve a greater long-term SR maintenance after an initially successful CA procedure [51,57][39][45]. The adoption of dedicated, tachycardia-oriented strategies for mapping and ablation of AAFL seem associated with even better results [46,51][35][45]. However, the greater the complexity of the atrial substrate to ablate, the higher the incidence of arrhythmia recurrence at follow-up. The worst long-term clinical outcome is commonly seen in patients with surgically corrected CHD (46–52% AAFL recurrence) [34,35][31][32], with better results observed after PVI (16–28%) [21,58][19][36] or in patients with apparently normal hearts (9–25% of tachycardia recurrence) [5,31][5][28].
5. The Winding Path to Improve the Procedure and the Overall Clinical Outcome
AAFL are typically sustained by critical isthmi anatomically [36][33] and functionally [4,38,39,46][4][35][48][50] defined. Regardless of the underlying structural heart disease and/or prior iatrogenic scars, these anatomical regions are bounded by anatomical/functional barriers and are associated with low bipolar voltages, fragmented electrograms [39,61][48][51], slow conduction velocity [51][45], and a typical mid-diastolic activation during ongoing tachycardia [10], which make these regions amenable to effective radiofrequency ablation [46][35]. For these reasons, as already described elsewhere [46[35][45],51], the integration of electro-anatomical information provided by these systems with surface and intracavitary signals would allow for the straightforward identification of the mid-diastolic isthmus amenable to radiofrequency ablation for effective arrhythmogenic substrate elimination. However, to avoid any misleading interpretation of the underlying circuit, accurate and high-density mapping of investigated atrial chambers is required to account for almost 90% of the tachycardia cycle length and thereby avoiding missing mapping segments potentially due to non-annotated low-amplitude and fragmented electrograms as low as 0.03–0.05 mV [27][25]. In this setting, the recent development of new tools, such as OctarayTM system (Biosense Webster Inc., Irvine, CA, USA) and the EnsiteTM Omnipolar Technology (OT) (Abbott, Chicago, IL, USA) might allow for even better results [62[52][53][54],63,64], provided that the multitude of signals collected is correctly acquired and interpreted. Although the implementation of these new technologies in CA procedures seems helpful in most cases, including mapping of re-entrant circuits with multiple loops [45,46][35][55], CA of the mid-diastolic isthmus may still be challenging due to its anatomical location and extension [59][47]. The ablation of roof-dependent circuits may be particularly challenging. The myocardial musculature surrounding the superior PV is generally thick and consistently displays adipose tissue separating the septopulmonary from the more endocardial septoatrial bundles [65][56]. This complex interweaving of myocardial fibers and their epicardial course may lead to non-transmural lesions and, thereby, to CA failure [66][57]. Regardless of the location of the tachycardia circuit or length of the ablative lesion, bidirectional conduction block does represent the essential endpoint of every CA procedure by evidence of detouring of the electrical wavefront around an anatomical barrier or scar through dedicated pacing maneuverers and/or demonstration of double potentials along the performed ablation lines. In some particular cases, demonstration of conduction block with the abovementioned criteria can be difficult and, therefore, complete disappearance of electrical signals at the target site can be considered a surrogate endpoint.References
- Bun, S.-S.; Latcu, D.G.; Marchlinski, F.; Saoudi, N. Atrial flutter: More than just one of a kind. Eur. Heart J. 2015, 36, 2356–2363.
- Twomey, D.; Sanders, P.; Roberts-Thomson, K.C. Atrial Macroreentry in Congenital Heart Disease. Curr. Cardiol. Rev. 2015, 11, 141–148.
- Enriquez, A.; Santangeli, P.; Zado, E.S.; Liang, J.; Castro, S.; Garcia, F.C.; Schaller, R.D.; Supple, G.E.; Frankel, D.S.; Callans, D.J.; et al. Postoperative atrial tachycardias after mitral valve surgery: Mechanisms and outcomes of catheter ablation. Heart Rhythm 2017, 14, 520–526.
- Deisenhofer, I.; Estner, H.; Zrenner, B.; Schreieck, J.; Weyerbrock, S.; Hessling, G.; Scharf, K.; Karch, M.R.; Schmitt, C. Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation: Incidence, electrophysiological characteristics, and results of radiofrequency ablation. Europace 2006, 8, 573–582.
- Fiala, M.; Chovančík, J.; Neuwirth, R.; Nevřalová, R.; Jiravský, O.; Škňouřil, L.; Dorda, M.; Januška, J.; Vodzinská, A.; Černý, J.; et al. Atrial macroreentry tachycardia in patients without obvious structural heart disease or previous cardiac surgical or catheter intervention: Characterization of arrhythmogenic substrates, reentry circuits, and results of catheter ablation. J. Cardiovasc. Electrophysiol. 2007, 18, 824–832.
- Natale, A.; Newby, K.H.; Pisanó, E.; Leonelli, F.; Fanelli, R.; Potenza, D.; Beheiry, S.; Tomassoni, G. Prospective randomized comparison of antiarrhythmic therapy versus first- line radiofrequency ablation in patients with atrial flutter. J. Am. Coll. Cardiol. 2000, 35, 1898–1904.
- Derval, N.; Takigawa, M.; Frontera, A.; Mahida, S.; Konstantinos, V.; Denis, A.; Duchateau, J.; Pillois, X.; Yamashita, S.; Berte, B.; et al. Characterization of Complex Atrial Tachycardia in Patients with Previous Atrial Interventions Using High-Resolution Mapping. JACC Clin. Electrophysiol. 2020, 6, 815–826.
- Pap, R.; Kohári, M.; Makai, A.; Bencsik, G.; Traykov, V.B.; Gallardo, R.; Klausz, G.; Zsuzsanna, K.; Forster, T.; Sághy, L. Surgical technique and the mechanism of atrial tachycardia late after open heart surgery. J. Interv. Card. Electrophysiol. 2012, 35, 127–135.
- Scaglione, M.; Caponi, D.; Ebrille, E.; Di Donna, P.; Di Clemente, F.; Battaglia, A.; Raimondo, C.; Appendino, M.; Gaita, F. Very long-term results of electroanatomic-guided radiofrequency ablation of atrial arrhythmias in patients with surgically corrected atrial septal defect. Europace 2014, 16, 1800–1807.
- Kalman, J.M.; VanHare, G.F.; Olgin, J.E.; Saxon, L.A.; Stark, S.I.; Lesh, M.D. Ablation of ‘Incisional’ Reentrant Atrial Tachycardia Complicating Surgery for Congenital Heart Disease. Circulation 1996, 93, 502–512.
- Abrams, D.; Schilling, R. Mechanism and mapping of atrial arrhythmia in the modified Fontan circulation. Heart Rhythm 2005, 2, 1138–1144.
- Li, D.; Fan, Q.; Hirata, Y.; Ono, M.; An, Q. Arrhythmias After Fontan Operation with Intra-atrial Lateral Tunnel Versus Extra-cardiac Conduit: A Systematic Review and Meta-analysis. Pediatr. Cardiol. 2017, 8, 873–880.
- Markowitz, S.M.; Brodman, R.F.; Stein, K.M.; Mittal, S.; Slotwiner, D.J.; Iwai, S.; Das, M.K.; Lerman, B.B. Lesional tachycardias related to mitral valve surgery. J. Am. Coll. Cardiol. 2002, 39, 1973–1983.
- Marazzato, J.; Cappabianca, G.; Angeli, F.; Crippa, M.; Golino, M.; Ferrarese, S.; Beghi, C.; De Ponti, R. Catheter ablation of atrial tachycardias after mitral valve surgery: A systematic review and meta-analysis. J. Cardiovasc. Electrophysiol. 2020, 31, 2632–2641.
- Marazzato, J.; Cappabianca, G.; Angeli, F.; Crippa, M.; Golino, M.; Ferrarese, S.; Beghi, C.; De Ponti, R. Ablation of atrial tachycardia in the setting of prior mitral valve surgery. Minerva Cardiol. Angiol. 2021, 69, 94–101.
- Morady, F.; Oral, H.; Chugh, A. Diagnosis and ablation of atypical atrial tachycardia and flutter complicating atrial fibrillation ablation. Heart Rhythm 2009, 6 (Suppl. 8), S29–S32.
- Akerström, F.; Bastani, H.; Insulander, P.; Schwieler, J.; Arias, M.A.; Jensen-Urstad, M. Comparison of Regular Atrial Tachycardia Incidence After Circumferential Radiofrequency versus Cryoballoon Pulmonary Vein Isolation in Real-Life Practice. J. Cardiovasc. Electrophysiol. 2014, 25, 948–952.
- Ciconte, G.; Baltogiannis, G.; De Asmundis, C.; Sieira, J.; Conte, G.; Di Giovanni, G.; Saitoh, Y.; Irfan, G.; Mugnai, G.; Hunuk, B.; et al. Circumferential pulmonary vein isolation as index procedure for persistent atrial fibrillation: A comparison between radiofrequency catheter ablation and second-generation cryoballoon ablation. Europace 2015, 17, 559–565.
- Wasmer, K.; Mönnig, G.; Bittner, A.; Dechering, D.; Zellerhoff, S.; Milberg, P.; Köbe, J.; Eckardt, L. Incidence, characteristics, and outcome of left atrial tachycardias after circumferential antral ablation of atrial fibrillation. Heart Rhythm 2012, 9, 1660–1666.
- Leitz, P.; Wasmer, K.; Andresen, C.; Güner, F.; Köbe, J.; Rath, B.; Reinke, F.; Wolfes, J.; Lange, P.S.; Ellermann, C.; et al. The Incidence, Electrophysiological Characteristics and Ablation Outcome of Left Atrial Tachycardias after Pulmonary Vein Isolation Using Three Different Ablation Technologies. J. Cardiovasc. Dev. Dis. 2022, 9, 50.
- Pappone, C.; Oreto, G.; Rosanio, S.; Vicedomini, G.; Tocchi, M.; Gugliotta, F.; Salvati, A.; Dicandia, C.; Calabrò, M.P.; Mazzone, P.; et al. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: Efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation 2001, 104, 2539–2544.
- Satomi, K.; Bänsch, D.; Tilz, R.; Chun, J.; Ernst, S.; Antz, M.; Greten, H.; Kuck, K.H.; Ouyang, F. Left atrial and pulmonary vein macroreentrant tachycardia associated with double conduction gaps: A novel type of man-made tachycardia after circumferential pulmonary vein isolation. Heart Rhythm 2008, 5, 43–51.
- Gerstenfeld, E.P.; Callans, D.J.; Dixit, S.; Russo, A.M.; Nayak, H.; Lin, D.; Pulliam, W.; Siddique, S.; Marchlinski, F. Mechanisms of organized left atrial tachycardias occurring after pulmonary vein isolation. Circulation 2004, 110, 1351–1357.
- Luther, V.; Sikkel, M.; Bennett, N.; Guerrero, F.; Leong, K.; Qureshi, N.; Ng, F.S.; Hayat, S.A.; Sohaib, S.A.; Malcolme-Lawes, L.; et al. Visualizing Localized Reentry with Ultra-High Density Mapping in Iatrogenic Atrial Tachycardia. Circ. Arrhythmia Electrophysiol. 2017, 10, e004724.
- Frontera, A.; Mahajan, R.; Dallet, C.; Vlachos, K.; Kitamura, T.; Takigawa, M.; Cheniti, G.; Martin, C.; Duchateau, J.; Lam, A.; et al. Characterizing localized reentry with high-resolution mapping: Evidence for multiple slow conducting isthmuses within the circuit. Heart Rhythm 2019, 16, 679–685.
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017, 14, e275–e444.
- Tai, C.-T.; Huang, J.-L.; Lin, Y.-K.; Hsieh, M.-H.; Lee, P.-C.; Ding, Y.-A.; Chang, M.-S.; Chen, S.-A. Noncontact three-dimensional mapping and ablation of upper loop re-entry originating in the right atrium. J. Am. Coll. Cardiol. 2002, 40, 746–753.
- Stevenson, I.H.; Kistler, P.M.; Spence, S.J.; Vohra, J.K.; Sparks, P.B.; Morton, J.B.; Kalman, J.M. Scar-related right atrial macroreentrant tachycardia in patients without prior atrial surgery: Electroanatomic characterization and ablation outcome. Heart Rhythm 2005, 2, 594–601.
- Marrouche, N.F.; Natale, A.; Wazni, O.M.; Cheng, J.; Yang, Y.; Pollack, H.; Verma, A.; Ursell, P.; Scheinman, M.M. Left septal atrial flutter: Electrophysiology, anatomy, and results of ablation. Circulation 2004, 109, 2440–2447.
- Kharbanda, R.K.; Özdemir, E.H.; Taverne, Y.J.; Kik, C.; Bogers, A.J.; de Groot, N.M. Current Concepts of Anatomy, Electrophysiology, and Therapeutic Implications of the Interatrial Septum. JACC Clin. Electrophysiol. 2019, 5, 647–656.
- Baker, B.M.; Lindsay, B.D.; Bromberg, B.I.; Frazier, D.W.; Cain, M.E.; Smith, J.M. Catheter ablation of clinical intraatrial reentrant tachycardias resulting from previous atrial surgery: Localizing and transecting the critical isthmus. J. Am. Coll. Cardiol. 1996, 28, 411–417.
- Triedman, J.K.; Bergau, D.M.; Saul, J.; Epstein, M.R.; Walsh, E.P. Efficacy of Radiofrequency Ablation for Control of Intraatrial Reentrant Tachycardia in Patients with Congenital Heart Disease. J. Am. Coll. Cardiol. 1997, 30, 1032–1038.
- Jaïs, P.; Shah, D.C.; Haïssaguerre, M.; Hocini, M.; Peng, J.T.; Takahashi, A.; Garrigue, S.; Le Métayer, P.; Clémenty, J. Mapping and ablation of left atrial flutters. Circulation 2000, 101, 2928–2934.
- Magnin-Poull, I.; De Chillou, C.; Miljoen, H.; Andronache, M.; Aliot, E. Mechanisms of Right Atrial Tachycardia Occurring Late After Surgical Closure of Atrial Septal Defects. J. Cardiovasc. Electrophysiol. 2005, 16, 681–687.
- De Ponti, R.; Verlato, R.; Bertaglia, E.; Del Greco, M.; Fusco, A.; Bottoni, N.; Drago, F.; Sciarra, L.; Ometto, R.; Mantovan, R.; et al. Treatment of macro-re-entrant atrial tachycardia based on electroanatomic mapping: Identification and ablation of the mid-diastolic isthmus. Europace 2007, 9, 449–457.
- Vlachos, K.; Efremidis, M.; Derval, N.; Martin, C.A.; Takigawa, M.; Bazoukis, G.; Frontera, A.; Gkalapis, C.; Duchateau, J.; Nakashima, T.; et al. Use of high-density activation and voltage mapping in combination with entrainment to delineate gap-related atrial tachycardias post atrial fibrillation ablation. Europace 2021, 23, 1052–1062.
- Esato, M.; Hindricks, G.; Sommer, P.; Arya, A.; Gaspar, T.; Bode, K.; Bollmann, A.; Wetzel, U.; Hilbert, S.; Kircher, S.; et al. Color-coded three-dimensional entrainment mapping for analysis and treatment of atrial macroreentrant tachycardia. Heart Rhythm 2009, 6, 349–358.
- Grubb, C.S.; Lewis, M.; Whang, W.; Biviano, A.; Hickey, K.; Rosenbaum, M.; Garan, H. Catheter Ablation for Atrial Tachycardia in Adults with Congenital Heart Disease: Electrophysiological Predictors of Acute Procedural Success and Post-Procedure Atrial Tachycardia Recurrence. JACC Clin. Electrophysiol. 2019, 5, 438–447.
- Liu, S.; Lin, Y.; Lee, P.; Vicera, J.J.; Chang, S.; Lo, L.; Hu, Y.; Chung, F.; Tuan, T.; Chao, T.; et al. The isthmus characteristics of scar-related macroreentrant atrial tachycardia in patients with and without cardiac surgery. J. Cardiovasc. Electrophysiol. 2021, 32, 1921–1930.
- Delacretaz, E.; Ganz, L.I.; Soejima, K.; Friedman, P.L.; Walsh, E.P.; Triedman, J.K.; Sloss, L.J.; Landzberg, M.J.; Stevenson, W.G. Multi atrial maco-re-entry circuits in adults with repaired congenital heart disease: Entrainment mapping combined with three-dimensional electroanatomic mapping. J. Am. Coll. Cardiol. 2001, 37, 1665–1676.
- Zhang, J.; Tang, C.; Zhang, Y.; Han, H.; Li, Z.; Su, X. Electroanatomic Characterization and Ablation Outcome of Nonlesion Related Left Atrial Macroreentrant Tachycardia in Patients without Obvious Structural Heart Disease. J. Cardiovasc. Electrophysiol. 2012, 24, 53–59.
- Chae, S.; Oral, H.; Good, E.; Dey, S.; Wimmer, A.; Crawford, T.; Wells, D.; Sarrazin, J.-F.; Chalfoun, N.; Kühne, M.; et al. Atrial Tachycardia After Circumferential Pulmonary Vein Ablation of Atrial Fibrillation: Mechanistic Insights, Results of Catheter Ablation, and Risk Factors for Recurrence. J. Am. Coll. Cardiol. 2007, 50, 1781–1787.
- Anter, E.; McElderry, T.H.; Contreras-Valdes, F.M.; Li, J.; Tung, P.; Leshem, E.; Haffajee, C.I.; Nakagawa, H.; Josephson, M.E. Evaluation of a novel high-resolution mapping technology for ablation of recurrent scar-related atrial tachycardias. Heart Rhythm 2016, 13, 2048–2055.
- Balt, J.C.; Klaver, M.N.; Mahmoodi, B.K.; van Dijk, V.F.; Wijffels, M.C.E.F.; Boersma, L.V.A. High-density versus low-density mapping in ablation of atypical atrial flutter. J. Interv. Card. Electrophysiol. 2021, 62, 587–599.
- De Ponti, R.; Marazzi, R.; Zoli, L.; Caravati, F.; Ghiringhelli, S.; Salerno-Uriarte, J.A. Electroanatomic mapping and ablation of macroreentrant atrial tachycardia: Comparison between successfully and unsuccessfully treated cases. J. Cardiovasc. Electrophysiol. 2010, 21, 155–162.
- Drago, F.; Russo, M.S.; Marazzi, R.; Salerno-Uriarte, J.A.; Silvetti, M.S.; De Ponti, R. Atrial tachycardias in patients with congenital heart disease: A minimally invasive simplified approach in the use of three-dimensional electroanatomic mapping. Europace 2011, 13, 689–695.
- Maheshwari, A.; Shirai, Y.; Hyman, M.C.; Arkles, J.S.; Santangeli, P.; Schaller, R.D.; Supple, G.E.; Nazarian, S.; Lin, D.; Dixit, S.; et al. Septal Versus Lateral Mitral Isthmus Ablation for Treatment of Mitral Annular Flutter. JACC Clin. Electrophysiol. 2019, 5, 1292–1299.
- Ouyang, F.; Ernst, S.; Vogtmann, T.; Goya, M.; Volkmer, M.; Schaumann, A.; Bänsch, D.; Antz, M.; Kuck, K.H. Characterization of reentrant circuits in left atrial macroreentrant tachycardia: Critical isthmus block can prevent atrial tachycardia recurrence. Circulation 2002, 105, 1934–1942.
- Tanner, H.; Lukac, P.; Schwick, N.; Fuhrer, J.; Pedersen, A.K.; Jansen, P.S.; Delacretaz, E. Irrigated-tip catheter ablation of intraatrial reentrant tachycardia in patients late after surgery of congenital heart disease. Heart Rhythm 2004, 1, 268–275.
- Nakagawa, H.; Shah, N.; Matsudaira, K.; Overholt, E.; Chandrasekaran, K.; Beckman, K.J.; Spector, P.; Calame, J.D.; Rao, A.; Hasdemir, C.; et al. Characterization of reentrant circuit in macroreentrant right atrial tachycardia after surgical repair of congenital heart disease: Isolated channels between scars allow “focal″ ablation. Circulation 2001, 103, 699–709.
- Bogun, F.; Bender, B.; Li, Y.-G.; Hohnloser, S.H. Ablation of atypical atrial flutter guided by the use of concealed entrainment in patients without prior cardiac surgery. J. Cardiovasc. Electrophysiol. 2000, 11, 136–145.
- Sroubek, J.; Rottmann, M.; Barkagan, M.; Leshem, E.; Shapira-Daniels, A.; Brem, E.; Bs, C.F.; Bs, J.M.; Basu, S.; Bar-Tal, M.; et al. A novel octaray multielectrode catheter for high-resolution atrial mapping: Electrogram characterization and utility for mapping ablation gaps. J. Cardiovasc. Electrophysiol. 2019, 30, 749–757.
- Sarkozy, A.; Vijgen, J.; De Potter, T.; Schilling, R.; Markides, V. An early multicenter experience of the novel high-density star-shaped mapping catheter in complex arrhythmias. J. Interv. Card. Electrophysiol. 2022, in press.
- Rillo, M.; Palamà, Z.; Punzi, R.; Vitanza, S.; Aloisio, A.; Polini, S.; Tucci, A.; Msc, A.P.; Zonno, F.; Anastasia, A.; et al. A new interpretation of nonpulmonary vein substrates of the left atrium in patients with atrial fibrillation. J. Arrhythmia 2021, 37, 338–347.
- Seiler, J.; Schmid, D.K.; Irtel, T.A.; Tanner, H.; Rotter, M.; Schwick, N.; Delacrétaz, E. Dual-loop circuits in postoperative atrial macro re-entrant tachycardias. Heart 2007, 93, 325–330.
- Ho, S.Y.; Anderson, R.H.; Sanchez-Quintana, D. Atrial structure and fibres: Morphologic bases of atrial conduction. Cardiovasc. Res. 2002, 54, 325–336.
- Pambrun, T.; Duchateau, J.; Delgove, A.; Denis, A.; Constantin, M.; Ramirez, F.D.; Chauvel, R.; Tixier, R.; Welte, N.; André, C.; et al. Epicardial course of the septopulmonary bundle: Anatomical considerations and clinical implications for roof line completion. Heart Rhythm 2021, 18, 349–357.
