Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Evgeny Nikitin.
Living organisms react to external stimuli to adapt their activity to the environment for survival. Acquired information is encoded by neurons by action potentials (APs) in a series of discrete electrical events. Rapid initiation of the AP is critical for fast reactions and strongly relies on voltage-activated Na+-selective channels (NaVs), which are widely expressed by both invertebrate and vertebrate neurons.
- neuron
- action potential
- sodium channel
1. Theoretical Role of NaV Channels in AP Initiation
The classic Hodgkin-Huxley model (1952) [1] postulates the universal crucial role of NaV channels in action potential (AP) initiation in neurons of both vertebrates and invertebrates. The model describes the transmembrane ionic currents underlying action potential initiation in the giant axons of squids. The universal model for the generation of electrical signals in nerve cells describes the electrical mechanisms underlying the initiation and development of action potentials. The Hodgkin-Huxley theory was originally based on mollusk experiments, while subsequent studies demonstrated that it applies to mammalian central neurons as well [2][3]. In the resting state, the membrane potential of a neuron is negative, ranging within −40 to −80 mV. Once the membrane potential reaches a threshold value, voltage-dependent Na+ currents activate and evoke escalating membrane depolarization (Figure 1). This results in the inactivation and closure of Na+ channels accompanied by the activation of voltage-dependent K+ currents aimed at bringing the membrane potential back to the polarized state. Normally, in neurons there are several types of voltage-gated K+ (KV) and Na+ (NaV) channels, expressed by the same cell, which are involved in AP generation.
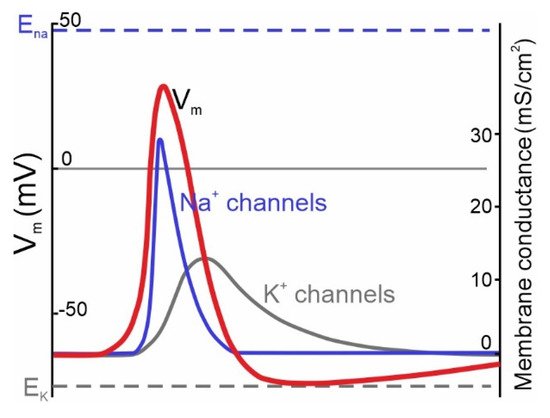
Figure 1. Ionic channels underlying the generation of APs in neurons described by the classic Hodgkin & Huxley model of the ionic mechanism of AP generation in a squid giant axon. Membrane potential (Vm) changes over time are accompanied by channel opening (modified from [1]). Na+ channels (blue curve) activate during the rise phase and initiate the AP (red curve). K+ channels (dark gray curve) provide membrane repolarization.
2. Normal Structure of Human NaV Channels and Their Pathological Mutations
Nine human NaV (1.1–1.9) channel genes encode a pore-forming α-subunit composed of four homologous domains (DI–DIV; Figure 2A). Each α-subunit contains six transmembrane α-helical segments (S1–S6) connected by intracellular linkers [4]. The structure of the transmembrane domains is highly conserved (Figure 2A). The sequentially linked transmembrane segments S1−S4 form a voltage-sensing domain, and segments S5–S6 form the pore-forming domain [5]. The DI-DIV domains are connected with intracellular cytoplasmic linkers (Figure 2A,B). The DIIIS6-DIVS1 linker is involved in closing the pore during fast inactivation (Figure 2A,B). Moreover, extracellular domains are involved in interactions with auxiliary β-subunits.
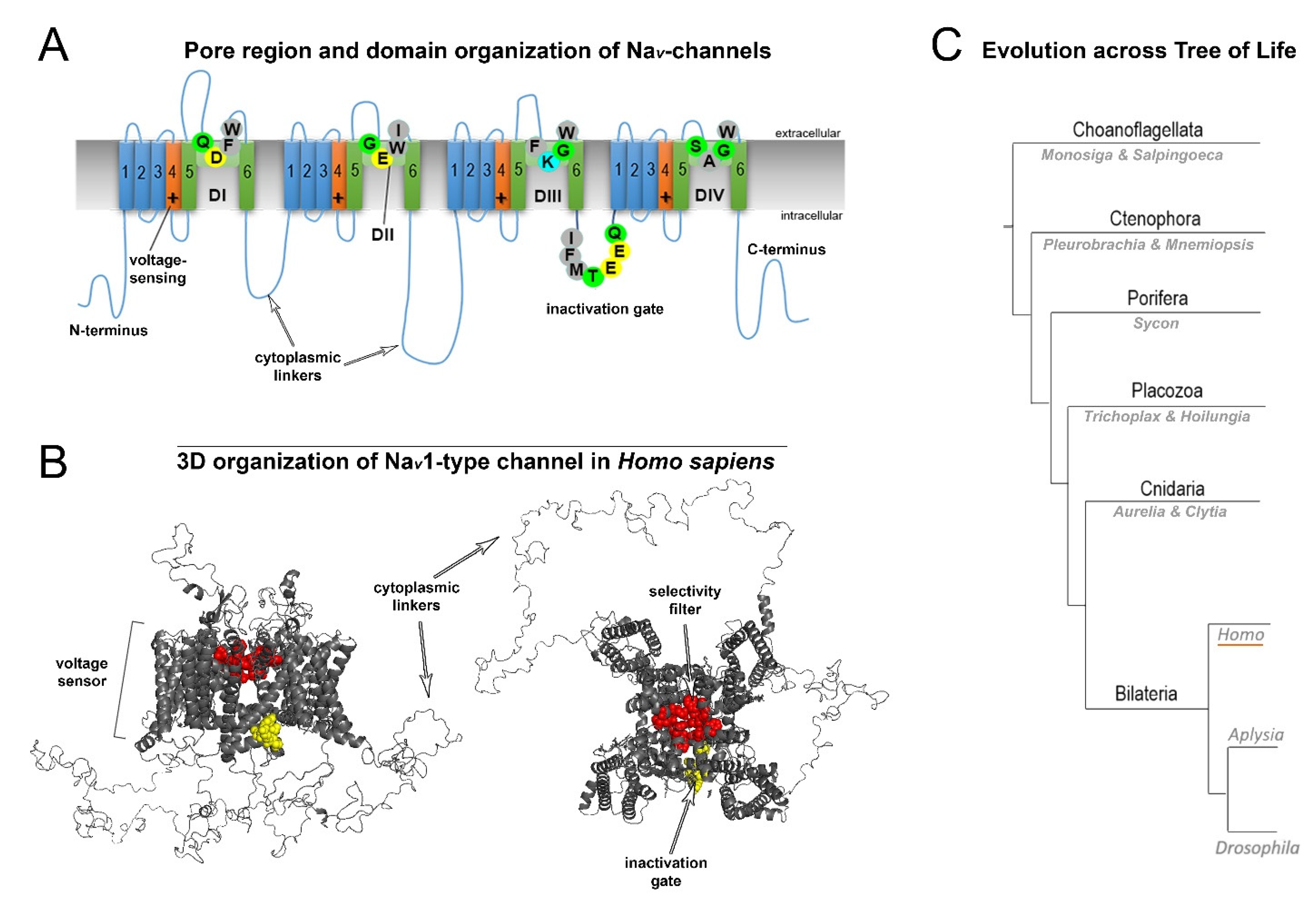
Figure 2. General structure and evolution of NaV channels. (A) Pore region and domain organization of NaV-channels (Homo sapiens). 2D model shows critical amino acids of NaV domains (D1-DIV), which contribute to pore selectivity motifs (marked as SF–Selectivity Filter) and inactivation gates. All species contain a critical lysine (K) in the pore region, which is responsible for sodium selectivity. Transmembrane α-helical segments are marked with numbers (1–6). Capital letters show single-letter amino acid code (B) 3D organization of NaV1-type channels (H. sapiens). The scheme includes all domains: the selectivity filter, inactivation gate and cytoplasmic linkers. (C) Evolution across the tree of life. Schematic phylogenetic relationships among representative animal clades of different classes of sodium channels. Homo (humans), Aplysia, and Drosophila represent mammals, mollusks, and insects, respectively.
Fast neuronal NaV channels (NaV1.1, NaV1.2, NaV1.3, NaV1.6) have the longest cytoplasmic linkers. In contrast, the cytoplasmic linkers of myocyte Nav1.4 channels are the shortest. NaV1.8, NaV1.9, and NaV1.5 channels display intermediate cytoplasmic linkers (Supplementary Sequence S1). Mutations of the most well studied channels, NaV1.1, NaV1.2 and NaV1.6, are found to be associated with specific functional epileptic disorders. More specifically, missense mutations in the cytoplasmic linkers and N-/C-termini of those channels are often found in patients with benign epileptic syndromes, whereas mutations in transmembrane segments and D-domains in most cases are related to severe epileptic encephalopathy [6].
3. Variability of Linkers of NaV Channels in the Evolutionary Tree
Organization of the neuronal membrane incorporates a large number of variable structural elements that are located close to one another. The structures of voltage-gated sodium channels significantly differ between evolutionarily distant branches of animals (Figure 2C). Importantly, cytoplasmic linkers are shorter in the ascidian species Sycon compared to in squids or humans. Vertebrates display a very conservative arrangement of the selectivity filter and transmembrane domains. Important differences between species arise in the length of linkers. In contrast, invertebrates show variable organization of the selectivity filter that does not include lysine in the DIII linker, which is characteristic of mammalian fast NaV channels. This suggests that the fast kinetics of the channel is not only due to the arrangement of the selectivity filter, but additional specific features of channel organization may also contribute. There is a substantial difference in the length of cytoplasmic linkers: in ascidians, the sequences are shorter compared to humans (1695 vs. 2009 aminoacids, respectively; Figure 3).
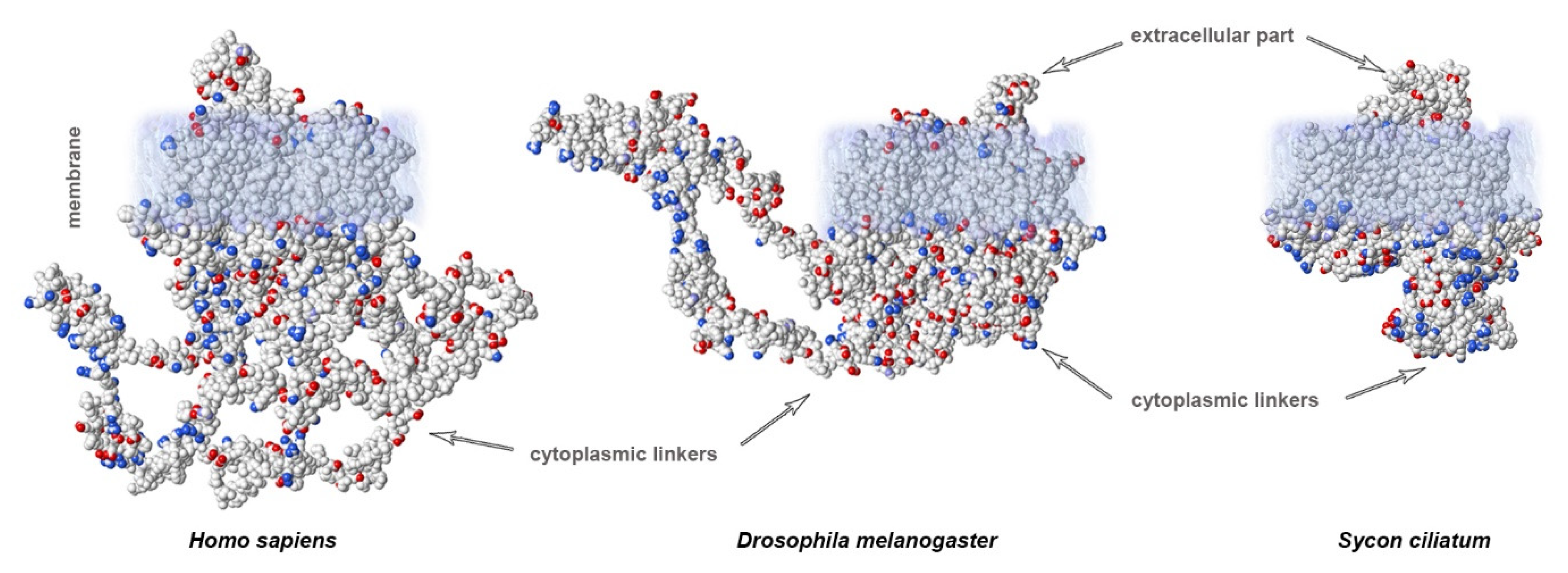
Figure 3. Calculated electrostatic potentials reveal different lengths of cytoplasmic linkers of NaV channels: mammals (H. sapiens) show the longest linkers compared to those of insects (Drosophila) or sponges (Sycon). Sequences obtained from GenBank.
4. Sites of AP Initiation in Mammalian Neurons
In mammalian pyramidal cells, the AP originates from the distal part of the non-myelinated axonal initial segment (AIS, 20–60 µm long; [7][8]. From the site of initiation, the AP propagates back to the soma and forward to axonal terminals (Figure 4). At the site of initiation at the distal AIS, AP onset displays a smooth exponential-like shape, which is indicative of its compliance with the Hodgkin-Huxley model [3][9]. By contrast, if recorded in the soma, APs display sharp non-exponential “kink”-like onset dynamics [10][11], which is thought to be due to passive AP invasion as a result of backpropagation [3]. Remarkably, APs with “kink”-like onset dynamics are also observed in mollusks, and are recorded when the AP is initiated at a distance from the recording site [12]. Apart from initiation at the AIS, APs are known to rejuvenate at the nodes of Ranvier as a result of saltatory conductance in myelinated axons. Moreover, de novo AP initiation at the proximal node of Ranvier has been shown in Purkinje neurons [13][14], which adds to the computational abilities of those neurons.
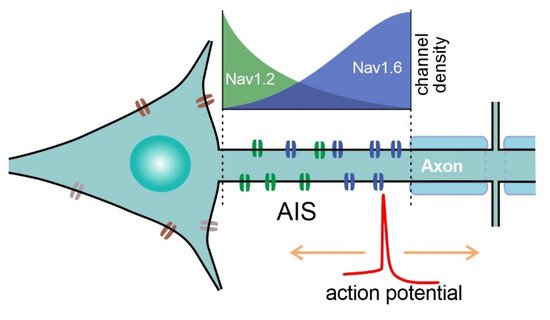
Figure 4. Schematic of sodium channel distribution at the AIS of pyramidal neurons. NaV1.6 density is higher at the distal part of the AIS, whereas NaV1.2 channels are clustered at the proximal part of the AIS. APs are initiated at the distal part of the AIS, which is enriched with low-threshold NaV1.6 channels.
5. Specific NaV Channels Involved in AP Initiation in the CNS of Mammals
Aggregation of NaV channels at the AIS and nodes of Ranvier is critical for the initiation, waveform and propagation of APs. High NaV density at the AIS is not required for axonal AP initiation, but it is crucial for a high bandwidth of information encoding and precise AP timing [15]. To initiate the AP, voltage-gated channels are required to display sufficiently fast activation at relatively low voltage thresholds. Computational studies of the contribution of axonal channels to computational abilities suggest that coding features strongly depend on the voltage sensitivity of axonal ion channels [16]. Voltage-gated NaV channels consist of a pore-forming α-subunit with ion selectivity, conductance and voltage sensing properties, and the accessory β-subunits, which are able to modify the kinetics and voltage dependence of gating. However, expression of the α-subunit alone in a heterologous expression system is sufficient to produce a voltage-gated sodium current [17]. In the adult mammalian CNS, three tetrodotoxin-sensitive NaV channel isoforms (NaV1.1, NaV1.2 and NaV1.6) underlie AP generation, and to a large extent determine the AP dynamics in central neurons. Adding low concentrations of tetrodotoxin, a selective blocker of fast NaV channels substantially compromises AP dynamics by reducing the number of available NaV channels [10]. Likewise, more precise experiments with local application of tetrodotoxin to the AIS or lowering Na+ concentration result in compromised AP dynamics and a decline in the frequency transfer function at higher frequency ranges [18]. Compared to invertebrates, physiologically dissected neuronal Na+ currents carried by NaV channels display much faster dynamics of activation (Table 1). The difference might be even stronger at physiological temperatures, as these experiments are normally performed at lower temperatures to preserve the mammalian neurons from quick degradation in electrophysiological experiments in situ.
Table 1. Electrical characteristics of NaV channels cloned into heterologous expression systems in situ (NaV1.6 to EukCatB) and physiologically dissected transient Na+ currents (INaTs) of molluscan neurons (Lymnaea to Aplysia) obtained using the voltage-clamp technique. The voltage characteristics of activation threshold and peak current, as well as times-to-halfpeak (the length of time it takes to reach half-peak activation) are shown. Voltage values obtained in patch-clamp experiments might contain errors due to liquid junction potentials.
| Channel/Current | Threshold: Approx. mV | Peak, mV | Time to ½ Peak, ms | References | ||
|---|---|---|---|---|---|---|
| NaV1.6/AnkG | −45 | −10 | ~0.25 | [19] | ||
| NaV1.2 | −40 | −5 | ~0.3 | [19] | ||
| NaV1.1 | −40 | 0 | ~0.28 | [20] | ||
| Ciona | NaV1a | −30 | 5 | ~0.5 | [21] | |
| Insect DmNaV1/tipE | −45 | −5 | ~1.1–1.2 | [22][23] | ||
| Plant EukCatB | −90 | −40 | ~0.6 | [24] | ||
| Lymnaea | I | NaT | −55 | −35 | ~2.5 | [25][26] |
| Loligo | I | NaT | −35 | −5 | ~0.6 | [27] |
| Aplysia | I | NaT | −30 | 0 | ~0.9 | [27] |
References
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544.
- Kole, M.H.; Letzkus, J.J.; Stuart, G.J. Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron 2007, 55, 633–647.
- McCormick, D.A.; Shu, Y.; Yu, Y. Neurophysiology: Hodgkin and Huxley model—Still standing? Nature 2007, 445, E1–E2; discussion E2–E3.
- Levitan, I.B.; Kaczmarek, L.K. The Neuron: Cell and Molecular Biology, 2nd ed.; Oxford University Press: New York, NY, USA, 1997; p. xii. 543p.
- de Lera Ruiz, M.; Kraus, R.L. Voltage-gated sodium channels: Structure, function, pharmacology, and clinical indications. J. Med. Chem. 2015, 58, 7093–7118.
- Encinas, A.C.; Watkins, J.C.; Longoria, I.A.; Johnson, J.P., Jr.; Hammer, M.F. Variable patterns of mutation density among NaV1.1, NaV1.2 and NaV1.6 point to channel-specific functional differences associated with childhood epilepsy. PLoS ONE 2020, 15, e0238121.
- Stuart, G.; Schiller, J.; Sakmann, B. Action potential initiation and propagation in rat neocortical pyramidal neurons. J. Physiol. 1997, 505, 617–632.
- Palmer, L.M.; Stuart, G.J. Site of action potential initiation in layer 5 pyramidal neurons. J Neurosci 2006, 26, 1854–1863.
- Nikitin, E.S.; Malyshev, A.Y.; Balaban, P.M.; Volgushev, M.A. Physiological aspects of the use of the Hodgkin-Huxley model of action potential generation for neurons in invertebrates and vertebrates. Neurosci. Behav. Physiol. 2017, 47, 751–757.
- Naundorf, B.; Wolf, F.; Volgushev, M. Unique features of action potential initiation in cortical neurons. Nature 2006, 440, 1060–1063.
- Shu, Y.; Duque, A.; Yu, Y.; Haider, B.; McCormick, D.A. Properties of action-potential initiation in neocortical pyramidal cells: Evidence from whole cell axon recordings. J. Neurophysiol. 2007, 97, 746–760.
- Jansen, R.F.; Pieneman, A.W.; Ter Maat, A. Spontaneous switching between ortho- and antidromic spiking as the normal mode of firing in the cerebral giant neurons of freely behaving Lymnaea stagnalis. J. Neurophysiol. 1996, 76, 4206–4209.
- Clark, B.A.; Monsivais, P.; Branco, T.; London, M.; Häusser, M. The site of action potential initiation in cerebellar Purkinje neurons. Nat. Neurosci. 2005, 8, 137–139.
- Foust, A.; Popovic, M.; Zecevic, D.; McCormick, D.A. Action potentials initiate in the axon initial segment and propagate through axon collaterals reliably in cerebellar Purkinje neurons. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 6891–6902.
- Lazarov, E.; Dannemeyer, M.; Feulner, B.; Enderlein, J.; Gutnick, M.J.; Wolf, F.; Neef, A. An axon initial segment is required for temporal precision in action potential encoding by neuronal populations. Sci. Adv. 2018, 4, eaau8621.
- Zhang, C.; Hofmann, D.; Neef, A.; Wolf, F. Ultrafast population coding and axo-somatic compartmentalization. PLoS Comput. Biol. 2022, 18, e1009775.
- Catterall, W.A.; Lenaeus, M.J.; Gamal El-Din, T.M. Structure and pharmacology of voltage-gated sodium and calcium channels. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 133–154.
- Ilin, V.; Malyshev, A.; Wolf, F.; Volgushev, M. Fast computations in cortical ensembles require rapid initiation of action potentials. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 2281–2292.
- Shirahata, E.; Iwasaki, H.; Takagi, M.; Lin, C.; Bennett, V.; Okamura, Y.; Hayasaka, K. Ankyrin-G regulates inactivation gating of the neuronal sodium channel, Nav1.6. J Neurophysiol 2006, 96, 1347–1357.
- Vanoye, C.G.; Lossin, C.; Rhodes, T.H.; George, A.L., Jr. Single-channel properties of human NaV1.1 and mechanism of channel dysfunction in SCN1A-associated epilepsy. J. Gen. Physiol. 2005, 127, 1–14.
- Kawai, T.; Hashimoto, M.; Eguchi, N.; Nishino, J.M.; Jinno, Y.; Mori-Kreiner, R.; Aspåker, M.; Chiba, D.; Ohtsuka, Y.; Kawanabe, A.; et al. Heterologous functional expression of ascidian Nav1 channels and close relationship with the evolutionary ancestor of vertebrate Nav channels. J. Biol. Chem. 2021, 296, 100783.
- Billen, B.; Vassilevski, A.; Nikolsky, A.; Debaveye, S.; Tytgat, J.; Grishin, E. Unique bell-shaped voltage-dependent modulation of Na+ channel gating by novel insect-selective toxins from the spider Agelena orientalis. J. Biol. Chem. 2010, 285, 18545–18554.
- Wang, L.; Nomura, Y.; Du, Y.; Dong, K. Differential effects of TipE and a TipE-homologous protein on modulation of gating properties of sodium channels from Drosophila melanogaster. PLoS ONE 2013, 8, e67551.
- Helliwell, K.E.; Chrachri, A.; Koester, J.A.; Wharam, S.; Taylor, A.R.; Wheeler, G.L.; Brownlee, C. A Novel Single-Domain Na(+)-Selective Voltage-Gated Channel in Photosynthetic Eukaryotes. Plant Physiol 2020, 184, 1674–1683.
- Staras, K.; Gyori, J.; Kemenes, G. Voltage-gated ionic currents in an identified modulatory cell type controlling molluscan feeding. Eur. J. Neurosci. 2002, 15, 109–119.
- Nikitin, E.S.; Kiss, T.; Staras, K.; O’Shea, M.; Benjamin, P.R.; Kemenes, G. Persistent sodium current is a target for cAMP-induced neuronal plasticity in a state-setting modulatory interneuron. J. Neurophysiol. 2006, 95, 453–463.
- Gilly, W.F.; Gillette, R.; McFarlane, M. Fast and slow activation kinetics of voltage-gated sodium channels in molluscan neurons. J. Neurophysiol. 1997, 77, 2373–2384.
More
