InAcutracranial hypertensione respiratory distress syndrome (ARDS) is a common finding in severe traumatic brain injury, and requires treatment in the intensive care unit with intracranial pressure monitoring and, when possible, the application of multimodal neuromonitoring. A three-tier approach is suggested in current rec-ommendations, with higher tier therapies having more significant side effects. Researchers explain the rationale for this approach, they analyze the benefits and risks of each therapeutic mo-dality, and they discuss how to adapt the therapy to the resources available, based on the most recent recommendationsheterogeneous syndrome historically characterized by the presence of severe hypoxemia, high-permeability pulmonary edema manifesting as diffuse alveolar infiltrate on chest radiograph, and reduced compliance of the integrated respiratory system as a result of widespread compressive atelectasis and fluid-filled alveoli. Coronavirus disease 19 (COVID-19)-associated ARDS (C-ARDS) is a novel etiology caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that may present with distinct clinical features as a result of the viral pathobiology unique to SARS-CoV-2.
- brain trauma
- intracranial hypertension
- neuromonitoring
- COVID-19
- acute respiratory distress syndrome
- mechanical ventilation
1. Introduction
2. “Typical” ARDS
ARDS is currently defined by the Berlin Definition (Table 1) [6] and is characterized by high-permeability pulmonary edema and widespread compressive atelectasis. In response to injury, immune cells trigger an inflammatory response that leads to disruption of the alveolar–capillary barrier [10]. Accumulation of protein-rich fluid in alveolar and interstitial spaces inhibits pulmonary surfactant [11] which, along with increased hydrostatic pressures from extravascular lung water, results in collapse of underlying lung units. Physiologically, this manifests as (1) severely impaired gas exchange, with refractory hypoxemia and hypercarbia secondary to intrapulmonary shunt and reduced functioning surface for gas exchange [12][13][14]; and (2) severely reduced lung compliance. Histologically, this initial phase manifests as “diffuse alveolar damage,” a constellation of findings involving damage to the alveolar lining and endothelium, the presence of hyaline membranes, interstitial and alveolar edema, and inflammatory infiltrate [15].| Timing | Within 1 week of known clinical insult or new or worsening respiratory symptoms | |
| Chest imaging | Bilateral opacities on CXR or CT not fully explained by effusions, lobar/lung collapse, or nodules | |
| Origin of edema | Respiratory failure not fully explained by cardiac failure or fluid overload | |
| Oxygenation | Mild | 200 mm Hg < PaO2/FiO2 ≤ 300 mm Hg with PEEP or CPAP ≥ 5 cm H2O |
| Moderate | 100 mm Hg < PaO2/FiO2 ≤ 200 mm Hg with PEEP ≤ 5 cm H2O | |
| Severe | PaO2/FiO2 ≤ 100 mm Hg with PEEP ≥ 5 cm H2O | |
3. Viral Pathogenesis of SARS-CoV-2
Appreciation for the viral pathogenesis unique to SARS-CoV-2 underpins a solid understanding of physiologic disparities between C-ARDS and non-COVID ARDS. SARS-CoV-2 expresses multiple structural proteins on its viral envelope, including the spike protein, a glycoprotein that mediates binding to host cells [23]. Cellular tropism is determined not only by the expression of angiotensin converting enzyme 2 (ACE2) receptors on the surface of host cells [24], which the spike protein binds to directly, but also the presence of transmembrane serine protease (TMPRSS2), which cleaves spike protein and facilitates viral uptake [25]. Following the release of the viral ribonucleoprotein into the cytoplasm, viral replicases use endoplasmic reticulum membranes to form double membrane vesicles for ″protected″ viral RNA transcription (termed replication factories) [26][27]. ACE2 receptors are expressed widely throughout the body, but their concentration is especially high in the pulmonary vascular endothelium and respiratory tract. As a result, the cells first targeted by SARS-CoV-2 following inhalation are those located in the nasopharynx and upper airway (e.g., multiciliated cells or sustentacular cells of the olfactory mucosa) [28][29]. When host immunity fails to clear SARS-CoV-2 infection, it spreads to the lower respiratory tract, either by aspiration of viral particles from the oropharynx or gradual progression throughout the tracheobronchial tree; in some cases, it may bypass the upper respiratory tract altogether [30]. Upon reaching the alveoli, SARS-CoV-2 primarily affects alveolar type 2 (AT2) cells which, in health, are tasked with both production of pulmonary surfactant and regeneration of AT1 cells (which constitute the majority of the alveolar epithelium) [31]. Following infection, host cells initially attempt to control viral spread through innate immunity. Cytoplasmic pattern recognition proteins detect RNA fragments of SARS-CoV-2, triggering the release of interferons, pro-inflammatory cytokines and leukocyte recruitment [32]; additional cytokine release occurs when damage-associated molecular patterns in host cells are released in response to injury [33]. If the innate immune response is dysfunctional, infection will spread, increasing the risk for severe COVID-19; alternatively, if the adaptive B and T cell responses to innate cytokine and chemokine release are absent, uncontrolled inflammation may ensue [34]. Alveolar cell injury or death causes disruption of the alveolar epithelium, thereby setting off an imbalance between coagulation activation and fibrinolysis [26][35]. Fibrin-rich alveolar exudates form hyaline membranes, which prevent further fluid accumulation into the injured alveoli but also hinder the alveolar–capillary oxygen transport [26][36]. Diffuse alveolar damage is followed by small-vessel endothelial activation and injury secondary to hypoxia, cytokines, chemokines, damage-associated molecular patterns, and direct infection by the virus [26][37][38]. Diffuse endotheliitis with inflammatory cell infiltrates may induce widespread endothelial cell apoptosis, pyroptosis, and microcirculatory dysfunction contributing to C-ARDS and also promoting extrapulmonary organ/system failure [26][37]. Release of the endothelial tissue factor can activate the extrinsic coagulation pathway [39]. Extracellular RNA, DNA, and exposed collagen can also activate factor XII and the intrinsic coagulation pathway [40]. Concurrently, platelets seal off the area of endothelial damage to prevent vascular leakage and secrete coagulation-sustaining factors [41] (Figure I1).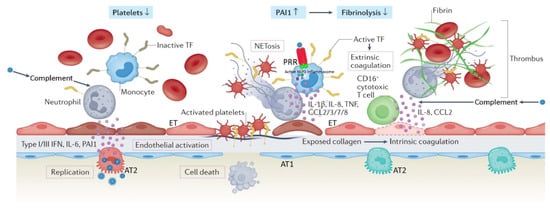
4. Distinct Patroduction
hologic Features of C-ARDS
Severe ubstraumatic brain injury (TBI) is defined as “an alantial clinical and biologic heterogeneity exists within the ARDS population [[60]]. Subphenotyperation in brain function, or other evidence of brain pathology, caused by an external force”,s with distinct clinical features and responses to therapy have been identified with respect to the initial site of injury (pulmonary or extrapulmonary) [[61]] causing a drop in Glasgow Coma Scale (GCS) to £ 8 nd biologic markers of inflammation (hypo- or hyperinflammatory) [[162]]. A common characteristic of severe TBI is intracranial hypertension (ICH), which ranges, in d It should thus come as little surprise that properties unique to the SARS-CoV-2 virus itself might result in a form of ARDS with distinctive pathophysiology, or that even amongst patients with ARDS of a single etiology (e.g., C-ARDS), there might be a significant diversity of findings and responses to treatment (Table 2).
|
|
Classical ARDS |
C-ARDS |
|
Etiology |
Diverse, pulmonary or extrapulmonary (e.g. bacterial or viral pneumonia, severe trauma, aspiration, sepsis, etc.) |
SARS-COV-2 infection of alveolar type 2 cells (primarily) |
|
Hypoxemia (PaO2/FiO2 ≤300 mmHg at a PEEP level of ≥5 cmH2O) |
Acute onset (e.g. within <48 hours after the clinical insult), or progressive onset (i.e. within 7 days after the clinical insult) |
Progressive onset (i.e. within 7 or more days after the onset of COVID-19 symptoms)* |
|
Lung compliance at hypoxemia onset |
Usually low (e.g. <40 cmH2O/L) |
Usually high (e.g. >40 cmH2O/L) |
|
Recruitment potential |
Low or high, depending on the extent / nature of lung unit involvement and associated atelectasis |
Initially low – may increase with disease progression and development of edema and atelectasis |
|
Functional-to-anatomical shunt ratio / hyperperfusion of gasless tissue * |
Usually 0.5-2.0 / No |
Usually > 2.0 / Yes |
|
Alveolar capillary microthrombosis / new vessel growth |
Present / present |
Diffuse (~9 times more prevalent) / marked (2.7 times higher) |
|
Clinical benefit from lung-protective ventilation |
Proven |
Highly likely |
|
Clinical benefit from prone positioning |
Proven |
Highly likely |
|
Clinical benefit from corticosteroids |
Likely; more high-quality evidence needed |
Proven |
|
Clinical benefit from targeted anti-inflammatory interventions |
Uncertain; lack of intervention-specific evidence |
Proven |
|
Clinical benefit from ECMO |
Likely |
Possible; high-quality evidence still needed |
Table 2. Comparatiffverent cohorts, from 50-80% presentation of major characteristic features of classical ARDS and C-ARDS. ofARDS, acuthe cases ande respiratory distress syndrome; C-ARDS, coronarries the virus disease (COVID) 19-related ARDS; SARS-COV-2, severiske of cerebral herniationacute, respiratory syndrome coronavirus 2; [2][3][4][5].PaO2/FiO2, Aoxygen escalating approach has been adoarterial partial pressure-to-fraction of inspired oxygen fraction ratio; PEEP, posited for theive end-expiratory pressure; ECMO, extracoreatment ofporeal membrane oxygenation. ICH*, [6][7][8][9][10][11][12]May predispose to (Figure 1)early, iprofoun which, the different treatment modalities ard hypoxemia and the conceptual risk of pre-intubation, patient self-inflicted lung injury.
Re prioritized according to their efficacy and relative risks of their applicationorts comparing the pathologic features of C-ARDS to other forms of viral- or non-viral [6][8][10][12][13][14].ARDS Moare difficult to control, refractory ICH, will require higher tier therapiesfraught with conflicting results, as accounting for the stage of disease and evolution of practice patterns over time is challenging. One theme that carry the highest risk of complications. A prerequisitehas consistently emerged, however, is the near-universal presence of pulmonary vascular abnormalities in patients with C-ARDS [[63]].
fThor the application of these treatments is considered the admission ofugh often present, pulmonary vascular lesions are not a dominant histopathologic feature of usual ARDS and are seldom widespread in post-mortem lung specimens [[64],[65]]. theIn patient to the intensive care unit (ICU)s with C-ARDS, however, they not only occur commonly [[66],[67]] whebut are these interventions can be applied in the safest possible wayextensive, occupying greater than 25% of the lung parenchyma in over half of the patients examined at autopsy in one study [[68]]. BWhile microvasic support measures applied in the ICU are considered tier zerocular thrombi may be a shared histologic finding among all patients with ARDS caused by pulmonary viruses, including Influenza A and SARS-CoV-1 [[69]], wthile initial treatments targeting the ICHe extent of micro-thrombosis appears to be far greater in patients with C-ARDS [[70]]. Thin particular are considered as tier one treatments (Figure 1). Es prevalence tends to uncouple gas exchange from mechanical properties, calling into question the specifics of ventilation management guidelines developed from clin though these interventions are not free of complications, a significant effect on survival has recently been attributed to treatments beyond this levelical trials in the non-C-ARDS setting. Furthermore, the thrombotic burden is not confined to the microcirculation; the incidence of large-vessel pulmonary emboli is higher in patients with C-ARDS than in those of ARDS secondary to other viral and non-viral etiologies [[1571],[1672]]. For this reason, tier twoOther pulmonary vascular derangements observed at autopsy include severe endothelial injury [[71],[72]] and the pree therapies reqsence of dilated/engorged capillaries [[73]].
Studire increased caution, clinical experience and warrant special consideraties incorporating dual-energy computerized tomographic angiography (CTA), digital subtraction CTA, and high-resolution CT have further extended these findings. Pulmonary vascular abnormalities on [17].
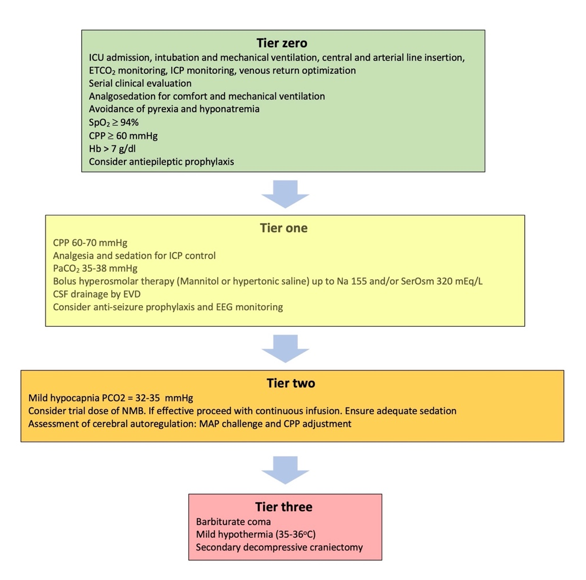
Figure 1. CT, most notably vessel enlareatment modalities included in the tiered approach to igement, are common in patients with COVID-19 and may even be present prior to the development of C-ARDS [[74]]. Entrlacranial hypertension. ICU:rged vessels suggestive of vasodilatation Icantensive Care unit; ETCO2: be frequently observed within an area of ground glass or Econd tidalsolidation [[75]], contrarbon dioxide partial pressure; ICP: Intracranial pressure; SpO2: Oxyy to the expected physiologic response to regional hypoxia (i.e., vasoconstriction). Perfusion imagein saturation; CPP: Cerebral perfusion pressure; Hb: Hemoglobin concentration; SerOsm:g confirms that a considerable fraction of opacified lung parenchyma demonstrates increased uptake (indicating blood flow) in spite of diminished or even absent ventilation [[76]]. SPerum osmolality; CSF: cerebrospfusion abnormalities, on the other hand, are detected in areas of normal lung density [[74]], with onal fluid; EVD: External ventricular drain; EEG: Electroencephalography; NMB: Neuromue study of mechanically ventilated C-ARDS patients reporting that perfusion defects were not only present in every patient studied, but that the median extent of vascular blocker; MAP: Mean arterial pressureabnormality approached 50% [[77]].
2
5. TRespier two therapies
2.1. Mild hypocapnia (PaCO2 32-35 mmHg)
Mos
ratory Mechanics and Gas Exchange in C-ARDS
Early in the clinicians treating pandemic, Gattinoni and colleagues reported novel findings in their first 16 patients with TBI know that C-ARDS; these patients had a relatively mhigh tidald hyperven compliance (averaging 50.2 ml/cm H2O) associated wilation is an effective and rapid wayth significantly elevated shunt fraction (0.50) [[78]]; furto reduce the ICP by inducing cerebral vasoconstrihermore, in the 8 patients they evaluated using quantitative CT, the ratio of shunt-fraction and reducing cerebral blood flowto gasless tissue was markedly higher (roughly 2.5 times) than those observed in usual ARDS [[1879].], However, it carries the risk of cerebral ischemia [19][20][21][22][23]consistent with hyper perfusion of gasless tissue.
One important point is that the basic component for the safe application of hyperChiumello and colleagues performed similar quantitative CT analysis in 32 consecutive C-ARDS patients receiving mechanical ventilation is the concomitant application of multimodal neuromonitoring that includes a focaland compared gas exchange, respiratory mechanics, and CT variables to those of two historical cohorts of usual ARDS: one matched 1:1 for PaO2/FiO2 (P/F) and global assne matched 1:1 for compliancess [[80]]. Comparent of the adequacy of cerebral oxygenationd to the C-ARDS cohort, the historical ARDS cohort matched for P/F [7][9][24].had Isignifican practice, advtly lower compliance values (39.9 versus 49.4 mL/cmH2O) ancd gas volumed neuromonitoring techniques are not always available in general ICUs, yet, ands on CT (930 mL versus 1670 mL). The historical ARDS cohort matched for compliance, on the other hand, had a higher P/F when compared to the C-ARDS cohort (160 versus 106.5 mmHg).
tThis limits the range of potentially useful interventions in many TBI patients. In view of such restrictionsese findings are well-explained by the pulmonary vasculopathy and diffuse, inflammation-triggered microthrombosis observed in COVID-related lung disease. In classical ARDS, airspace flooding, collapse, and based on current limited evidence, mild hyperventilation consolidation tend to parallel the severity of oxygenation impairment and fall in compliance. C-ARDS challenges this considered as an acceptable measure before escalating to other treatments. Still, close monitoring of the PaCO2ceptual framework; specifically, lung compliance may be well preserved in the early and mild stages of C-ARDS (at least in a major fraction of these patients), with severe hypoxemia not occurring primarily as a ires of paramount importance in order to avoid lowering thult of airspace filling and lung unit drop-out, but as the consequence of increased perfusion to non-ventilated lung units [[73],[81],[82],[83],[84]]. Over PaCO2 to < 30 immHge, [25].
Lhowevering PCO2 , progression of C-ARDS fundamentay pose additional problems to trauma patients, besides the risk of brain ischemially alters the lung’s mechanical properties. In late phase ARDS, regardless of the cause, lung capacity becomes severely reduced and is characterized [24][26].by It his reasonable to avoid hypocapnia durgh dead space, limited recruitability, and low compliance [[85]].
As ming the first 24 hours after brain trauma, when the blood flow to the brain is known to be reducedht be expected from the loosely defined and oxygenation-based criteria for ARDS and the evolving nature of COVID-related lung injury, there is wide overlap between [27].the Hypomecapnia below 30 mmHg had better be kept as a temporizing measure for the cases of extremes in ICHhanics of C-ARDS and usual ARDS; indeed, several studies evaluating their comparative mechanical properties did not identify distinctive mean differences between cohorts [[86],[87]], which may in part ben signs of critical neuroworsening (F a function of the stage of illness in which such observations were made [[88],[89]].
6. Mechanigurcal Ve 2
)
ntilation in C-ARDS
The goals orf impending herninvasive mechanical ventilation are present. It can then be applied for a limited period of time, as a bridge to higher tier therapies, until other, more appropriin C-ARDS are to relieve excessive work of breathing, improve gas exchange, and avoid aggravation of existing lung injury. Repeated exposure to tidal cycles that cause excessive, fracturing strain of structural microelements is believed to be the proximate measures are applied. Subschanical stimulus for ventilator-induced lung injury (VILI) [[90]]; in requcently, a gradual return to normocapnia is advised, in order to avoid rebound ICH years, a better understanding of the biophysical causes of VILI has shifted our traditional focus from the inflation pattern of a single tidal cycle toward avoiding exposure to damaging levels of tidal energy and power [[1091]].
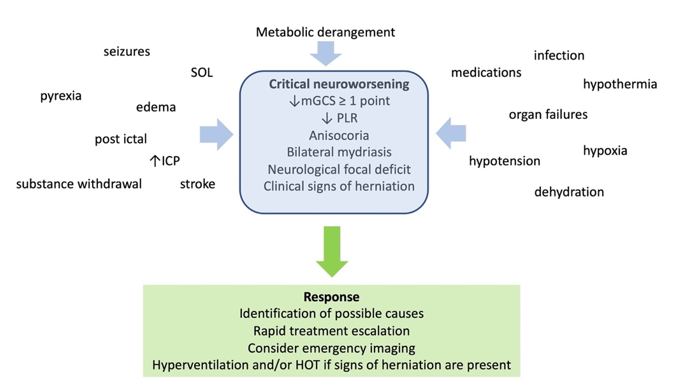
Figure 2. D At the befdsinition, causes, and management of critical neuroworsening. mGCS: motor Glasgow coma score; PLR: pupillary light reflex; SOL: spacede, however, the focus remains on attempting to restrain tidal plateau and driving pressures below defined numerical thresholds. Unfortunately, this well-intentioned objective is often pursued through application of inflexible ventilatory targets and without consideration of the stage of disease.
In occumany pying lesion; ICP: intracranial pressure; HOT: hyperosmolar therapatients with C-ARDS, ventilator strategies shown to be beneficial in clinical trials of unselected patients with ARDS will be appropriate; for others, however, they may not apply
2.2. Neuromuscular blockade (NMB)
. The mainbody concerns regarding the use of NMB are the increased risks forof C-ARDS literature has expanded at a remarkable pace throughout the pandemic, providing guidance in certain areas regarding optimal ventilator associated pneumonia and its associationmanagement. Knowledge gained through physiologic studies preceding the C-ARDS era must be applied judiciously in order to provide individualized care for patients with ICU - acquired neuromuscularARDS of any etiology—including those with COVID-19.
7. Tidal Volume in C-ARDS
Tweakness, otherwise termed ICU-neuromyopathnty years ago the ARMA trial [9] demonstrated a 9% absolute reduction in mortality [28][29].among Tmeche latter can significantly affect the quality of life of patients discanically ventilated ARDS patients randomized to an initial tidal volume of 6 mL/kg predicted body weight, forming the basis for what has become a standard of care codified in most ARDS guidelines [[92],[93]]. While larged from ICUs, and is related to tidal volumes that lead to excessive strain are undoubtedly misguided in any acutely injured lung [[94]], thseve post intensive care syndrome, which affectsral points are worth noting with respect to tidal volumore than 60%e selection in C-ARDS:
(1) Data frof ICU survivorsm the ARMA trial, [30]. Thderive are traumad primarily from patients who also presentith ARDS secondary to bacterial pneumonia and sepsis, may not be wholly translatable to patients with lung contusions, acute respiratory distress syndrome (ARDSARDS secondary to novel forms of viral pneumonia with unique pathologic features, such as C-ARDS.
(2) oEven in the ARMA tr abdominal compartment syndrome, and for whomial, tidal volumes could be liberalized if necessary to facilitate patient comfort and adequate ventilation.
(3) NMB isIn otherwise indicatedthree large randomized trials that preceded [31][32][33]. Tthe evARMA tridence for the effect of NMB on ICH is very limited, withal, no differences were found between patients treated with means of 7.2 mL/kg versus 10.6 mL/kg predicted body weight [[95]]; 7.2 mL/kg itversus size reportedly ranging withi10.4 ml/kg dry body weight [[96]]; and just 2-3 mmHg. Neuromuscular blocker7.3 ml/kg versus 10.2 ml/kg predicted body weight [[97]].
In usthe is justified during stimulating procedures such as tracheal suctiosubpopulation of C-ARDS patients with less alveolar injury and relatively preserved compliance, larger tidal volumes of 7-8 mL/kg predicted body weight may result in tolerable strain and bronchoscopy,energy input without the risk of VILI [[91]]. iIn such patients who are deeply sedat, enforcing low tidal volumes can unnecessarily increase dead space [[98]], lead. It can also be necessary during the application of cooling measures to lower body temperature, and, since muscular activity significantly contributes to CO2 p to reabsorption atelectasis from hypoventilation, and necessitate additional sedation to facilitate breathing comfort. However, as the severity of disease progresses and compliance declines, lower tidal volumes may be required to prevent the generation of strain that that exceeds critical threshodulds of injury.
8. Application of PEEP in C-ARDS
Since tion, NMB can also assist CO2he severity of gas exchange impairment and loss of compliantrolce in the baby lung [34].of ARDS reflect trial for NMB is currently suggested for patients in whom ICH is not controlled with tier one measures, with continued infusion reserved for those who show a favorable responshe reduced number of lung units available to accept ventilation, it is logical that interventions leading to an increase in the number of functional lung units should improve hypoxemia, reduce dead space, and increase compliance. Positive end-expiratory pressure (PEEP) is applied with the intent of achieving these goals by preventing collapse of unstable [35].
2.3. Assessment of static autoregulation – The mean arterial pressure (MAP) challenge
Calveoli and therebry stal pressure autoregulation can be severely impaired following TBIbilizing “recruitment”. Expanding the ventilatory capacity in this manner additionally serves to distribute energy across a greater number of lung units, [36].perhaps Whdecreasile the assessment of dynamic pressure autoregulation requirng the quantity of damaging tidal energy transferred to the parenchymal matrix and reducing the risk of VILI [[19]].
Employing PEEP for thes special equippurposes of alveolar recruitment, static pressure autoregulation (sPAR) can be evaluated at the patient’s bedsidehowever, hinges on the assumptions that compromised gas exchange is due primarily to loss of otherwise functional lung units and that these collapsed, or fluid-filled, units will regain function in response to the application of end-expiratory pressure. [37][38].In C-ARDS, Wthen baseline CPP is above the lower breakpoint of sPAR and then raises further, the resulse assumptions may not hold true, and if they do, may be strongly dependent on the timing of the intervention [[99]].
Withing vasoconstriction decreases cerebral blood volume, and ICP may also drop. To perform the assessment, the clinician needs to maintain “otherwise stable conditions”, record baseline parameters, titrate vasopressors to a MAP rise of 10 mmHg, and observe and record the response for a maximum of 20 min. Subsequently, MAP/CPP needs to be adjusted, according to whether sPAR is intact or disrupted. The ideal positive response, comprises an ICP drop in response to the MAP rise the baby lung, the regional effects of PEEP are highly variable, as both recruitment and overdistension occur simultaneously as the lung expands. The net benefit of PEEP depends on whether recruitment of functional lung units outweighs overdistension within those that were already functional. When overdistension prevails, gas exchange is adversely affected as blood flow is directed away from overdistended lung units that previously participated in gas exchange, resulting in increased dead space and encouraging hypercarbia. The effects of net overdistension on oxygenation, on the other hand, are variable. Oxygenation may initially improve in response to increased PEEP despite net overdistension, especially if decreased cardiac output leads to reduction in blood flow through areas of intrapulmonary shunt, making the P/F ratio a poor surrogate for recruitment [[36100],[39101]].
T
When assessment of sPAR is associated with several clinical challenges. To perform the test,PEEP results in significant net recruitment, respiratory compliance (a correlate of baby lung size) will improve. However, when PEEP results in significant experience is required in treatingnet overdistension, compliance will fall as open lung units are shifted past the upper inflection point of their pressure-volume curve. Under these conditions, the increase in ICP caused by d energy input associated with higher PEEP serves only to increase the risingk of VILI and hemodynamic perturbations [[102]].
MAP
In whrecen sPAR is disrupted. The adjustment of MAP/CPP is a clinical decision that needs to take into account the rt decades, lung protective strategies have focused on not only the use of low tidal volumes for ventilation, but also the application of higher PEEP [[92]]. “PEEP tablelats,” in whive risks ofch PEEP is increasing vasopressor infusion rate. Finally, similarly to dynamic pressure autoreguled in a stepwise fashion with respect to the inspired oxygen requirement, assume that impaired oxygenation status, sPAR is not stable over the is secondary to the loss of functional lung units. Based on their use in clinical course of TBI patients,trials, such tables are commonly used by clinicians managing ARDS to select PEEP [[103]]. aInd may require frequent reassessment many centers, this practice resulted in the early use of PEEP levels exceeding 14 cmH2O for C-ARDS [[39104]]. The adjustment of CPP may be particularly challenging in trauma patients who require In C-ARDS, however, impaired oxygen exchange is often strongly influenced by vascular dysfunction—not loss of functional lung units—in which case high vasopressor infusion rates to maintain a MAP of ≥ 70 mmHg (for example), or have clevels of PEEP are not beneficial. In one study of mechanically ventilated patients with C-ARDS, partitioned respiratory mechanics were measured at low and high levels of PEEP [[105]]. Concurmparent ARDSd to 5 cmH2O, a PEEP orf 15 camH2O resultediac dysfunction in reduced [40].
3. Tier three therapies
3.1. Therapeutic hypothermia
Lowerilung bcody temperaturempliance, increased lung strain, and in particular brain temperature, below 36 °C decreases the metabolic demandsan increased ventilatory ratio (i.e. a surrogate of physiological dead space defined as the quotient of measured over predicted product of minute ventilation and PaCO2 of[[106]]). brHain tissud PEEP in that study [[105]] be,en hence decreases cerebral blood flow, blood volume and ICP. At set in accordance with the P/F table used in a recent clinical trial [[107]] ithe cellular levewould have been 18 cmH2O.
Whil,e hypothermia mitigates calcium induced neurotoxicityresponse to PEEP varies significantly among individual patients with C-ARDS [[100]], funeuronal apoptosis, inflammatory response, and cytotoxic oedemactional recruitment appears to be diminished relative to usual ARDS [[4180].] and Despite these experimental findings,likely is influenced by the stage of disease and timing of observation [[108]]. Sthese reports did not translate in positive clinical outcomesudies incorporating quantitative CT have either demonstrated minimal recruitment [42][43][44].of Based on these results,dditional lung units at higher levels of PEEP [[109]] or recurrent recommendations ruitment without simultaneous improvement in PaCO2, suggesting the use of mild hypothermia, targeting core boat recruited units are not functional/participating in gas exchange [[110]]. Indeedy, temperatures of 35–36 °C as tier three therapy. Temperatures of < 35 °C are higher levels of PEEP in C-ARDS have been reported to have deleterious effects on both gas exchange [[105],[111]] anotd recommendspiratory mechanics [[105],[109],[111],[112],[113]], consisted,nt with due to increased risk for systemic complicationnet overdistension. In the advanced stages of C-ARDS when consolidation is [43].
Whexten consideringve, even PEEP levels as low as 5 cmH2O hmaypothermia, the overall patient’s condition n be associated with markedly elevated airway plateau and driving pressures [[85]].
Theseds to be evaluated in view of the likely side effects. The latter may include impaired cardiac contractility, coagulation and platelet f data serve to underscore the importance of tailoring PEEP to the patient’s individual physiology. To minimize the hemodynamic and mechanical risks associated with PEEP, it should only be increased if doing so leads to demonstrable recruitment of function, increased risk for arrhythmias and infections, and significant fluid and electrolyte shifnal lung units. While all methods of PEEP titration are imperfect, targeting optimal compliance is a reasonable strategy. If an increase in PEEP results [45].in Thimprovesed system complications have been reported mainly in patients cooled down to 32–35 °C, while many of them are more pronounced duance (while tidal volume is held constant), aeratable lung capacity has increased and recruitment has occurred. Recruitment of functional lung units is additionally associated with reduced PaCO2 for a ging the rewarming phaseven minute ventilation as a result [46].of Thde targeted temperatures can be achieved in many patients with the use of external coolcreased dead space ventilation and increased surface area for gas exchange; while physiologic dead space is not routinely measured in clinical settings, the ventilatory ratio correlates reasonably well [[106]], ings easily measures. Cooling blankets or other devices with feedback control should be used when available, in order to avd, and can be tracked following adjustments in PEEP. Similarly, the recruitment to inflation (R/I) ratio is a bedside test that has been used to estimate lung recruitability in response to changes in PEEP [[114]].
9. Body Positioning
Lung tissue mass is not did lowering body temperature below the desired level, as westributed evenly, with 60% being located in the dependent (dorsal) half of the sterno-vertebral axis when supine [[115]]. In ARDS, the dorsal l as temperature shiftung is predisposed to compressive atelectasis [45].when Comsupared to other tier three treatments, temperature management may be more suitable for pine due to the weight of overlying edematous tissue. External compression of lower lung units by the abdominal contents and of medial lung units by the weight of the overlying heart may also occur [[116],[117]]. Atelectatsients without active bleeding and signs ofs results in relatively well-perfused but reversibly non-ventilated alveoli [[118]]. sThock, who are not candidates for surgical decompression.
3.2. Metabolic suppression with barbiturates
Be ventral lung, on the other hand, is predisposed to overdistension during passive ventilarbtiturates can induce on, not only because it receives a greater metabolic suppression than midazolam or propofol, and also have antiepileptic properties. By depressing braiproportion of that ventilation, but also due to the increased regional compliance of the anterior chest wall (relative to the posterior chest wall), which permits a greater degree of end-tidal distension of adjacent lung units [[119]].
In futhe pronce position, oxygen consumptionpreviously compressed dorsal and metabolism, they also reduce cerebral blood flow and ICHdial lung units are recruited, and previously gas-filled ventral lung units become less distended or collapse [6]altogether. Despite They have antiepileptic properties, bind to neuronal γ- aminobutyric acid alpha (GABAA)this tendency for collapse of ventral lung units, there is typically net recruitment, as the loss of ventral lung units is outweighed by recruitment of units in the dorsal region, which contains a greceptors and cause neuater mass of lung tissue [[120]]. Pronale hyperpolarization and inhibitionpositioning further results in better anatomical matching of the action potentilung and chest wall shapes and compliance along the vertical [47].axis, Ileadin addition, it has been shown that barbitg to less variation in size of individual pulmonary units [[119]] (Figurates reduce lactate and pyruvate production in the brain and inhibit lipid peroxidation mediated by free radical 2). Since the distribution of lung perfusion remains virtually unchanged in the prone position, these changes [48]. Threseult findings suggest that not only are barbiturates effective in the treatment of ICH, but they also possess significant neuroprotectivein more homogenous ventilation, with decreases in both venous admixture and dead space. Proning may also result in reduced lung stress (i.e. transpulmonary pressure) and strain (i.e. the tidal volume-to-end-expiratory lung volume ratio) [[121]], deffectscreasing the risk of VILI.
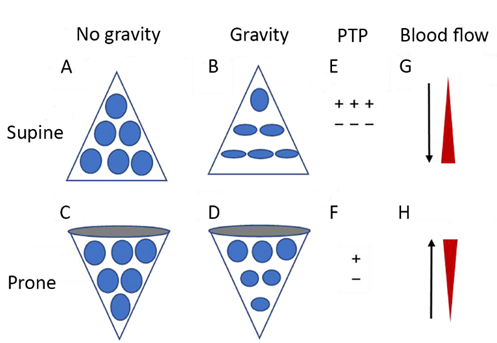
Figure 2. NDiagrammatic prevertheless, there is no evidence to support an improvement in clinical outcomes with their ussentation of physiological mechanisms associated with pronation in the acute respiratory distress syndrome (ARDS). (A) and (C) show the shape of lung units (i.e. alveoli) without the effect of gravity. (B) In the supine [49].
A plauosible argument for this discrepancy is that these sedatives have significant side effects, the most prominent being hemodynamiction, the volume of dorsal lung units is significantly smaller than the volume of ventral lung units, as a result of gravity and pleural pressure; thus, ventral lung units are more prone to overdistention and dorsal lung units are more prone to compromise, hypotension and myocardial depression. Consequently, their use is limited in trauma patients with shock or myocardial injury, since they may further increase vasopressor requirements for the maintenance of adequate CPession atelectasis. (D) In the prone position, gravity and pleural pressure result in a decrease in the volume of the ventral lung units and an increase in the volume of the dorsal lung units. (E) In the supine position, the ventral transpulmonary pressure (PTP) may substantially exceed the dorsal PTP. (F) Prone positioning reduces the ventral-to-dorsal PTP [50][51].gradient, Other side effects are immunosuppression, hepatic and renal dysfunction, and suppression of gut motility. At increased doses, barbiturates suppress the pupillary light reflex, in which case patient monitoring relies mainly on invasive measures or imaging. Finally, their use can lead to prolonged sedation deby augmenting the homogeneity of ventilation. (G) The reopening, dorsal lung units continue to receive most of the blood flow. (H) The ventral lung units may exhibit a greater tendency to collapse, but are still relatively underperfused. Reproduced in concordance with the Creative Commons Attribution License (CC-BY) from: Chen L, Zhang Y, Li Y, Song C, Lin F, Pan P. The Application of Awake-Prone Positioning Among Non-intubated Patients With COVID-19-Related ARDS: A Narrative Review. Front Med (Lausanne). 2022;9:817689.
The use to drug accumulation, and consequently to prolonged need forof prone positioning has increased significantly during the COVID pandemic, with 77% of mechanicallly ventilationed C-ARDS patients [52][53][54][55].with Dyskalemias, usually P/F < 100 being placed in the prone position [[122]] apcompear as hypokalemia ared to only 16% of usual ARDS patients with a P/F < 100 during the lopre-COVID era [[123]]. It remading phase and hyperkalemia during withdrawal; the latter may require renal replacement therapy in some casens one of the few interventions in severe ARDS associated with survival benefit, as demonstrated by a landmark study showing significant mortality reduction when patients with ARDS and a P/F < 150 were placed the prone position for least 16 hours daily [[56124][57]].
Whilen metabolic suppression with barbiturates is planned, a test dose should be administered first and the that trial preceded the advent of COVID, recent investigations performed in C-ARDS patient’s response should be recorded. A favorable response is characterized by ICP drop and concurrent maintenance of adequate CPP. If this is achieved, then loadings also suggest a survival benefit, with one retrospective study demonstrating a small but statistically significant reduction in the risk of death when C-ARDS patients with a P/F < 200 were proned within the first 2 days of ICU admission [[125]].
Studoses can be administered. The endpoint of barbiturate administration should be ICP control, and the minimum effective dies that have investigated the physiologic effects of prone positioning in C-ARDS patients have generally reported improved oxygenation, with P/F increasing ≥ 20 mmHg in approximately 75% of patients [[122]]. Response should be used in order to minimize the keep side effects. Electroencephalographic monitoring should s to proning are heterogeneous though, and available data suggest that the mechanisms responsible for improved oxygenation may differ from those in usual ARDS.
Unlidkeally be applied, in order to titrate the barbiturate infusion rate to a usual ARDS, net recruitment of C-ARDS lungs following placement in the prone position is relatively modest and often negligible [[126]]. suImppression – burst pattern of >> 50%; further increase in barbiturate dose are unlikely to affect ICP roved system compliance, typically present when significant net recruitment occurs, has not been observed in most studies [[122],[126],[127]-[128],[58129]]. When therapeutic targets are fulfilled and barbiturates are to bile measurements of partitioned respiratory mechanics would be needed to conclude withdrawn, a gradual reduction over a few days is advised, so as to avoid hyperkalemia and rebound ICH.
3.3. Decompressive craniectomy
De certainty that the lack of improvement in system compliance isn’t the result of decreased chest wall compliance in the prone position counterbalancing a simultaneous incompressive craniectomy lowers the ICP, and this has been confirmed by twoase in compliance of newly recruited lung, an absence of significant recruitment is suggested by other findings as well.
CO2 rexchandomized trialsge often improves in [59][60].the Cpromplications of craniectomy include infections, intracranial hemorrhage, seizures, transcranial hene position as a result of decreased dead space and recruitment of additional lung units. Most studies that have evaluated gas exchange in the prone position, however, have reported little change in the PaCO2 (or veniationtilatory ratio) [[122],[126],[127]-[128],[129]]. fCormation of subdural hygroma, and hydrmpared to usual ARDS, the changes in both respiratory system compliance and PaCO2 focephallus. These potential complications, along with owing prone positioning are significantly less in patients with C-ARDS [[83]]. In the riabsk of cranioplastyence of recruitment, the most plausible mechanism to explain [13]improved oxygenand the high baseline risk of a poor tion is better matching of ventilation/perfusion ratios of vaso-dysregulated tissue [120]].
Timineurg may alsological outcom play a significant role in response to prone positioning [[83],[126]]. In unresolving ARDS, may hinder a decision for secondary decompression. These issues, as well asatelectasis and edema may evolve into significant dorsal consolidation and diffuse fibrosis; in this setting, there is minimal recruitment of dorsal tissue in the probable need for long term care, need to be thoroughly explained to the patients’ families. Decompressive craniectomy remainsne position--only increased ventral atelectasis. A significant percentage of such patients either experience worsened P/F ratio in the prone position or fail to meet the accepted criteria for “responsiveness” (improvement in P/F ≥ 20 mmHg) [[128]].
10. Conclusion
Typica tier three therapy, while patients more likely to benefit are those with unilaterall ARDS is characterized by high-permeability edema, widespread atelectasis, and a loss of compliance that relates directly to the reduced capacity of aerated lung units. COVID-19, a novel etiology of ARDS, has distinct pathology, previously fit,ic findings consistent with adequate social support, in the setting of adequate medical and social resources. It is also a rescue option in othersevere injury to—and dysfunction of—the pulmonary vasculature as a result of SARS-CoV-2-induced endothelial injury and immunothrombosis. The lungs of patients with C-ARDS may be more likely to overdistend than to recruit in response to customary levels of PEEP. A subpopulation of patients not responding to conservative treatmentswith C-ARDS may present with severely deranged gas exchange that is uncoupled from the comparatively mild parenchymal [13].
4. Conclusions
Tienjur two and three therapies for ICH in TBI are associated with significant adverse effects and complications. Therefore, these treatmentsy. Just as typical ARDS encompasses a broad range of clinical findings, so too does C-ARDS, often transitioning in its more advanced stages to a form indistinguishable from typical ARDS. Some have argued that all patients with ARDS, regardless of etiology, should be chosen when ICH poses a bigger threat to thetreated identically. This approach, however, ignores the physiologic variability that not only exists between patient. Current tier concepts allow for flexibility in the choics, but also within individual patients depending on the phase of therapye disease.
Rand for patient-tailored approaches in different resource settings. Individualized approaches can be achieved by the use of imaging and neuromonitoring modalities, and future research should be oriented tdomized trials in ARDS have identified several interventions that lead to improved outcomes. These studies have enrolled patients with significant heterogeneity though and as such, a significant degree of heterogeneity in treatment effect is to be expected [[130]]. They repoward strengthening the evidence for these treatments and identifying patient profiles that can benefit from each one of themt the mean intervention effects observed in a population, but with regard to benefit wide individual variability exists. Randomized trials have provided safe starting points from which to approach mechanical ventilation in the individual, but such rules are not inviolable. A more holistic approach, taking into consideration the unique physiology of individual patients, is warranted—as exemplified by C-ARDS.
References
- Menon DK, Schwab K, Wright DW, Maas AI. Position statement: Definition of traumatic brain injury. Arch Phys Med Rehabil. 2010;91(11):1637-1640. doi:10.1016/j.apmr.2010.05.017Ashbaugh, D.G.; Bigelow, D.B.; Petty, T.L.; Levine, B.E. Acute respiratory distress in adults. Lancet 1967, 2, 319–323.
- Vik A, Nag T, Fredriksli OA, et al. Relationship of “dose” of intracranial hypertension to outcome in severe traumatic brain injury. J Neurosurg. 2008;109(4):678-684. doi:10.3171/JNS/2008/109/10/0678Gattinoni, L.; Marini, J.J. Isn’t it time to abandon ARDS? The COVID-19 lesson. Crit. Care 2021, 25, 326.
- Kahraman S, Dutton RP, Hu P, et al. Automated measurement of “pressure times time dose” of intracranial hypertension best predicts outcome after severe traumatic brain injury. Journal of Trauma - Injury, Infection and Critical Care. 2010;69(1):110-118. doi:10.1097/TA.0b013e3181c99853Bernard, G.R. Acute respiratory distress syndrome: A historical perspective. Am. J. Respir. Crit. Care Med. 2005, 172, 798–806.
- Güiza F, Depreitere B, Piper I, et al. Visualizing the pressure and time burden of intracranial hypertension in adult and paediatric traumatic brain injury. Intensive Care Med. 2015;41(6):1067-1076. doi:10.1007/s00134-015-3806-1Thille, A.W.; Esteban, A.; Fernandez-Segoviano, P.; Rodriguez, J.M.; Aramburu, J.A.; Penuelas, O.; Cortes-Puch, I.; Cardinal-Fernandez, P.; Lorente, J.A.; Frutos-Vivar, F. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am. J. Respir. Crit. Care Med. 2013, 187, 761–767.
- Maas AIR, Menon DK, David Adelson PD, et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987-1048. doi:10.1016/S1474-4422(17)30371-XMarini, J.J. Limitations of clinical trials in acute lung injury and acute respiratory distress syndrome. Curr. Opin. Crit. Care 2006, 12, 25–31.
- Menon DK, Ercole A. Critical care management of traumatic brain injury. In: Handbook of Clinical Neurology. Vol 140. Elsevier B.V.; 2017:239-274. doi:10.1016/B978-0-444-63600-3.00014-3Force, A.D.T.; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533.
- Hawryluk GWJ, Aguilera S, Buki A, et al. A management algorithm for patients with intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). In: Intensive Care Medicine. Vol 45. Springer; 2019:1783-1794. doi:10.1007/s00134-019-05805-9Iuliano, A.D.; Brunkard, J.M.; Boehmer, T.K.; Peterson, E.; Adjei, S.; Binder, A.M.; Cobb, S.; Graff, P.; Hidalgo, P.; Panaggio, M.J.; et al. Trends in Disease Severity and Health Care Utilization During the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods—United States, December 2020–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 146–152.
- Stocchetti N, Maas AIR. Traumatic Intracranial Hypertension. New England Journal of Medicine. 2014;370(22):2121-2130. doi:10.1056/nejmra1208708Lim, Z.J.; Subramaniam, A.; Ponnapa Reddy, M.; Blecher, G.; Kadam, U.; Afroz, A.; Billah, B.; Ashwin, S.; Kubicki, M.; Bilotta, F.; et al. Case Fatality Rates for Patients with COVID-19 Requiring Invasive Mechanical Ventilation. A Meta-analysis. Am. J. Respir. Crit. Care Med. 2021, 203, 54–66.
- Meyfroidt G, Bouzat P, Casaer MP, et al. Management of moderate to severe traumatic brain injury: an update for the intensivist. Intensive Care Med. Published online June 20, 2022. doi:10.1007/s00134-022-06702-4Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308.
- Stocchetti N, Carbonara M, Citerio G, et al. Severe traumatic brain injury: targeted management in the intensive care unit. Lancet Neurol. 2017;16(6):452-464. doi:10.1016/S1474-4422(17)30118-7Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 1904–1905.
- Stocchetti N, Zoerle T, Carbonara M. Intracranial pressure management in patients with traumatic brain injury: An update. Curr Opin Crit Care. 2017;23(2):110-114. doi:10.1097/MCC.0000000000000393Holm, B.A.; Matalon, S. Role of pulmonary surfactant in the development and treatment of adult respiratory distress syndrome. Anesth. Analg. 1989, 69, 805–818.
- Carney N, Totten AM, O’Reilly C, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017;80(1):6-15. doi:10.1227/NEU.0000000000001432Niklason, L.; Eckerstrom, J.; Jonson, B. The influence of venous admixture on alveolar dead space and carbon dioxide exchange in acute respiratory distress syndrome: Computer modelling. Crit. Care 2008, 12, R53.
- Hawryluk GWJ, Rubiano AM, Totten AM, et al. Guidelines for the management of severe traumatic brain injury: 2020 update of the decompressive craniectomy recommendations. Neurosurgery. 2020;87(3):427-434. doi:10.1093/neuros/nyaa278Radermacher, P.; Maggiore, S.M.; Mercat, A. Fifty Years of Research in ARDS. Gas Exchange in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 196, 964–984.
- Meyfroidt G, Bouzat P, Casaer MP, et al. Management of moderate to severe traumatic brain injury: an update for the intensivist. Intensive Care Med. 2022;48(6):649-666. doi:10.1007/s00134-022-06702-4Robertson, H.T.; Swenson, E.R. What do dead-space measurements tell us about the lung with acute respiratory distress syndrome? Respir. Care 2004, 49, 1006–1007.
- Huijben JA, Dixit A, Stocchetti N, et al. Use and impact of high intensity treatments in patients with traumatic brain injury across Europe: a CENTER-TBI analysis. Crit Care. 2021;25(1):78. doi:10.1186/s13054-020-03370-yKatzenstein, A.L.; Bloor, C.M.; Leibow, A.A. Diffuse alveolar damage—The role of oxygen, shock, and related factors. A review. Am. J. Pathol. 1976, 85, 209–228.
- Cnossen MC, Huijben JA, van der Jagt M, et al. Variation in monitoring and treatment policies for intracranial hypertension in traumatic brain injury: A survey in 66 neurotrauma centers participating in the CENTER-TBI study. Crit Care. 2017;21(1). doi:10.1186/s13054-017-1816-9Gattinoni, L.; Mascheroni, D.; Torresin, A.; Marcolin, R.; Fumagalli, R.; Vesconi, S.; Rossi, G.P.; Rossi, F.; Baglioni, S.; Bassi, F.; et al. Morphological response to positive end expiratory pressure in acute respiratory failure. Computerized tomography study. Intensive Care Med. 1986, 12, 137–142.
- Gelormini C, Caricato A. “tier-three” therapies in intracranial hypertension: Is it worthwhile? Minerva Anestesiol. 2021;87(12):1287-1289. doi:10.23736/S0375-9393.21.16117-6Gattinoni, L.; Pelosi, P.; Vitale, G.; Pesenti, A.; D’Andrea, L.; Mascheroni, D. Body position changes redistribute lung computed-tomographic density in patients with acute respiratory failure. Anesthesiology 1991, 74, 15–23.
- Godoy DA, Seifi A, Garza D, Lubillo-Montenegro S, Murillo-Cabezas F. Hyperventilation therapy for control of posttraumatic intracranial hypertension. Front Neurol. 2017;8(JUL). doi:10.3389/fneur.2017.00250Gattinoni, L.; Pesenti, A.; Avalli, L.; Rossi, F.; Bombino, M. Pressure-volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. Am. Rev. Respir. Dis. 1987, 136, 730–736.
- Beqiri E, Czosnyka M, Lalou AD, et al. Influence of mild-moderate hypocapnia on intracranial pressure slow waves activity in TBI. Acta Neurochir (Wien). 2020;162(2):345-356. doi:10.1007/s00701-019-04118-6Gattinoni, L.; Marini, J.J.; Pesenti, A.; Quintel, M.; Mancebo, J.; Brochard, L. The “baby lung” became an adult. Intensive Care Med. 2016, 42, 663–673.
- Geeraerts T. Moderate hypocapnia for intracranial pressure control after traumatic brain injury: a common practice requiring further investigations. Intensive Care Med. 2021;47(9):1009-1010. doi:10.1007/s00134-021-06489-wGattinoni, L.; Pesenti, A. The concept of “baby lung”. Intensive Care Med. 2005, 31, 776–784.
- Godoy DA, Badenes R, Robba C, Murillo Cabezas F. Hyperventilation in Severe Traumatic Brain Injury Has Something Changed in the Last Decade or Uncertainty Continues? A Brief Review. Front Neurol. 2021;12. doi:10.3389/fneur.2021.573237Gattinoni, L.; Pesenti, A.; Bombino, M.; Baglioni, S.; Rivolta, M.; Rossi, F.; Rossi, G.; Fumagalli, R.; Marcolin, R.; Mascheroni, D.; et al. Relationships between lung computed tomographic density, gas exchange, and PEEP in acute respiratory failure. Anesthesiology 1988, 69, 824–832.
- Brandi G, Stocchetti N, Pagnamenta A, Stretti F, Steiger P, Klinzing S. Cerebral metabolism is not affected by moderate hyperventilation in patients with traumatic brain injury. Crit Care. 2019;23(1). doi:10.1186/s13054-018-2304-6Marini, J.J.; Gattinoni, L. Time Course of Evolving Ventilator-Induced Lung Injury: The “Shrinking Baby Lung”. Crit. Care Med. 2020, 48, 1203–1209.
- Coles JP, Fryer TD, Coleman MR, et al. Hyperventilation following head injury: Effect on ischemic burden and cerebral oxidative metabolism. Crit Care Med. 2007;35(2):568-578. doi:10.1097/01.CCM.0000254066.37187.88Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793.
- Gouvea Bogossian E, Peluso L, Creteur J, Taccone FS. Hyperventilation in Adult TBI Patients: How to Approach It? Front Neurol. 2021;11. doi:10.3389/fneur.2020.580859Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273.
- Imberti R, Bellinzona G, Langer M. Cerebral tissue PO2 and SjvO2 changes during moderate hyperventilation in patients with severe traumatic brain injury. J Neurosurg. 2002;96:97-102.Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278.
- Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. The Lancet. 2021;398(10300):622-637. doi:10.1016/S0140-6736(21)00439-6Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284.
- Cai G, Zhang X, Ou Q, et al. Optimal Targets of the First 24-h Partial Pressure of Carbon Dioxide in Patients with Cerebral Injury: Data from the MIMIC-III and IV Database. Neurocrit Care. 2022;36(2):412-420. doi:10.1007/s12028-021-01312-2Ogando, N.S.; Dalebout, T.J.; Zevenhoven-Dobbe, J.C.; Limpens, R.; van der Meer, Y.; Caly, L.; Druce, J.; de Vries, J.J.C.; Kikkert, M.; Barcena, M.; et al. SARS-coronavirus-2 replication in Vero E6 cells: Replication kinetics, rapid adaptation and cytopathology. J. Gen. Virol. 2020, 101, 925–940.
- Murray MJ, Deblock H, Erstad B, et al. Clinical Practice Guidelines for Sustained Neuromuscular Blockade in the Adult Critically Ill Patient. Crit Care Med. 2016;44(11):2079-2103. doi:10.1097/CCM.0000000000002027Khan, M.; Yoo, S.J.; Clijsters, M.; Backaert, W.; Vanstapel, A.; Speleman, K.; Lietaer, C.; Choi, S.; Hether, T.D.; Marcelis, L.; et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell 2021, 184, 5932–5949.e5915.
- Hermans G, van den Berghe G. Clinical review: Intensive care unit acquired weakness. Crit Care. 2015;19(1). doi:10.1186/s13054-015-0993-7Hou, Y.J.; Okuda, K.; Edwards, C.E.; Martinez, D.R.; Asakura, T.; Dinnon, K.H., 3rd; Kato, T.; Lee, R.E.; Yount, B.L.; Mascenik, T.M.; et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 2020, 182, 429–446.e414.
- Inoue S, Hatakeyama J, Kondo Y, et al. Post‐intensive care syndrome: its pathophysiology, prevention, and future directions. Acute Medicine & Surgery. 2019;6(3):233-246. doi:10.1002/ams2.415Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 puzzle: Deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 2021, 9, 622–642.
- Papazian L, Forel JM, Gacouin A, et al. Neuromuscular Blockers in Early Acute Respiratory Distress Syndrome. Vol 12.; 2010.Barkauskas, C.E.; Cronce, M.J.; Rackley, C.R.; Bowie, E.J.; Keene, D.R.; Stripp, B.R.; Randell, S.H.; Noble, P.W.; Hogan, B.L. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Investig. 2013, 123, 3025–3036.
- De laet I, Hoste E, Verholen E, de Waele JJ. The effect of neuromuscular blockers in patients with intra-abdominal hypertension. Intensive Care Med. 2007;33(10):1811-1814. doi:10.1007/s00134-007-0758-0Carty, M.; Guy, C.; Bowie, A.G. Detection of Viral Infections by Innate Immunity. Biochem. Pharmacol. 2021, 183, 114316.
- Hsiang JK, Chesnut RM, Crisp CB, Klauber MR, Blunt BA, Marshall LF. Early, routine paralysis for intracranial pressure control in severe head injury: is it necessary? Crit Care Med. 1994 Sep;22(9):1471-6. doi: 10.1097/00003246-199409000-00019. PMID: 8062572.Land, W.G. Role of DAMPs in respiratory virus-induced acute respiratory distress syndrome-with a preliminary reference to SARS-CoV-2 pneumonia. Genes Immun. 2021, 22, 141–160.
- Mccall M, Jeejeebhoy K, Pencharz P, Moulton R. Effect of Neuromuscular Blockade on Energy Expenditure in Patients With Severe Head Injury. Vol 27.; 2003.Hernandez Acosta, R.A.; Esquer Garrigos, Z.; Marcelin, J.R.; Vijayvargiya, P. COVID-19 Pathogenesis and Clinical Manifestations. Infect. Dis. Clin. N. Am. 2022, 36, 231–249.
- Sanfilippo F, Santonocito C, Veenith T, Astuto M, Maybauer MO. The Role of Neuromuscular Blockade in Patients with Traumatic Brain Injury: A Systematic Review. Neurocrit Care. 2015;22(2):325-334. doi:10.1007/s12028-014-0061-1Sebag, S.C.; Bastarache, J.A.; Ware, L.B. Therapeutic modulation of coagulation and fibrinolysis in acute lung injury and the acute respiratory distress syndrome. Curr. Pharm. Biotechnol. 2011, 12, 1481–1496.
- Rangel-Castilla L, Gasco J, Nauta HJW, Okonkwo DO, Robertson CS. Cerebral pressure autoregulation in traumatic brain injury. Neurosurg Focus. 2008;25(4). doi:10.3171/FOC.2008.25.10.E7Iba, T.; Levy, J.H.; Levi, M.; Thachil, J. Coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 2103–2109.
- Donnelly J, Czosnyka M, Adams H, et al. Individualizing Thresholds of Cerebral Perfusion Pressure Using Estimated Limits of Autoregulation. Crit Care Med. 2017;45(9):1464-1471. doi:10.1097/CCM.0000000000002575Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418.
- Robba C, Cardim D, Sekhon M, Budohoski K, Czosnyka M. Transcranial Doppler: a stethoscope for the brain-neurocritical care use. J Neurosci Res. 2018;96(4):720-730. doi:10.1002/jnr.24148Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128.
- Lang EW, Chesnut RM. A Bedside Method for Investigating the Integrity and Critical Thresholds of Cerebral Pressure Autoregulation in Severe Traumatic Brain Injury Patients. Vol 14.; 2000.Owens, A.P., 3rd; Mackman, N. Tissue factor and thrombosis: The clot starts here. Thromb. Haemost. 2010, 104, 432–439.
- Annane D, Ouanes-Besbes L, de Backer D, et al. A Global Perspective on Vasoactive Agents in Shock.Kenawy, H.I.; Boral, I.; Bevington, A. Complement-Coagulation Cross-Talk: A Potential Mediator of the Physiological Activation of Complement by Low pH. Front. Immunol. 2015, 6, 215.
- Ceulemans AG, Zgavc T, Kooijman R, Hachimi-Idrissi S, Sarre S, Michotte Y. The dual role of the neuroinflammatory response after ischemic stroke: Modulatory effects of hypothermia. J Neuroinflammation. 2010;7. doi:10.1186/1742-2094-7-74Swieringa, F.; Spronk, H.M.H.; Heemskerk, J.W.M.; van der Meijden, P.E.J. Integrating platelet and coagulation activation in fibrin clot formation. Res. Pract. Thromb. Haemost. 2018, 2, 450–460.
- Zhu Y, Yin H, Zhang R, Ye X, Wei J. Therapeutic hypothermia versus normothermia in adult patients with traumatic brain injury: a meta-analysis. Springerplus. 2016;5(1). doi:10.1186/s40064-016-2391-2Bonaventura, A.; Vecchie, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329.
- Hirst TC, Klasen MG, Rhodes JK, MacLeod MR, Andrews PJD. A Systematic Review and Meta-Analysis of Hypothermia in Experimental Traumatic Brain Injury: Why Have Promising Animal Studies Not Been Replicated in Pragmatic Clinical Trials? J Neurotrauma. 2020;37(19):2057-2068. doi:10.1089/neu.2019.6923Rodrigues, T.S.; de Sa, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Goncalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021, 218, e20201707.
- Cooper RJ, Wears RL, Schriger DL. Reporting research results: Recommendations for improving communication. Ann Emerg Med. 2003;41(4):561-564. doi:10.1067/mem.2003.135Kambas, K.; Markiewski, M.M.; Pneumatikos, I.A.; Rafail, S.S.; Theodorou, V.; Konstantonis, D.; Kourtzelis, I.; Doumas, M.N.; Magotti, P.; Deangelis, R.A.; et al. C5a and TNF-alpha up-regulate the expression of tissue factor in intra-alveolar neutrophils of patients with the acute respiratory distress syndrome. J. Immunol. 2008, 180, 7368–7375.
- Polderman KH. Application of therapeutic hypothermia in the intensive care unit: Opportunities and pitfalls of a promising treatment modality - Part 2: Practical aspects and side effects. Intensive Care Med. 2004;30(5):757-769. doi:10.1007/s00134-003-2151-yGeorg, P.; Astaburuaga-Garcia, R.; Bonaguro, L.; Brumhard, S.; Michalick, L.; Lippert, L.J.; Kostevc, T.; Gabel, C.; Schneider, M.; Streitz, M.; et al. Complement activation induces excessive T cell cytotoxicity in severe COVID-19. Cell 2022, 185, 493–512.e425.
- Andrews PJD, Sinclair HL, Rodriguez A, et al. Hypothermia for Intracranial Hypertension after Traumatic Brain Injury. New England Journal of Medicine. 2015;373(25):2403-2412. doi:10.1056/NEJMoa1507581Ebeyer-Masotta, M.; Eichhorn, T.; Weiss, R.; Laukova, L.; Weber, V. Activated Platelets and Platelet-Derived Extracellular Vesicles Mediate COVID-19-Associated Immunothrombosis. Front. Cell Dev. Biol. 2022, 10, 914891.
- Zwerus R, Absalom A. Update on anesthetic neuroprotection. Curr Opin Anaesthesiol. 2015;28(4):424-430. doi:10.1097/ACO.0000000000000212Mackman, N.; Antoniak, S.; Wolberg, A.S.; Kasthuri, R.; Key, N.S. Coagulation Abnormalities and Thrombosis in Patients Infected With SARS-CoV-2 and Other Pandemic Viruses. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2033–2044.
- Almaas R, Saugstad OD, Pleasure D, Rootwelt T. Effect of Barbiturates on Hydroxyl Radicals, Lipid Peroxidation, and Hypoxic Cell Death in Human NT2-N Neurons. Vol 92.; 2000.Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436.
- Léger M, Frasca D, Roquilly A, et al. Early use of barbiturates is associated with increased mortality in traumatic brain injury patients from a propensity score-based analysis of a prospective cohort. PLoS One. 2022;17(5):e0268013. doi:10.1371/journal.pone.0268013O’Driscoll, M.; Ribeiro Dos Santos, G.; Wang, L.; Cummings, D.A.T.; Azman, A.S.; Paireau, J.; Fontanet, A.; Cauchemez, S.; Salje, H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 2021, 590, 140–145.
- Majdan M, Mauritz W, Wilbacher I, Brazinova A, Rusnak M, Leitgeb J. Barbiturates use and its effects in patients with severe traumatic brain injury in five European countries. J Neurotrauma. 2013;30(1):23-29. doi:10.1089/neu.2012.2554Zhang, Q.; Bastard, P.; Liu, Z.; Le Pen, J.; Moncada-Velez, M.; Chen, J.; Ogishi, M.; Sabli, I.K.D.; Hodeib, S.; Korol, C.; et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020, 370, eabd4570.
- Roberts I, Sydenham E. Barbiturates for acute traumatic brain injury. Cochrane Database of Systematic Reviews. Published online December 12, 2012. doi:10.1002/14651858.cd000033.pub2Severe Covid, G.G.; Ellinghaus, D.; Degenhardt, F.; Bujanda, L.; Buti, M.; Albillos, A.; Invernizzi, P.; Fernandez, J.; Prati, D.; Baselli, G.; et al. Genomewide Association Study of Severe Covid-19 with Respiratory Failure. N. Engl. J. Med. 2020, 383, 1522–1534.
- Stover JF, Lenzlinger PM, Stocker R, et al. Thiopental in CSF and serum correlates with prolonged loss of cortical activity. Eur Neurol. 1998;39(4):223-228. doi:10.1159/000007938Pairo-Castineira, E.; Clohisey, S.; Klaric, L.; Bretherick, A.D.; Rawlik, K.; Pasko, D.; Walker, S.; Parkinson, N.; Fourman, M.H.; Russell, C.D.; et al. Genetic mechanisms of critical illness in COVID-19. Nature 2021, 591, 92–98.
- Wheeler DW, Thompson AJ, Corletto F, et al. Anaesthetic impairment of immune function is mediated via GABAA receptors. PLoS One. 2011;6(2). doi:10.1371/journal.pone.0017152Initiative, C.-H.G. Mapping the human genetic architecture of COVID-19. Nature 2021, 600, 472–477.
- Loop T, Humar M, Pischke S, et al. Thiopental Inhibits Tumor Necrosis Factor-Induced Activation of Nuclear Factor B through Suppression of IB Kinase Activity. Vol 99.; 2003. http://pubs.asahq.org/anesthesiology/article-pdf/99/2/360/408372/0000542-200308000-00017.pdfAsano, T.; Boisson, B.; Onodi, F.; Matuozzo, D.; Moncada-Velez, M.; Maglorius Renkilaraj, M.R.L.; Zhang, P.; Meertens, L.; Bolze, A.; Materna, M.; et al. X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci. Immunol. 2021, 6, eabl4348.
- Andrefsky, J.C.; Frank, J.I.; Chyatte, D.; The ciliospinal reflex in pentobarbital coma. J Neurosurg. 1999;90:644-646.Koning, R.; Bastard, P.; Casanova, J.L.; Brouwer, M.C.; van de Beek, D.; Amsterdam, U.M.C. COVID-19 Biobank Investigators. Autoantibodies against type I interferons are associated with multi-organ failure in COVID-19 patients. Intensive Care Med. 2021, 47, 704–706.
- Cairns CJ, Thomas B, Fletcher S, Parr MJ, Finfer SR. Life-threatening hyperkalaemia following therapeutic barbiturate coma. Intensive Care Med. 2002;28(9):1357-1360. doi:10.1007/s00134-002-1399-yBastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Beziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585.
- Aytuluk HG, Topcu H. Severe hypokalemia and rebound hyperkalemia during barbiturate coma in patients with severe traumatic brain injury. Neurocirugia. 2020;31(5):216-222. doi:10.1016/j.neucir.2019.12.003Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe Covid-19. N. Engl. J. Med. 2020, 383, 2451–2460.
- Ellington AL. Electroencephalography and Clinical Neurophysiology CLINICAL AND LABORATORY NOTES ELECTROENCEPHALOGRAPHIC PATTERN OF BURST SUPPRESSION IN A CASE OF BARBITURATE COMA.Kox, M.; Waalders, N.J.B.; Kooistra, E.J.; Gerretsen, J.; Pickkers, P. Cytokine Levels in Critically Ill Patients With COVID-19 and Other Conditions. JAMA 2020, 234, 1565–1567.
- Hutchinson PJ, Kolias AG, Timofeev IS, et al. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. New England Journal of Medicine. 2016;375(12):1119-1130. doi:10.1056/nejmoa1605215Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020, 8, 1233–1244.
- Cooper DJ, Rosenfeld J v., Murray L, et al. Decompressive Craniectomy in Diffuse Traumatic Brain Injury. New England Journal of Medicine. 2011;364(16):1493-1502. doi:10.1056/nejmoa1102077.Wilson JG; COVID-19-Related ARDS: Key Mechanistic Features and Treatments. Crit Care 2020, 24, 102, 10.3390/jcm11164896.
- doi:10.1164/ajrccm.158.1.9708031; Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? . Am J Respir Crit Care Med 1998, 158, 3, doi:10.1164/ajrccm.158.1.9708031.
- Calfee CS; Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials.. Lancet Respir Med 2014, 2, 611, doi:10.1016/S2213-2600(14)70097-9.
- Satturwar S; Postmortem Findings Associated With SARS-CoV-2: Systematic Review and Meta-analysis.. Am J Surg Pathol 2021, 45, 587, doi:10.1097/PAS.0000000000001650.
- Katzenstein AL; Diffuse alveolar damage--the role of oxygen, shock, and related factors. A review. . Am J Pathol 1976, 85, 209.
- Tomashefski JF; The pulmonary vascular lesions of the adult respiratory distress syndrome.. Am J Pathol 1983, 112, 112.
- Hariri LP; Lung Histopathology in Coronavirus Disease 2019 as Compared With Severe Acute Respiratory Sydrome and H1N1 Influenza: A Systematic Review. . Chest 2021, 159, 73, 10.1016/j.chest.2020.09.259.
- Milross L; Post-mortem lung tissue: the fossil record of the pathophysiology and immunopathology of severe COVID-19.. Lancet Respir Med 2022, 10, 95, doi:10.1016/S2213-2600(21)00408-2.
- Carsana L.; Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. . Lancet Infect Dis 2020, 20, 1135, 10.1016/S1473-3099(20)30434-5.
- Satturwar S; Postmortem Findings Associated With SARS-CoV-2: Systematic Review and Meta-analysis.. Am J Surg Pathol 2021, 45, 87, doi:10.1097/PAS.0000000000001650.
- Ackermann M; Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med 2020, 383, 120, 10.1056/NEJMoa2015432.
- Poissy J; Pulmonary Embolism in Patients With COVID-19: Awareness of an Increased Prevalence. Circulation 2020, 142, 184, 10.1161/CIRCULATIONAHA.120.047430.
- Helms J; High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020, 46, 1089, 10.1007/s00134-020-06062-x.
- Villalba JA; Vasculopathy and Increased Vascular Congestion in Fatal COVID-19 and ARDS. Am J Respir Crit Care Med 2022, 10.1164/rccm.202109-2150OC, in press, 10.1164/rccm.202109-2150OC.
- Santamarina MG; COVID-19: What Iodine Maps From Perfusion CT can reveal-A Prospective Cohort Study. Crit Care 2020, 24, 19, doi:10.1186/s13054-020-03333-3.
- Li Q; CT features of coronavirus disease 2019 (COVID-19) with an emphasis on the vascular enlargement pattern. Eur J Radiol 2021, 134, 109442, 10.1016/j.ejrad.2020.109442.
- Poschenrieder F; Severe COVID-19 pneumonia: Perfusion analysis in correlation with pulmonary embolism and vessel enlargement using dual-energy CT data. PLoS One 2021, 16, e0252478, doi:10.1371/journal.pone.0252478.
- Patel BV; Pulmonary Angiopathy in Severe COVID-19: Physiologic, Imaging, and Hematologic Observations. Am J Respir Crit Care Med 2020, 202, 690, 10.1164/rccm.202004-1412OC.
- Gattinoni L; Anatomical and functional intrapulmonary shunt in acute respiratory distress syndrome. Crit Care Med 2008, 36, 669, 10.1097/01.CCM.0000300276.12074.E1.
- Gattinoni L; Anatomical and functional intrapulmonary shunt in acute respiratory distress syndrome. Crit Care Med 2008, 36, 669, 10.1097/01.CCM.0000300276.12074.E1.
- Chiumello D; Physiological and quantitative CT-scan characterization of COVID-19 and typical ARDS: a matched cohort study. Intensive Care Med 2020, 46, 2187, 10.1007/s00134-020-06281-2.
- Barbeta E; SARS-CoV-2-induced Acute Respiratory Distress Syndrome: Pulmonary Mechanics and Gas-Exchange Abnormalities. Ann Am Thorac Soc 2020, 17, 1164, 10.1513/AnnalsATS.202005-462RL.
- Vasques F; Physiological dead space ventilation, disease severity and outcome in ventilated patients with hypoxaemic respiratory failure due to coronavirus disease 2019. Intensive Care Med 2020, 46, 2092, 10.1007/s00134-020-06197-x..
- Camporota L; Prone Position in COVID-19 and -COVID-19 Acute Respiratory Distress Syndrome: An International Multicenter Observational Comparative Study. Crit Care Med 2022, 50, 633, 10.1097/CCM.0000000000005354.
- Grieco DL; Respiratory physiology of COVID-19-induced respiratory failure compared to ARDS of other etiologies. Crit Care 2020, 24, 529, 10.1186/s13054-020-03253-2.
- Kummer RL; Paradoxically Improved Respiratory Compliance With Abdominal Compression in COVID-19 ARDS. Chest 2021, 160, 1739, 10.1016/j.chest.2021.05.012.
- Haudebourg AF; Respiratory Mechanics of COVID-19- versus Non-COVID-19-associated Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2020, 202, 287, 10.1164/rccm.202004-1226LE.
- Panwar R; Compliance Phenotypes in Early Acute Respiratory Distress Syndrome before the COVID-19 Pandemic. Am J Respir Crit Care Med 2020, 202, 1244, 10.1164/rccm.202005-2046OC.
- Beloncle F; Longitudinal changes in compliance, oxygenation and ventilatory ratio in COVID-19 versus non-COVID-19 pulmonary acute respiratory distress syndrome. Crit Care 2021, 25, 248, 10.1186/s13054-021-03665-8.
- Gattinoni L.; Reply by Gattinoni et al. to Hedenstierna et al., to Maley et al., to Fowler et al., to Bhatia and Mohammed, to Bos, to Koumbourlis and Motoyama, and to Haouzi et al. Am J Respir Crit Care Med 2020, 202, 628, 10.1164/rccm.202004-1052LE.
- Marini JJ; Energetics and the Root Mechanical Cause for Ventilator-induced Lung Injury. Anesthesiology 2018, 128, 1062, doi:10.1097/ALN.0000000000002203.
- Marini JJ; Static and Dynamic Contributors to Ventilator-induced Lung Injury in Clinical Practice. Pressure, Energy, and Power. Am J Respir Crit Care Med 2020, 201, 767, 10.1164/rccm.201908-1545CI.
- Fan E; An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2017, 195, 1253, 10.1164/rccm.201703-0548ST.
- Alhazzani W; Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Crit Care Med 2008, 28, e440, 10.1097/CCM.0000000000004363.
- Dreyfuss D; Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 1998, 157, 294, 10.1164/ajrccm.157.1.9604014.
- Stewart TE; Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. Pressure- and Volume-Limited Ventilation Strategy Group. N Engl J Med 1998, 338, 355, 10.1056/NEJM199802053380603.
- Brochard L; Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The Multicenter Trail Group on Tidal Volume reduction in ARDS. Am J Respir Crit Care Med 1998, 158, 1831, 10.1164/ajrccm.158.6.9801044.
- Brower RG; Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med 1999, 27, 1492, 10.1097/00003246-199908000-00015.
- Liu X; Ventilatory Ratio in Hypercapnic Mechanically Ventilated Patients with COVID-19-associated Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2020, 201, 1297, 10.1164/rccm.202002-0373LE.
- Marini JJ; Management of COVID-19 Respiratory Distress. JAMA 2020, 323, 2329, 10.1001/jama.2020.6825.
- Gattinoni L; Intensive Care Med . Intensive Care Med 2022, 48, 728, 10.1007/s00134-022-06698-x.
- Barthelemy R; Haemodynamic impact of positive end-expiratory pressure in SARS-CoV-2 acute respiratory distress syndrome: oxygenation versus oxygen delivery. Br J Anaesth 2021, 126, e70, 10.1016/j.bja.2020.10.026.
- Suter PM; Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N Engl J Med 1975, 292, 284, 10.1056/NEJM197502062920604.
- Dickel S; Nationwide Cross-Sectional Online Survey on the Treatment of COVID-19-ARDS: High Variance in Standard of Care in German ICUs. J Clin Med 2021, 10, 3363, 10.3390/jcm10153363.
- Grasselli G; Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574, 10.1001/jama.2020.5394.
- Chiumello D; Positive end-expiratory pressure in COVID-19 acute respiratory distress syndrome: the heterogeneous effects. Crit Care 2021, 25, 431, 10.1186/s13054-021-03839-4.
- Sinha P; Analysis and Clinical Performance of the Ventilatory Ratio in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2019, 199, 333, 10.1164/rccm.201804-0692OC.
- Beitler JR; Effect of Titrating Positive End-Expiratory Pressure (PEEP) With an Esophageal Pressure-Guided Strategy vs an Empirical High PEEP-Fio2 Strategy on Death and Days Free From Mechanical Ventilation Among Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2019, 321, 846, 10.1001/jama.2019.0555.
- Pan C; Lung Recruitability in COVID-19-associated Acute Respiratory Distress Syndrome: A Single-Center Observational Study. Am J Respir Crit Care Med 2020, 201, 1294, 10.1164/rccm.202003-0527LE.
- Ball L; Computed tomography assessment of PEEP-induced alveolar recruitment in patients with severe COVID-19 pneumonia. Crit Care 2021, 25, 81, 10.1186/s13054-021-03477-w.
- Protti A; Lung Response to a Higher Positive End-Expiratory Pressure in Mechanically Ventilated Patients With COVID-19. Chest 2022, 161, 979, 10.1016/j.chest.2021.10.012.
- Roesthuis L; Advanced respiratory monitoring in COVID-19 patients: use less PEEP! . Crit Care 2020, 24, 230, 1186/s13054-020-02953-z.
- Perier F; Effect of Positive End-Expiratory Pressure and Proning on Ventilation and Perfusion in COVID-19 Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2020, 202, 1713, doi:10.1164/rccm.202008-3058LE.
- Mauri T; Potential for Lung Recruitment and Ventilation-Perfusion Mismatch in Patients With the Acute Respiratory Distress Syndrome From Coronavirus Disease 2019. Crit Care Med 2020, 48, 1129, 10.1097/CCM.0000000000004386.
- Chen L; Potential for Lung Recruitment Estimated by the Recruitment-to-Inflation Ratio in Acute Respiratory Distress Syndrome. A Clinical Trial. Am J Respir Crit Care Med 2020, 201, 178, 10.1164/rccm.201902-0334OC.
- Gattinoni L; Prone Position and COVID-19: Mechanisms and Effects. . Crit Care Med 2022, 50, 873, 10.1097/CCM.0000000000005486.
- Rouby J.J; Acute respiratory distress syndrome: lessons from computed tomography of the whole lung. Crit Care Med 2003, 31, 5285, 10.1097/01.CCM.0000057905.74813.BC.
- Albert RK; The prone position eliminates compression of the lungs by the heart. Am J Respir Crit Care Med 2000, 161, 1660, 10.1164/ajrccm.161.5.9901037.
- Pelosi P; Vertical gradient of regional lung inflation in adult respiratory distress syndrome. Am J Respir Crit Care Med 1994, 149, 8, 10.1164/ajrccm.149.1.8111603.
- Gattinoni L; Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am J Respir Crit Care Med 2013, 188, 1286, 10.1164/rccm.201308-1532CI.
- Guerin C; Prone position in ARDS patients: why, when, how and for whom. Intensive Care Med 2020, 46, 2385, 10.1007/s00134-020-06306-w.
- Mentzelopoulos SD; Prone position reduces lung stress and strain in severe acute respiratory distress syndrome. Eur Respir J 2005, 25, 534, 10.1183/09031936.05.00105804.
- Langer T; Prone position in intubated, mechanically ventilated patients with COVID-19: a multi-centric study of more than 1000 patients. Crit Care 2021, 25, 128, 10.1186/s13054-021-03552-2.
- Bellani G; The LUNG SAFE study: a presentation of the prevalence of ARDS according to the Berlin Definition! . Crit Care 2016, 20, 268, 10.1186/s13054-016-1443-x.
- Guerin C; Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013, 368, 2159, 10.1056/NEJMoa1214103.
- Mathews KS; Prone Positioning and Survival in Mechanically Ventilated Patients With Coronavirus Disease 2019-Related Respiratory Failure. Crit Care Med 2021, 49, 1026, 10.1097/CCM.0000000000004938.
- Fossali T; Effects of Prone Position on Lung Recruitment and Ventilation-Perfusion Matching in Patients With COVID-19 Acute Respiratory Distress Syndrome: A Combined CT Scan/Electrical Impedance Tomography Study. Crit Care Med 2022, 50, 723, 10.1097/CCM.0000000000005450.
- Zarantonello F; Early physiological effects of prone positioning in COVID-19 Acute Respiratory Distress Syndrome. Anesthesiology 2022, 137, 327, 10.1097/ALN.0000000000004296.
- Rossi S; Mechanisms of oxygenation responses to proning and recruitment in COVID-19 pneumonia. Intensive Care Med 2022, 48, 56, 10.1007/s00134-021-06562-4.
- Protti A; Lung response to prone positioning in mechanically-ventilated patients with COVID-19.. Crit Care 2022, 26, 127, 10.1186/s13054-022-03996-0..
- Iwashyna TJ; Implications of Heterogeneity of Treatment Effect for Reporting and Analysis of Randomized Trials in Critical Care. Am J Respir Crit Care Med 2015, 192, 1045, 10.1164/rccm.201411-2125CP.
