Clinically significant portal hypertension is associated with most complications of advanced chronic liver disease (ACLD), including variceal bleeding, ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, and hepatic encephalopathy. Gut dysbiosis is a hallmark of ACLD with portal hypertension and consists of the overgrowth of potentially pathogenic bacteria and a decrease in autochthonous bacteria; additionally, congestion makes the intestinal barrier more permeable to bacteria and their products, which contributes to the development of complications through inflammatory mechanisms within the gut–liver axis. The identification of the gut–liver liver-axis-related metabolic and molecular pathways may serve as a target for new therapeutic strategies through the modulation of the intestinal environment.
- gut microbiome
- portal hypertension
- cirrhosis
- HVPG
- CSPH
- ACLD
1. Introduction
2. Pathogenesis of Portal Hypertension in Liver Disease
3. Gut–Liver Axis Composition and Function
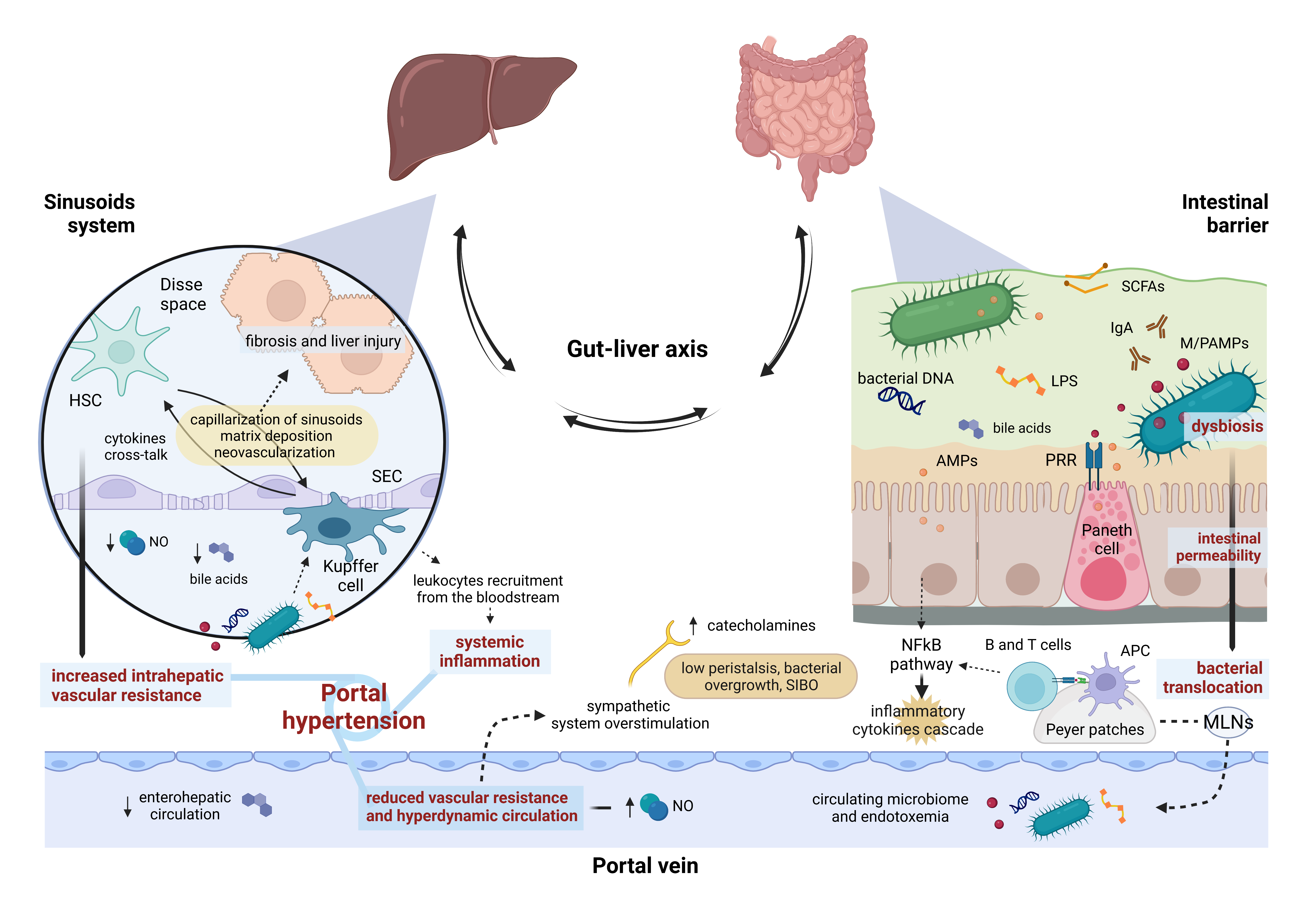
4. Gut–Liver Axis Impairment and Portal Hypertension: A Two-Way Street
5. Influence of the Gut Microbiome on Portal Hypertension
| Study | Endpoint | Patients | Analysis | Microbiota Profile |
|---|---|---|---|---|
| Gedgaudas R et al., 2022 [71] | Circulating bacterial DNA signatures of PH severity | 58 cirrhotic pts 46 healthy controls |
16S rRNA | Circulating microbiome profile could not predict CSPH or severe PH |
| Virseda-Berdices A et al., 2022 [72] | Association between baseline-specific bacterial taxa and HVPG decrease in pts with HCV-related cirrhosis after successful DAA therapy | 32 cirrhotic pts (21 HIV-positive) with CSPH (HVPG ≥ 10 mmHg) | 16S rRNA | ↑ in Corynebacteriales and Diplorickettsiales orders, Diplorickettsiaceae family, Corynebacterium and Aquicella genera, and Undibacterium parvum species ↓ in Oceanospirillales and Rhodospirillales orders, Halomonadaceae family, and Massilia genus |
| Yokoyama K et al., 2020 [73] | To find gut microbiota changes associated with PH in cirrhotic pts | 12 pts with cirrhosis and PH 24 controls |
16S rRNA | ↑ in Lactobacillales order, ↓ in Clostridium cluster IV and cluster IX in pts with cirrhosis and PH compared to controls |
| Gómez-Hurtado I et al., 2019 [74] | To explore portal hemodynamics changes in experimental portal hypertensive cirrhosis/BDL rats after B. pseudocatenulatum CECT 7765 administration | 6 sham-operated, 6 BDL, and 8 BDL rats previously treated with B. pseudocatenulatum |
16S rRNA | ↑ in Clostridiales and Bacteroidales orders was independently associated with variations in portal vein area and portal flow, while changes in the Proteobacteria phylum were independently associated with congestion. B. pseudocatenulatum significantly decreased Proteobacteria and increased Bacteroidetes |
| Huang HC et al., 2021 [75] | Outcomes of FMT in BDL cirrhotic rats | BDL rats received either vehicle, fecal, or gut (terminal ileum) microbiota transplantation | 16S rRNA | Both microbiota transplants increased Bifidobacteria. Microbiota transplantation in cirrhotic rats was associated with reduced PP |
| Garcıa-Lezana T et al., 2018 [76] | Role of intestinal microbiota in PH onset in NASH | 23 control rats (13 receiving FMT from HFGFD rats) and 27 HFGFD rats (14 receiving FMT from control rats) | 16S rRNA | Clostridium and Adlercreutzia abundance was inversely related to PP |
References
- Schnabl, B.; Brenner, D.A. Interactions between the Intestinal Microbiome and Liver Diseases. Gastroenterology 2014, 146, 1513–1524.
- Adak, A.; Khan, M.R. An Insight into Gut Microbiota and Its Functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493.
- Gentile, C.L.; Weir, T.L. The Gut Microbiota at the Intersection of Diet and Human Health. Science 2018, 362, 776–780.
- Sekirov, I.; Russell, S.L.; Caetano M Antunes, L.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904.
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836.
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients 2019, 11, 923.
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14.
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-Mediated Dysbiosis Regulates Progression of NAFLD and Obesity. Nature 2012, 482, 179–185.
- Albillos, A.; de Gottardi, A.; Rescigno, M. The Gut-Liver Axis in Liver Disease: Pathophysiological Basis for Therapy. J. Hepatol. 2020, 72, 558–577.
- Seo, Y.S.; Shah, V.H. The Role of Gut-Liver Axis in the Pathogenesis of Liver Cirrhosis and Portal Hypertension. Clin. Mol. Hepatol. 2012, 18, 337–346.
- Wiest, R.; Garcia-Tsao, G. Bacterial Translocation (BT) in Cirrhosis. Hepatology 2005, 41, 422–433.
- Goel, A.; Gupta, M.; Aggarwal, R. Gut Microbiota and Liver Disease. J. Gastroenterol. Hepatol. 2014, 29, 1139–1148.
- Arab, J.P.; Martin-Mateos, R.M.; Shah, V.H. Gut–Liver Axis, Cirrhosis and Portal Hypertension: The Chicken and the Egg. Hepatol. Int. 2017, 12, 24–33.
- Baffy, G. Potential Mechanisms Linking Gut Microbiota and Portal Hypertension. Liver Int. 2019, 39, 598–609.
- Garcia-Tsao, G. Portal Hypertension. Curr. Opin. Intern. Med. 2006, 5, 399–407.
- de Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C.; Abraldes, J.G.; Albillos, A.; Baiges, A.; Bajaj, J.; Bañares, R.; et al. Baveno VII—Renewing Consensus in Portal Hypertension. J. Hepatol. 2022, 76, 959–974.
- Bosch, J.; Berzigotti, A.; Garcia-Pagan, J.C.; Abraldes, J.G. The Management of Portal Hypertension: Rational Basis, Available Treatments and Future Options. J. Hepatol. 2008, 48, S68–S92.
- Bloom, S.; Kemp, W.; Lubel, J. Portal Hypertension: Pathophysiology, Diagnosis and Management. Intern. Med. J. 2015, 45, 16–26.
- Mauro, E.; Gadano, A. What’s New in Portal Hypertension? Liver Int. 2020, 40, 122–127.
- Gunarathne, L.S.; Rajapaksha, H.; Shackel, N.; Angus, P.W.; Herath, C.B. Cirrhotic Portal Hypertension: From Pathophysiology to Novel Therapeutics. World J. Gastroenterol. 2020, 26, 6111–6140.
- EASL Clinical Practice Guidelines on Prevention and Management of Bleeding and Thrombosis in Patients with Cirrhosis. J. Hepatol. 2022, 76, 1151–1184.
- Wanless, I.R.; Wong, F.; Blendis, L.M.; Greig, P.; Heathcote, E.J.; Levy, G. Hepatic and Portal Vein Thrombosis in Cirrhosis: Possible Role in Development of Parenchymal Extinction and Portal Hypertension. Hepatology 1995, 21, 1238–1247.
- Turco, L.; Garcia-Tsao, G. Portal Hypertension: Pathogenesis and Diagnosis. Clin. Liver Dis. 2019, 23, 573–587.
- Gracia-Sancho, J.; Marrone, G.; Fernández-Iglesias, A. Hepatic Microcirculation and Mechanisms of Portal Hypertension. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 221–234.
- Villa, E.; Cammà, C.; Marietta, M.; Luongo, M.; Critelli, R.; Colopi, S.; Tata, C.; Zecchini, R.; Gitto, S.; Petta, S.; et al. Enoxaparin Prevents Portal Vein Thrombosis and Liver Decompensation in Patients with Advanced Cirrhosis. Gastroenterology 2012, 143, 1253–1260.
- Turco, L.; Schepis, F.; Villa, E. The Role of Anticoagulation in Treating Portal Hypertension. Curr. Hepatol. Rep. 2018, 17, 200–208.
- Kim, Y.S.; Ho, S.B. Intestinal Goblet Cells and Mucins in Health and Disease: Recent Insights and Progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330.
- van Itallie, C.M.; Holmes, J.; Bridges, A.; Gookin, J.L.; Coccaro, M.R.; Proctor, W.; Colegio, O.R.; Anderson, J.M. The Density of Small Tight Junction Pores Varies among Cell Types and Is Increased by Expression of Claudin-2. J. Cell Sci. 2008, 121, 298–305.
- Gautreaux, M.D.; Dietch, E.A.; Berg, R.D. T Lymphocytes in Host Defense against Bacterial Translocation from the Gastrointestinal Tract. Infect. Immun. 1994, 62, 2874–2884.
- Gautreaux, M.D.; Gelder, F.B.; Deitch, E.A.; Berg, R.D. Adoptive Transfer of T Lymphocytes to T-Cell-Depleted Mice Inhibits Escherichia Coli Translocation from the Gastrointestinal Tract. Infect. Immun. 1995, 63, 3827–3834.
- Hapfelmeier, S.; Lawson, M.A.E.; Slack, E.; Kirundi, J.K.; Stoel, M.; Heikenwalder, M.; Cahenzli, J.; Velykoredko, Y.; Balmer, M.L.; Endt, K.; et al. Reversible Microbial Colonization of Germ-Free Mice Reveals the Dynamics of IgA Immune Responses. Science 2010, 328, 1705–1709.
- Spadoni, I.; Zagato, E.; Bertocchi, A.; Paolinelli, R.; Hot, E.; di Sabatino, A.; Caprioli, F.; Bottiglieri, L.; Oldani, A.; Viale, G.; et al. A Gut-Vascular Barrier Controls the Systemic Dissemination of Bacteria. Science 2015, 350, 830–834.
- Wiest, R.; Lawson, M.; Geuking, M. Pathological Bacterial Translocation in Liver Cirrhosis. J. Hepatol. 2014, 60, 197–209.
- Duparc, T.; Plovier, H.; Marrachelli, V.G.; van Hul, M.; Essaghir, A.; Ståhlman, M.; Matamoros, S.; Geurts, L.; Pardo-Tendero, M.M.; Druart, C.; et al. Hepatocyte MyD88 Affects Bile Acids, Gut Microbiota and Metabolome Contributing to Regulate Glucose and Lipid Metabolism. Gut 2017, 66, 620–632.
- Lorenzo-Zúñiga, V.; Bartolí, R.; Planas, R.; Hofmann, A.F.; Viñado, B.; Hagey, L.R.; Hernández, J.M.; Mañé, J.; Alvarez, M.A.; Ausina, V.; et al. Oral Bile Acids Reduce Bacterial Overgrowth, Bacterial Translocation, and Endotoxemia in Cirrhotic Rats. Hepatology 2003, 37, 551–557.
- Bertók, L. Bile Acids in Physico-Chemical Host Defence. Pathophysiology 2004, 11, 139–145.
- di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public Health 2021, 18, 12836.
- Tilg, H.; Cani, P.D.; Mayer, E.A. Gut Microbiome and Liver Diseases. Gut 2016, 65, 2035–2044.
- Lee, N.; Kim, W.U. Microbiota in T-Cell Homeostasis and Inflammatory Diseases. Exp. Mol. Med. 2017, 49, e340.
- Ponziani, F.R.; Zocco, M.A.; Cerrito, L.; Gasbarrini, A.; Pompili, M. Bacterial Translocation in Patients with Liver Cirrhosis: Physiology, Clinical Consequences, and Practical Implications. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 641–656.
- Duffield, J.S.; Forbes, S.J.; Constandinou, C.M.; Clay, S.; Partolina, M.; Vuthoori, S.; Wu, S.; Lang, R.; Iredale, J.P. Selective Depletion of Macrophages Reveals Distinct, Opposing Roles during Liver Injury and Repair. J. Clin. Investig. 2005, 115, 56–65.
- Seki, E.; de Minicis, S.; Österreicher, C.H.; Kluwe, J.; Osawa, Y.; Brenner, D.A.; Schwabe, R.F. TLR4 Enhances TGF-β Signaling and Hepatic Fibrosis. Nat. Med. 2007, 13, 1324–1332.
- Brandl, K.; Kumar, V.; Eckmann, L. MINI-REVIEW Microbiome and Host Interactions Gut-Liver Axis at the Frontier of Host-Microbial Interactions. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312.
- Fox, E.S.; Thomas, P.; Broitman, S.A. Clearance of Gut-Derived Endotoxins by the Liver. Release and Modification of 3H, 14C-Lipopolysaccharide by Isolated Rat Kupffer Cells. Gastroenterology 1989, 96, 456–461.
- Seki, E.; Schnabl, B. Role of Innate Immunity and the Microbiota in Liver Fibrosis: Crosstalk between the Liver and Gut. J. Physiol. 2012, 590, 447–458.
- Norman, K.; Pirlich, M. Gastrointestinal Tract in Liver Disease: Which Organ Is Sick? Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 613–619.
- Tranah, T.H.; Edwards, L.A.; Schnabl, B.; Shawcross, D.L. Targeting the Gut-Liver-Immune Axis to Treat Cirrhosis. Gut 2021, 70, 982–994.
- Trebicka, J.; Macnaughtan, J.; Schnabl, B.; Shawcross, D.L.; Bajaj, J.S. The Microbiota in Cirrhosis and Its Role in Hepatic Decompensation. J. Hepatol. 2021, 75, S67–S81.
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the Human Gut Microbiome in Liver Cirrhosis. Nature 2014, 513, 59–64.
- Maslennikov, R.; Ivashkin, V.; Efremova, I.; Poluektova, E.; Shirokova, E.; Maslennikov, R.; Poluektova, E.; Maslennikov, R. Gut-Liver Axis in Cirrhosis: Are Hemodynamic Changes a Missing Link? World J. Clin. Cases 2021, 9, 9320–9332.
- Henriksen, J.H.; Møller, S.; Ring-Larsen, H.; Christensen, N.J. The Sympathetic Nervous System in Liver Disease. J. Hepatol. 1998, 29, 328–341.
- Lin, R.S.; Lee, F.Y.; Lee, S.D.; Tsai, Y.T.; Lin, H.C.; Rei-Hwa, L.; Wan-Ching, H.; Cheng-Chun, H.; Sun-Sang, W.; Kwang-Juei, L. Endotoxemia in Patients with Chronic Liver Diseases: Relationship to Severity of Liver Diseases, Presence of Esophaegeal Varices, and Hyperdynamic Circulation. J. Hepatol. 1995, 22, 165–172.
- Bellot, P.; García-Pagán, J.C.; Francés, R.; Abraldes, J.G.; Navasa, M.; Pérez-Mateo, M.; Such, J.; Bosch, J. Bacterial DNA Translocation Is Associated with Systemic Circulatory Abnormalities and Intrahepatic Endothelial Dysfunction in Patients with Cirrhosis. Hepatology 2010, 52, 2044–2052.
- Garcia-Tsao, G.; Lee, F.Y.; Barden, G.E.; Cartun, R.; Brian West, A. Bacterial Translocation to Mesenteric Lymph Nodes Is Increased in Cirrhotic Rats with Ascites. Gastroenterology 1995, 108, 1835–1841.
- Cirera, I.; Martin Bauer, T.; Miguel, N.; Vila, J.; Grande, L.; Taurá, P.; Fuster, J.; García-Valdecasas, J.C.; Lacy, A.; María Jesús, S.; et al. Bacterial Translocation of Enteric Organisms in Patients with Cirrhosis. J. Hepatol. 2001, 34, 32–37.
- Nicoletti, A.; Ponziani, F.R.; Biolato, M.; Valenza, V.; Marrone, G.; Sganga, G.; Gasbarrini, A.; Miele, L.; Grieco, A. Intestinal Permeability in the Pathogenesis of Liver Damage: From Non-Alcoholic Fatty Liver Disease to Liver Transplantation. World J. Gastroenterol. 2019, 25, 4814–4834.
- Mcdermott, A.J.; Huffnagle, G.B. The Microbiome and Regulation of Mucosal Immunity. Immunology 2014, 142, 24–31.
- Marrone, G.; Shah, V.H.; Gracia-Sancho, J. Sinusoidal Communication in Liver Fibrosis and Regeneration. J. Hepatol. 2016, 65, 608–617.
- Tsuchida, T.; Friedman, S.L. Mechanisms of Hepatic Stellate Cell Activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411.
- Mehta, G.; Gustot, T.; Mookerjee, R.P.; Garcia-Pagan, J.C.; Fallon, M.B.; Shah, V.H.; Moreau, R.; Jalan, R. Inflammation and Portal Hypertension—The Undiscovered Country. J. Hepatol. 2014, 61, 155–163.
- Baffy, G. Origins of Portal Hypertension in Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2018, 63, 563–576.
- Puoti, C.; Bellis, L. Steatosis and Portal Hypertension. Eur. Rev. Med. Pharmacol. Sci. 2005, 9, 285–290.
- Zhang, W.; Wu, Y.; Mu, D.; Gong, J.; Wu, C.; Huang, C. Kupffer Cells: Increasingly Significant Role in Nonalcoholic Fatty Liver Disease. Ann. Hepatol. 2014, 13, 489–495.
- Heymann, F.; Tacke, F. Immunology in the Liver-from Homeostasis to Disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 88–110.
- Bosch, J.; García-Pagán, J.C. Complications of Cirrhosis. I. Portal Hypertension. J. Hepatol. 2000, 32 (Suppl. 1), 141–156.
- Iwakiri, Y.; Shah, V.; Rockey, D.C. Vascular Pathobiology in Chronic Liver Disease and Cirrhosis—Current Status and Future Directions. J. Hepatol. 2014, 61, 912–924.
- Wiest, R.; Groszmann, R.J. The Paradox of Nitric Oxide in Cirrhosis and Portal Hypertension: Too Much, Not Enough. Hepatology 2002, 35, 478–791.
- Iwakiri, Y.; Groszmann, R.J. Vascular Endothelial Dysfunction in Cirrhosis. J. Hepatol. 2007, 46, 927–934.
- Francés, R.; Muñoz, C.; Zapater, P.; Uceda, F.; Gascón, I.; Pascual, S.; Pérez-Mateo, M.; Such, J. Bacterial DNA Activates Cell Mediated Immune Response and Nitric Oxide Overproduction in Peritoneal Macrophages from Patients with Cirrhosis and Ascites. Gut 2004, 53, 860–864.
- Paratore, M.; Santopaolo, F.; Cammarota, G.; Pompili, M.; Gasbarrini, A.; Ponziani, F.R. Fecal Microbiota Transplantation in Patients with Hbv Infection or Other Chronic Liver Diseases: Update on Current Knowledge and Future Perspectives. J. Clin. Med. 2021, 10, 2605.
- Gedgaudas, R.; Bajaj, J.S.; Skieceviciene, J.; Varkalaite, G.; Jurkeviciute, G.; Gelman, S.; Valantiene, I.; Zykus, R.; Pranculis, A.; Bang, C.; et al. Circulating Microbiome in Patients with Portal Hypertension. Gut Microbes 2022, 14, 2029674.
- Virseda-Berdices, A.; Brochado-Kith, O.; Díez, C.; Hontañon, V.; Berenguer, J.; González-García, J.; Rojo, D.; Fernández-Rodríguez, A.; Ibañez-Samaniego, L.; Llop-Herrera, E.; et al. Blood Microbiome Is Associated with Changes in Portal Hypertension after Successful Direct-Acting Antiviral Therapy in Patients with HCV-Related Cirrhosis. J. Antimicrob. Chemother. 2022, 77, 719–726.
- Yokoyama, K.; Tsuchiya, N.; Yamauchi, R.; Miyayama, T.; Uchida, Y.; Shibata, K.; Fukuda, H.; Umeda, K.; Takata, K.; Tanaka, T.; et al. Exploratory Research on the Relationship between Human Gut Microbiota and Portal Hypertension. Intern. Med. 2020, 59, 2089–2094.
- Moratalla, A.; Gómez-Hurtado, I.; Moya-Pérez, Á.; Zapater, P.; Peiró, G.; González-Navajas, J.M.; Gómez Del Pulgar, E.M.; Such, J.; Sanz, Y.; Francés, R. Bifidobacterium Pseudocatenulatum CECT7765 Promotes a TLR2-Dependent Anti-Inflammatory Response in Intestinal Lymphocytes from Mice with Cirrhosis. Eur. J. Nutr. 2016, 55, 197–206.
- García-Lezana, T.; Raurell, I.; Bravo, M.; Torres-Arauz, M.; Salcedo, M.T.; Santiago, A.; Schoenenberger, A.; Manichanh, C.; Genescà, J.; Martell, M.; et al. Restoration of a Healthy Intestinal Microbiota Normalizes Portal Hypertension in a Rat Model of Nonalcoholic Steatohepatitis. Hepatology 2018, 67, 1485–1498.
- Verbeke, L.; Farre, R.; Trebicka, J.; Komuta, M.; Roskams, T.; Klein, S.; Elst, I.V.; Windmolders, P.; Vanuytsel, T.; Nevens, F.; et al. Obeticholic Acid, a Farnesoid X Receptor Agonist, Improves Portal Hypertension by Two Distinct Pathways in Cirrhotic Rats. Hepatology 2014, 59, 2286–2298.
- Fernandez, M.; Vizzutti, F.; Garcia-Pagan, J.C.; Rodes, J.; Bosch, J. Anti-VEGF Receptor-2 Monoclonal Antibody Prevents Portal-Systemic Collateral Vessel Formation in Portal Hypertensive Mice. Gastroenterology 2004, 126, 886–894.
- Fernández, M.; Semela, D.; Bruix, J.; Colle, I.; Pinzani, M.; Bosch, J. Angiogenesis in Liver Disease. J. Hepatol. 2009, 50, 604–620.
- Moghadamrad, S.; Mccoy, K.D.; Geuking, M.B.; Sägesser, H.; Kirundi, J.; Macpherson, A.J.; de Gottardi, A. Attenuated Portal Hypertension in Germ-Free Mice: Function of Bacterial Flora on the Development of Mesenteric Lymphatic and Blood Vessels. Hepatology 2015, 61, 1685–1695.
- Wang, R. Physiological Implications of Hydrogen Sulfide: A Whiff Exploration That Blossomed. Physiol. Rev. 2012, 92, 791–896.
- Distrutti, E.; Mencarelli, A.; Santucci, L.; Renga, B.; Orlandi, S.; Donini, A.; Shah, V.; Fiorucci, S. The Methionine Connection: Homocysteine and Hydrogen Sulfide Exert Opposite Effects on Hepatic Microcirculation in Rats. Hepatology 2008, 47, 659–667.
- Fiorucci, S.; Antonelli, E.; Mencarelli, A.; Orlandi, S.; Renga, B.; Rizzo, G.; Distrutti, E.; Shah, V.; Morelli, A. The Third Gas: H2S Regulates Perfusion Pressure in Both the Isolated and Perfused Normal Rat Liver and in Cirrhosis. Hepatology 2005, 42, 539–548.
- Singh, S.B.; Lin, H.C. Hydrogen Sulfide in Physiology and Diseases of the Digestive Tract. Microorganisms 2015, 3, 866–889.
- Chen, Y.; Qin, N.; Guo, J.; Qian, G.; Fang, D.; Shi, D.; Xu, M.; Yang, F.; He, Z.; van Nostrand, J.D.; et al. Functional Gene Arrays-Based Analysis of Fecal Microbiomes in Patients with Liver Cirrhosis. BMC Genom. 2014, 15, 1–13.
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The Severity of Nonalcoholic Fatty Liver Disease Is Associated with Gut Dysbiosis and Shift in the Metabolic Function of the Gut Microbiota. Hepatology 2016, 63, 764–775.
- Peleman, C.; Camilleri, M. Rifaximin, Microbiota Biology, and Hepatic Encephalopathy. Clin. Transl. Gastroenterol. 2016, 7, e195.
- Koh, A.; de Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345.
- Mitten, E.K.; Baffy, G. Microbiota Transplantation in Portal Hypertension: Promises and Pitfalls. Clin. Sci. 2022, 136, 425–429.
- Kakiyama, G.; Pandak, W.M.; Gillevet, P.M.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; Takei, H.; Muto, A.; Nittono, H.; Ridlon, J.M.; et al. Modulation of the Fecal Bile Acid Profile by Gut Microbiota in Cirrhosis. J. Hepatol. 2013, 58, 949–955.
- Hang, S.; Paik, D.; Yao, L.; Kim, E.; Trinath, J.; Lu, J.; Ha, S.; Nelson, B.N.; Kelly, S.P.; Wu, L.; et al. Author Correction: Bile Acid Metabolites Control TH17 and Treg Cell Differentiation (Nature, (2019), 576, 7785, (143-148), 10.1038/S41586-019-1785-z). Nature 2020, 576, 143–148.
- Giannelli, V.; di Gregorio, V.; Iebba, V.; Giusto, M.; Schippa, S.; Merli, M.; Thalheimer, U. Microbiota and the Gut-Liver Axis: Bacterial Translocation, Inflammation and Infection in Cirrhosis. World J. Gastroenterol. 2014, 20, 16795–16810.
- Schubert, K.; Olde Damink, S.W.M.; von Bergen, M.; Schaap, F.G. Interactions between Bile Salts, Gut Microbiota, and Hepatic Innate Immunity. Immunol. Rev. 2017, 279, 23–35.
- Kakiyama, G.; Hylemon, P.B.; Zhou, H.; Pandak, W.M.; Heuman, D.M.; Kang, D.J.; Takei, H.; Nittono, H.; Ridlon, J.M.; Fuchs, M.; et al. Colonic Inflammation and Secondary Bile Acids in Alcoholic Cirrhosis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014, 306, G929–G937.
- Lazzaro, B.P.; Zasloff, M.; Rolff, J. Antimicrobial peptides: Application informed by evolution. Science 2020, 368, eaau5480.
- Hancock, R.E.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334.
- Zong, X.; Fu, J.; Xu, B.; Wang, Y.; Jin, M. Interplay between gut microbiota and antimicrobial peptides. Anim. Nutr. 2020, 6, 389–396.
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105.
- Yang, S.C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 241.
- Abrudan, M.I.; Smakman, F.; Grimbergen, A.J.; Westhoff, S.; Miller, E.L.; van Wezel, G.P.; Rozen, D.E. Socially mediated induction and suppression of antibiosis during bacterial coexistence. Proc. Natl. Acad. Sci. USA 2015, 112, 11054–11059.
- Wang, L.; Fouts, D.E.; Stärkel, P.; Hartmann, P.; Chen, P.; Llorente, C.; DePew, J.; Moncera, K.; Ho, S.B.; Brenner, D.A.; et al. Intestinal REG3 Lectins Protect against Alcoholic Steatohepatitis by Reducing Mucosa-Associated Microbiota and Preventing Bacterial Translocation. Cell Host Microbe. 2016, 19, 227–239.
- Teltschik, Z.; Wiest, R.; Beisner, J.; Nuding, S.; Hofmann, C.; Schoelmerich, J.; Bevins, C.L.; Stange, E.F.; Wehkamp, J. Intestinal bacterial translocation in rats with cirrhosis is related to compromised Paneth cell antimicrobial host defense. Hepatology 2012, 55, 1154–1163.
- Chen, Y.; Ji, F.; Guo, J.; Shi, D.; Fang, D.; Li, L. Dysbiosis of Small Intestinal Microbiota in Liver Cirrhosis and Its Association with Etiology. Sci. Rep. 2016, 6, 34055.
- Ponziani, F.R.; Bhoori, S.; Castelli, C.; Putignani, L.; Rivoltini, L.; del Chierico, F.; Sanguinetti, M.; Morelli, D.; Paroni Sterbini, F.; Petito, V.; et al. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 107–120.
