Magnesium alloys are widely employed in various applications due to their high strength-to-weight ratio and superior mechanical properties as compared to unalloyed Magnesium. Alloying is considered an important way to enhance the strength of the metal matrix composite but it significantly influences the damping property of pure magnesium, while controlling the rate of corrosion for Mg-based material remains critical in the biological environment. Therefore, it is essential to reinforce the magnesium alloy with a suitable alloying element that improves the mechanical characteristics and resistance to corrosion of Mg-based material. Biocompatibility, biodegradability, lower stress shielding effect, bio-activeness, and non-toxicity are the important parameters for biomedical applications other than mechanical and corrosion properties. The development of various surface modifications is also considered a suitable approach to control the degradation rate of Mg-based materials, making lightweight Mg-based materials highly suitable for biomedical implants.
- alloying elements
- corrosion properties
- surface modifications
- biocompatibility
- EW42
1. Introduction
| S.NO | Types of Surface Modification Approach |
Modification Technique | Description | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Chemical Modification | Acid Etching | 1. Removing the initial oxide layer and adding homogenous, and compact layers afterward that slowed the rate of degradation. 2. The impact of acid etching as a pretreatment on the rate of degradation of the magnesium alloy AZ31 in SBF solutions analyzed by Munro et al. 3. In comparison to unetched alloys, homogenous and dense films of Mg | 3 | (PO | 4 | ) | 2 | layers were created on etched magnesium alloys, which significantly reduced the rate of degradation. | [11] |
| 2 | Chemical Modification | Alkali Treatment |
1. After being submerged in an alkaline solution, a new passive layer made up of MgCO | 3 | , Mg(OH) | 2 | , and MgO is deposited on the surface of magnesium alloys that improving the corrosion resistance. 2. Gu et al. analyzed the reduction in the degradation rate of Mg-based materials in SBF. |
[12] | ||
| 3 | Chemical Modification | Fluoride Treatment |
| S.NO | Material | Benefits | Drawbacks | Applications |
|---|
| S.NO | Material Property | Human Bone | Mg Alloys | Ti Alloys | Co-Cr Alloys | Stainless Steels | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Co-Cr alloy | 1. Provides good resistance to fatigue, wear, and corrosion. 2. Provides high strength. 3. Long-span biocompatibility. |
1. Very expensive. | ||||||||||
| 1 | Density (g/cm | 3 | ) | 1.8–2.1 | 2. Machining is difficult. |
1. Bone implantation (wires and plates). 2. Hip replacement. |
|||||||
| 1.74–2.00 | 4.4–4.5 | 8.3–9.2 | 7.9–8.1 | 2 | Ti alloy | 1. Enables good corrosion resistance and has low young’s modulus with suitable biocompatibility. 2. High strength than 316L stainless steel. |
1. High wear rate. 2. Very expensive. |
1. Fracture fixation. 2. Total joint replacement. |
|||||
| 2 | Yield Strength (MPa) | 30–114.3 | 20–200 | 896–1034 | 448–1606 | 221–1213 | 1. The most effective and practical method for surface modification of magnesium alloys at the moment is fluorination. | 3 | 316L Stainless steel |
1. Easily available, high toughness, suitable biocompatibility, inexpensive, 2. In order to create samples of HF-coated magnesium alloys, Li et al. created screws and tensile specimens using magnesium alloys as substrates. Even after a month of immersion, MgF | 2 | -coated samples maintained good mechanical characteristics due to a reduced rate of corrosion than bare samples. | [13] |
| and good fabrication characteristics. | 1. High corrosion, and wear rate, high-stress shielding effect. | 1. Bone implantation. | |||||||||||
| 3 | Compressive Strength (MPa) | 164–240 | 55–130 | N/A | N/A | N/A | 4 | Physical Modification | Sol-gel | 4 |
Mg-alloyCoating | 1. Biocompatible, bioresorbable, biodegradable, and bio-active. 2. Elastic modulus resembling human bone. 3. Low-stress shielding effect and lightweight.1. For a variety of metal substrates, including aluminum, steel, copper, magnesium, and their alloys, sol–gel coatings have been shown to have great chemical stability, excellent oxidation control, and increased corrosion resistance. 2. To reduce the degree of degradation of magnesium alloys, Gaur et al. created a sol–gel silane coating using a combination of diethyl phosphate ethyl tri-ethoxy silane (DEPETES) and bis- [3- (tri-ethoxy silyl) propyl] tetra-sulfide (BTESPT). 3. The samples with the sol–gel coating showed the greatest improvement in the rate of degradation. |
[14] |
| 1. High H | 2 | evolution during degradation | 1. Bone implantation, screws, and plates. |
| 4 | ||||||||||||
| Young’s Modulus (GPa) | ||||||||||||
| 3–23 | 41–45 | 110–117 | 210–232 | 189–205 | ||||||||
| 5 | Physical Modification | Organosilane coatings | 1. For orthopedic purposes, it acts as a protective and biocompatible coating on Mg alloys. 2. The hydrophobic Si-O-Si networks of organosilanes, minor galvanic reactions with Mg, increased adhesive strength, ease of chemical modification, and low cytotoxicity are only a few benefits of employing organosilanes as protective barrier coatings. |
[14] | ||||||||
| 5 | Fracture toughness (MPa/m | 1/2 | ) | 3–7 | 15–40 | 55–115 | 50–200 | NA | 6 | Physical Modification | Calcium Phosphate Coating |
1. Different procedures have been used to deposit different forms of calcium phosphate coatings, such as hydroxyapatite (HA), fluorinated hydroxyapatite (FHA), and brushite on Mg substrates. 2. One of the most effective methods discovered for covering the surface of orthopedic implants is calcium phosphate deposition. |
| 6 | Tensile Strength (MPa) | 70–150 | 3. The corrosion resistance of Mg alloys covered with Ca-P was greatly increased compared to untreated magnesium alloys, according to Cao et al. who created a Ca-P coating on AZ31 alloys in SBF. Additionally, there were no harmful effects of the Ca-P coating on cells. | [15] | ||||||||
| 7 | Physical Modification | Superhydrophobic Coating | 1. One of the most current techniques to increase the corrosion resistance of Mg alloys is a superhydrophobic surface. 2. A superhydrophobic surface with a 157.6° contact angle with water was created by Zhang et al. using AZ31 magnesium alloy. The superhydrophobic coatings considerably increased the corrosion resistance of the AZ31 alloy, according to the corrosion results. 3. Superhydrophobic surfaces inhibit the contact between cells and implants, which limits the implant’s capacity to stimulate bone repair and slows the rapid deterioration of magnesium alloys. |
[15] |
| 86–280 | ||||||
| 760–1140 | ||||||
| 655–1896 | ||||||
| 586–1351 | ||||||
| 7 | Elongation (%) | 1.07–2.10 | 12–21 | 12 | N/A | N/A |
2. Classification of Mg Alloy and Influence of Alloying Element on Various Mg Alloys
The alloying element such as aluminum, titanium, and magnesium are widely used in technologies and are the most suitable substitute for steel employed in structural applications [3]. Mg alloys have the lightest structure of all the non-ferrous element metals [5]. Therefore, one research study suggested that the usage of Mg-based alloys has increased in biomedical and structural applications [6]. During the formation of Mg-based alloys in biomedical applications, biocompatibility is the most important factor that governed the effectiveness of bone-implant and tissue regeneration without any toxic effects on living tissues [28][79]. The Mg-based alloys can be classified as wrought and cast magnesium alloys based on the processing approaches required for engineering applications and research and development perspectives [29][80]. The Mg alloys obtained via die and various casting approaches are referred to as-cast alloys [30][81]. The research study suggested that cast Mg alloys can be employed in limited areas where reasonable mechanical properties are required and are commonly used in industrial and commercial applications [6]. The wrought Mg alloys are obtained via secondary manufacturing processes such as Rolling, Equal-Channel Angular Pressing (ECAP), Extrusion, and Severe Plastic Deformation (SPD), while SPD is the most suitable approach for a reduction in grain size in microstructure characterization [31][82]. The researcher’s approach suggested that the wrought Mg alloys had remarkable mechanical properties as compared to cast Mg alloys [30][81]. The wrought alloys enable the homogenous composition of alloy along with grain refinement having no pores on the surface of the alloy [29][80]. The classification of various magnesium alloys was illustrated in Figure 1. The Mg alloy can be binary, ternary, and quaternary subject to the presence of several phases and alloying elements in the alloy [32][83].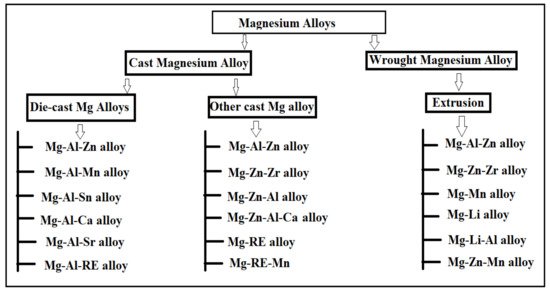
2.1. Mg-Al Based Alloys
When aluminum is dissolved in Mg, the formation of the α-Mg phase and ϒ-Mg17Al12 phases (eutectic ternary phases) occur which enhances the solid-solution strength [33][84]. Mg-Al alloy is the most predominantly used Mg alloy due to its high specific stiffness, high specific strength, good electromagnetic shielding, good vibration properties, good anti-radiation properties, and other characteristics. Thus, Mg-Al alloys, also referred to as the “green engineering materials of the twenty-first century,” have a wide range of potential applications in automobiles, electrical equipment, electronics, transportation, aviators, and biomedical application fields [33][84]. Mg-Al alloys are vitally used in bone implantation with good tissue healing and low-stress shielding effect on the implant. The literature suggests that the mechanical properties (compressive and tensile strength) of Mg-Al binary alloys with Al content that exceeded the solubility limit significantly increased. Furthermore, the addition of more than 6 wt.% of Ca in the Mg-Al alloy significantly improves the tensile and compressive strength of the Mg-Al alloy. The Mg–Al-based alloys have superior castability with average mechanical properties [34][85]. The most commonly used alloying element in Mg-Al alloys is zinc and manganese with a concentration of around 1 wt.% [33][84]. The Mg-Al-based alloys usually have AM, AE, and AZ series which are biodegradable in nature that involves AM60, AZ31, AZ81, AZ61, and AZ91 alloys [34][85]. The AZ31 alloy indicates A as aluminum and Z as zinc and number 3 and 1 indicate 3% of Aluminum and 1% of zinc, respectively [28][79]. The AM series comprises various alloys involving Mg inclusive of aluminum and manganese such as AM30, AM40, AM50, and AM60, etc. [35][86]. The AM-based alloy has higher mechanical properties and suitable extrudability in comparison with AZ alloys having an absence of eutectic ternary phases containing zinc [33][84]. The Mg-based alloys involve the addition of zinc to provide strength to solute particles, but Manganese is added to improve the anti-corrosion properties of the alloy by eliminating the patches of iron from the alloy [36][87]. The Mg-Al-based alloys have negligible age-hardening due to the formation of the β-Mg17Al12 phase [33][84]. About 70% of the grains identified in microstructures are recrystallized to an average grain size of 1.2 μm [34][35][36][37][85,86,87,88]. The various Mg-Al alloys along with the effect of the alloying element are illustrated in the below section.2.1.1. AZ Alloy Series
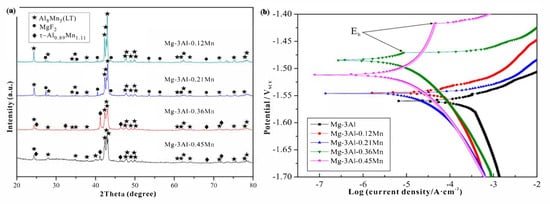
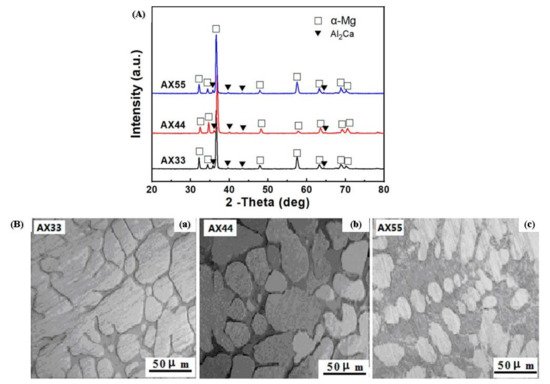
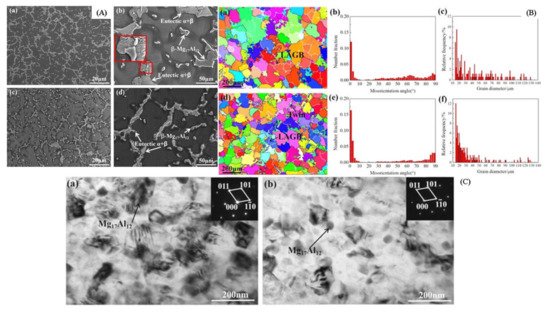
The addition of alloying element lithium in AZ31 alloy has optimized the microstructure of the alloy, as lithium enables the formation of a cross and non-basal slip [38][89]. Lithium is highly soluble in AZ31 alloy, allowing the alteration in lattice parameters of a solid solution of magnesium [38][89]. For an increase in the amount of lithium in AZ31 alloy, the random distribution of grains around the boundaries and a large reduction in basal structure intensity are obtained [39][90]. Additionally, lithium also promotes an enhancement in ductility and reduction in anisotropy of AZ31 alloy due to the recrystallization of grains around the boundary [38][89]. The tensile strength of the alloy remains the same with the addition of lithium [39][90]. The research study by Meng et al. suggested that a high value of elongation and tensile strength were found in Mg-1Al-1Zn-8Li, these being 9.2% and 233.38 MPa, respectively [40][41][91,92]. The addition of tin in Mg-Al alloy enhanced the compressive and tensile properties of Mg-Al-based alloy composite due to a reduction in the stacking energy in the alloy [42][93]. Wu et al. suggested that the grain size in AZ91 is refined via the addition of tin in hot extrusion due to the restriction in the formation of Mg2Sn precipitates around the grain boundary [43][94]. The rare earth elements in Mg-Al alloys allow the formation of thermally stable phase (Mg, Al)xREy as a result of that the enhancement in the mechanical properties of wrought Mg alloys that occurs [43][94]. The microstructure of the alloy AE44 (Mg-4.0Al-4.1RE-0.3Mn, wt.%) produced here mostly consists of α-Mg grains and Al11RE3 intermetallic phase at grain boundaries. Additionally, TEM images depict a lamellar phase with primarily Al11Ce3 indicated in Figure 2 [44][95]. The TEM also found trace phases. Both the lamellar phase and the feather-like phase contain La, Ce, Nd, and Al with an Al/RE ratio near 11:3, according to the results of the EDX micro-analysis, and the XRD pattern further demonstrates that the inter-metallics are a part of the Al11RE3 phase (Al11Ce3) [44][95]. Pan et al. analyzed the effects of yttrium on the microstructure and mechanical properties of AZ31 alloy [45][68]. At 0.5 wt.% of yttrium in Mg-Al alloy, high yield strength of around 209 MPa was observed due to the formation of the Al2Y phase [46][71]. At 0.9 wt.% of yttrium in AZ31, the optimal mechanical properties were obtained, i.e., YS (yield strength), UTS (ultimate tensile strength), and elongation (%) are 258.9 MPa, 371.9 MPa, and 7.33% due to refinement in grain size of the Al2Y phase [46][71]. The coarse and brittle Al2Y phase was obtained at 1.4 wt.% of yttrium in AZ31 which causes a reduction in the tensile strength of the alloy [46][71].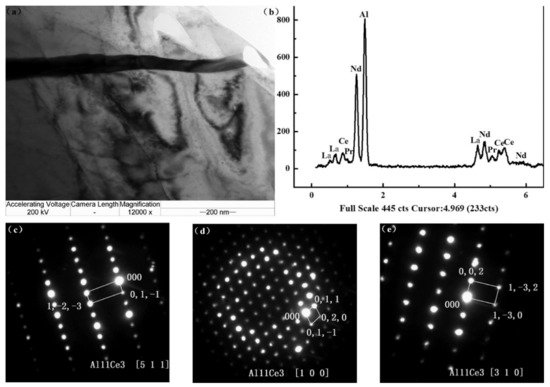
2.2. Mg-Zn Based Alloys
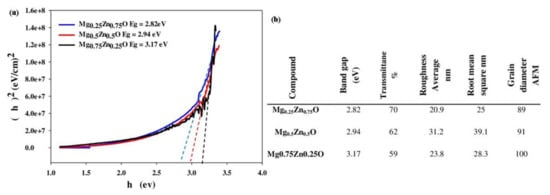
2.2.1. Mg-Zn-Ca Alloys