Hypopigmented mycosis fungoides (HMF) is a variant of cutaneous T-cell lymphoma (CTCL), a heterogeneous group of extranodal non-Hodgkin’s lymphomas. HMF and classic (erythematous patch/plaque) Mycosis Fungoides (MF) display contrasting clinical characteristics: (i) HMF presents with light colored to achromic patches, as opposed to classic MF, which presents with erythematous scaly patches, plaques, tumors or erythroderma, (ii) HMF primarily affects individuals with darker skin types (Fitzpatrick phototypes IV-VI), while classic MF affects mostly Caucasians, (iii) HMF is commonly seen in pediatric/adolescents and young adults, whereas classic MF is more prevalent in elderly individuals, and (iv) the predominant malignant cells in HMF are CD8+T-cells, as opposed to CD4+T-cells in classic MF. Our recent review paper highlights that active antitumor immune response, specifically a Th1/cytotoxic antitumor immune response seen robustly in HMF, is likely responsible for the differential behavior between these two MF variants. Furthermore, we propose that the hypopigmentation (clinical sign) may serve as a surrogate marker for the presence of antitumor immune response and may portend better prognosis.
- mycosis fungoides
- cutaneous T-cell lymphomas
- hypopigmentation
- hypopigmented mycosis fungoides
- Th1
- antitumor immune response
- cytotoxic cells
- immunosurveillance
- immunoediting
1. Hypopigmented Mycosis Fungoides
Mycosis fungoides(MF) is a form of cutaneous T-cell lymphoma (CTCL), a heterogeneous group of extranodal non-Hodgkin’s lymphomas characterized by the expansion of monoclonal T-cells involving the skin [1][2][3]. MF can present with several variants including the classic MF (also known as the conventional Alibert-Bazin), poikilodermatous/poikiloderma vasculare atrophicans, granulomatous slack skin, hypopigmented MF (HMF) [4][5] among others. Each variant has its own set of clinical and pathologic characteristics. In the current summary we focus on Hypopigmented Mycosis Fungoides (HMF).
1.1 Clinical presentation of Hypopigmented Mycosis Fungoides
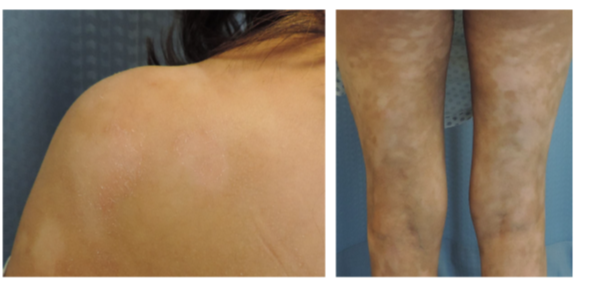
The main clinical defining feature of HMF are the light colored to achromic patches seen in patients (Figure 1) [6][7], however a few tumors cases have been reported [8][9]. Current evidence suggests that the hypopigmentation is caused by a damaged and reduced number of melanocytes in addition to an abnormal melanogenesis [6][10].
Figure 1. Clinical images of hypopigmented mycosis fungoides (HMF) patient, where the light colored patches can be observed. Figure adapted from [11].
Patients commonly present with lesions on the trunk, buttocks, and extremities in, what is called, a “bathing-suit” distribution pattern [5] [12]. Although not common, facial involvement has been reported [7]. Lesions range from a single patch [13] to generalized lesions covering a large body surface area (BSA). Patients may experience pruritus. However, systemic symptoms such as weight loss, fever or night sweats are uncommon [6][7][14].
1.2 Demographic characteristics of Hypopigmented Mycosis Fungoides
HMF has an earlier mean age of onset than classic MF. While classic MF commonly appears in patients > 55 years of age, HMF is typically reported at younger age, with patients in pediatric, adolescent and early adulthood populations [6]. The female to male ratio most commonly reported is approximately 1:1 [15]. HMF has a higher prevalence in Fitzpatrick IV-VI skin phototypes (i.e., populations with darker skin, including but not limited to African-American, South Asian, Middle Eastern, and Hispanic individuals) [7][16]. However, cases in fair skin patients, particularly Caucasian populations, are not uncommon [17].
1.3 Prognosis and Treatment of Hypopigmented Mycosis Fungoides
Given that HMF is a variant of MF, stratification and staging is the same for both and prognosis is closely associated with clinical disease stage. Stratification and staging are determined according to BSA involved and extracutaneous involvement [1]. MF cases diagnosed at early stages (IA-IIA) have an indolent course and slow progression [16], and cases diagnosed at advanced stages (≥IIB) often have reduced life expectancy of 3.2 to 9.9 years [2]. HMF is typically recognized as a variant with an excellent prognosis. Most individuals present at IA stage (i.e., <10% BSA involvement) to IB (≥10% BSA) stages and progression beyond IB clinical stage is rare. However, HMF recurrence is often reported and may occur months or even years after a complete remission [6].
The majority of HMF cases are treated with skin-directed therapies. In general, the first line of treatment for this variant is phototherapy (e.g., narrowband ultraviolet B or in select cases, ultraviolet A1), photochemotherapy (e.g., psoralen and ultraviolet A (PUVA)), and topical products (e.g., topical steroids, retinoids, imiquimod, or nitrogen mustard). Use of narrowband ultraviolet B light, usually in a cabinet, (NUVB; 311 nm) suppresses malignant cell proliferation by increasing keratinocytes cytokine production and by inhibiting antigen-presenting cells. PUVA involves exposure to ultraviolet A (UVA) radiation (320–400 nm) after ingestion of 8-methoxypsoralen and induces DNA damage, reduction of Langerhan cell population, apoptosis of malignant cells and suppression of keratinocyte cytokine production [5][6].
The most common topical treatment products include steroids, nitrogen mustard, retinoids and imiquimod. Steroids decrease cytokine production and induce apoptosis. Topical nitrogen mustard induces DNA damage due to its alkylating properties. Retinoids, specifically bexarotene, bind to retinoid receptors (Retinoid X Receptor or RXR in the case of bexarotene) that regulate cellular differentiation and apoptosis. Imiquimod augments antitumor immune response by activating Toll-Like Receptor-7 (TLR7), which in turn induces local interferon-α (IFN-α) and interferon-β (IFN-β) production[6].
2. Immunopathogenesis of Alibert-Bazin and Hypopigmented Mycosis Fungoides
Currently, the pathogenesis of MF and HMF is incompletely understood, however external triggers have been identified. Staphylococcus aureus toxins have been proposed as an external trigger. Cell autonomous factors promoting carcinogenesis and cancer progression include but are not limited to activation/deregulation of JAK-STAT, NOTCH, MAPK, and other signaling pathways [3][18][19][20][21][22].It is believed that this malignancy arises in T-cells with a mature resident CD45RO+ phenotype [23].
Environmental or pathogen-driven damage to the skin, cellular injury, or stress triggers a pro-inflammatory response initiated by keratinocytes. Keratinocytes release cytokines that activate both, innate and adaptive immune system. The innate immune response is executed by immune cells, such as dendritic cells (DCs), mast cells, and macrophages, which have direct effects on pathogens and the activation of Antigen Presenting Cells (APCs) [23][24].
The specialized APCs in the epidermis are the Langerhans cells and their dermal counterparts are the dermal DCs, and once activated by the innate immune response cells, will migrate to the skin-draining lymph nodes. In the lymph nodes, APCs will encounter naive T-cells that can be activated [24]. Active T-cells are antigen-specific, express cutaneous lymphocyte antigen (CLA) as well as CC chemokine receptor 4 (CCR4), which induces a skin-targeted migration [25]. The skin-targeted migration is facilitated by the dermal vessels keratinocyte-induced expression of the adhesion molecules complementary to the CLA and CCR4 receptors, E-selectin and CC chemokine ligand 17 (CCL17), respectively. Specific receptor-ligand recognition allows active T-cells to tether and roll along the endothelium and extravasate into the dermis. Once in the dermis, active T-cells mediate the inflammatory response [24][25]. Under normal conditions, activated T-cells should be eventually eliminated; however, MF cancer T-cells continue to proliferate driven by activation/deregulation of JAK-STAT, NOTCH, MAPK, and other signaling pathways, stimulated by exposure to S. aureus enterotoxins, upregulation of oncogenic miRNAs, among others [3][18][19][20][21][22][26].
3. Hypopigmented Mycosis Fungoides presents an active antitumor immune response
3.1 Antitumor immune response in Mycosis Fungoides
The three phases of cancer immunoediting have been proposed in MF in part explaining its progression while detailing varying predominant cytokine profiles [11]. Briefly, during the elimination phase malignant T-cells remain occult/incognito and under the control of the immune system [27][28]. However, no specific antigens have been discovered in early stage MF patients [29]. The next phase, the equilibrium phase, is a period of latency characterized by a balance between surviving and dying cancer cells. This phase corresponds to stage IA-IB in HMF, and has been characterized by the presence of tumor infiltrating CD8+ T-cells and a Th1 cytokine profile in lesional skin [27][28]. Finally, the escape phase is enabled by the genomic instability of the cells and a Darwinian pressure by the immune system, enabling the malignant cells to resist/evade immunosurveillance [28]. This phase is characterized by a shift to a Th2 cytokine profile with the concomitant expression of pro-eosinophilic/immunosuppressive molecules and additional molecules such as Fas ligand. As a result, malignant cells proliferate in the skin and beyond, in lymph nodes, blood and visceral organs [27].
3.2 Hypopigmented Mycosis Fungoides remains in the equilibrium phase of cancer immunoediting
In the recently published review paper [11], we proposed that HMF remains in the equilibrium phase of cancer immunoediting. This is supported by research regarding cytokines and cytotoxic molecules secreted by both neoplastic and infiltrating cells and by a low infiltration number of regulatory T-cells (Tregs). As highlighted previously, the equilibrium phase in MF is characterized by a Th1 cytokine profile and among these cytokines, high levels of TNF-α expression have been reported at mRNA [30] and protein [31] level in HMF. Furthermore, blocking this cytokine promotes CTCL progression in patients [32].
Tumor Infiltrating Lymphocytes (TILs), particularly CD8+ cytotoxic T-cells, have a major role in cancer prognosis in general, and specifically in classic MF [33]. CD8+ cytotoxic T-cells produce a set of molecules that have an effect on tumor cells, and several of these molecules have been assessed in HMF. Specifically, it has been reported that TILs in HMF samples express: T-cell intracytoplasmic antigen 1 (TIA1) [34], a cytotoxic molecule constitutively expressed by CD8+ cytotoxic cells; granzyme B [31], a serine protease which induces apoptosis on its target cells; and granulysin [34], which is expressed by activated cytotoxic lymphocytes and NK cells. The expression of these cytotoxic molecules in HMF suggests an active antitumor immune response and indicates that this variant remains in the equilibrium phase of cancer immunoediting.
Another key player in antitumor immune response are the Tregs. Tregs inhibit natural or therapeutic immune response against tumors and can be identified by their immunophenotype CD4+ CD25+ FOXP3+ [27]. Lower number of infiltrating Tregs have been found in HMF, when compared to classic MF [34], suggesting an active antitumor response. The question remains how HMF patients maintain a controlled antitumor immune response.
3.3 Hypopigmented Mycosis Fungoides characteristics as a result of an active antitumor immune response
HMF has its own set of clinical characteristics. Current evidence suggests several of them can be partially understood within the scope of an active antitumor immune response. In the current publication, we have addressed three characteristics: 1. Hypopigmentation, 2. Earlier age of onset and 3. Overall favorable prognosis.
We have hypothesized that reactive CD8+ cytotoxic T-lymphocytes are causing damage and alteration of melanocyte function and differentiation, impacting two melanocyte pathways: the pathway activated by basic fibroblast growth factor (bFGF) and the pathway activated by the stem cell factor (SCF) (i.e., c-kit ligand). Under normal circumstances, both pathways result in melanocyte growth and survival [6]. When compared to MF, a decreased expression of molecules within these pathways have been reported in HMF. HMF has lower levels of expression of bFGF mRNA [30] and CD117, tyrosinase, MART-1/melan-A [6][35], gp100 [35], and MiTF [6] proteins, leading to hypopigmentation. Furthermore, an association between low levels of bFGF mRNA and increased TNF-α in stage I HMF patients has been stablished [30] indicating a likely impact of this Th1 cytokine on hypopigmentation. Indeed, skin hypopigmentation or in other cases, depigmentation, can be associated with inflammatory/autoimmune diseases such as Darier disease, vitiligo [36] and with immune-related adverse events in cancer patients receiving immunotherapy [37][38]. Notably, re-pigmentation in HMF patients after treatment is commonly reported [6], suggesting that the depletion of malignant T-cells allows the re-establishment of functional melanocyte activity.
Childhood/juvenile MF is not common and it ranges from 2.7% to 16.6% of all MF cases and HMF is commonly overrepresented in pediatric case series [39][40][41]. In general, elderly patients' immune system is declining due to immunosenescence. Among the signs of immunosenescence, a declining adaptive immune response and a decreased number of CD8+ cells make the elderly individuals susceptible to deleterious changes that may enable carcinogenesis [42].
It has been consistently reported that HMF has a better prognosis than classic MF [6]. Among all HMF cases reported, we have identified that the majority with an immunophenotype other than CD8+ presenting with early (≤IB) disease still demonstrated a favorable prognosis, where disease remained in early stages. This indolent nature of HMF is seen regardless of the cell immunophenotype of the infiltrating malignant T-cells. Thus, we conclude that the differential behavior of HMF is not associated with the cell immunophenotype of the malignant T-cells. Furthermore, mixed MF (hypopigmented lesions in addition to other types of lesions) also has a better prognosis when compared to classic MF. African-American and dark-skinned patients with hypopigmented lesions in general have a longer overall survival rate [43]. Therefore, hypopigmentation alone may be viewed as a favorable prognostic marker [44].
4. Conclusions
Different clinical features of conventional Alibert-Bazin MF vs. HMF can be explained by the presence of active antitumor immune response in younger individuals, who have not experienced immunosenescence. In particular, HMF in juvenile/adolescents and young adults remains in the equilibrium phase of cancer immunoediting. Several lines of evidence support this hypothesis: the immunopathogenesis of MF implies the activation of the cytotoxic immune response; the three phases of cancer immunoediting have been identified with specific cellular and cytokine profiles, where HMF was shown to have a higher expression of TNF-α the Th1 cytokines associated with the equilibirum phase. Moreover, HMF TILs express cytotoxic molecules such as TIA1, granzyme B, and granulysin and a lower Treg infiltration that together support the notion of an active antitumor immune response. Furthermore, we propose that hypopigmentation in HMF is a marker for an active antitumor immune response, and therefore a favorable prognostic indicator.
References
- Melissa Pulitzer; Cutaneous T-cell Lymphoma. Clinics in Laboratory Medicine 2017, 37, 527-546, 10.1016/j.cll.2017.06.006.
- Nooshin Bagherani; Bruce R. Smoller; An overview of cutaneous T cell lymphomas. F1000Research 2016, 5, 1882, 10.12688/f1000research.8829.1.
- Feras M. Ghazawi; Nebras AlGhazawi; Michelle Le; Elena Netchiporouk; Steven J. Glassman; Denis Sasseville; Ivan V. Litvinov; Environmental and Other Extrinsic Risk Factors Contributing to the Pathogenesis of Cutaneous T Cell Lymphoma (CTCL). Frontiers in Oncology 2019, 9, online, 10.3389/fonc.2019.00300.
- Michael S. Howard; Bruce R. Smoller; Mycosis fungoides: classic disease and variant presentations.. Seminars in Cutaneous Medicine and Surgery 2000, 19, 91-99, 10.1016/s1085-5629(00)80005-x.
- Sarah I. Jawed; Patricia L. Myskowski; Steven Horwitz; Alison Moskowitz; Christiane Querfeld; Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome). Journal of the American Academy of Dermatology 2014, 70, 223.e1-223.e17, 10.1016/j.jaad.2013.08.033.
- Fabricio Cecanho Furlan; José Antonio Sanches Jr; Hypopigmented mycosis fungoides: a review of its clinical features and pathophysiology. Anais Brasileiros de Dermatologia 2013, 88, 954-960, 10.1590/abd1806-4841.20132336.
- I.J. Rodney; C. Kindred; K. Angra; O.N. Qutub; A.R. Villanueva; Rebat M. Halder; Hypopigmented mycosis fungoides: a retrospective clinicohistopathologic study. Journal of the European Academy of Dermatology and Venereology 2016, 31, 808-814, 10.1111/jdv.13843.
- Ekaterina Castano; Sharon Glick; Lucia Wolgast; Rizwan Naeem; Jaya Sunkara; Dirk Elston; Mark Jacobson; Hypopigmented mycosis fungoides in childhood and adolescence: a long-term retrospective study. Journal of Cutaneous Pathology 2013, 40, 924-934, 10.1111/cup.12217.
- Brandon Rowe; Alina Shevchenko; G. Yosipovitch; Leser-Trélat Sign in Tumor-Stage Mycosis Fungoides.. Dermatology online journal 2016, 22, online.
- Fabricio C. Furlan; Bruna A. De Paula Pereira; Luiz F. Da Silva; José Antonio Sanches Jr; Loss of melanocytes in hypopigmented mycosis fungoides: a study of 18 patients. Journal of Cutaneous Pathology 2013, 41, 101-107, 10.1111/cup.12262.
- Amelia Martínez Villarreal; Jennifer Gantchev; François Lagacé; Augustin Barolet; Denis Sasseville; Niels Ødum; Yann Vincent Charli-Joseph; Amparo Hernández Salazar; Ivan V. Litvinov; Hypopigmented Mycosis Fungoides: Loss of Pigmentation Reflects Antitumor Immune Response in Young Patients. Cancers 2020, 12, 2007, 10.3390/cancers12082007.
- Mohammad A El-Darouti; Salonaz A Marzouk; Omar Azzam; Marwa Mohsen Fawzi; Mona R E Abdel-Halim; Amira A Zayed; Tahra M Leheta; Vitiligo vs. hypopigmented mycosis fungoides (histopathological and immunohistochemical study, univariate analysis).. European Journal of Dermatology 2006, 16, 17-22.
- E. Hodak; E. Phenig; B. Amichai; M. Feinmesser; A. Kuten; L. Maron; D. Sahar; R. Bergman; M. David; Unilesional Mycosis fungoides. Dermatology 2000, 201, 300-306, 10.1159/000051542.
- Gustavo M. Amorim; Joao P. Niemeyer-Corbellini; Danielle C. Quintella; Tullia Cuzzi; Marcia Ramos-E-Silva; Hypopigmented mycosis fungoides: a 20-case retrospective series. International Journal of Dermatology 2018, 57, 306-312, 10.1111/ijd.13855.
- Laila El Shabrawi-Caelen; Lorenzo Cerroni; L. Jeffrey Medeiros; Timothy H. McCalmont; Hypopigmented Mycosis Fungoides. American Journal of Surgical Pathology 2002, 26, 450-457, 10.1097/00000478-200204000-00006.
- Christiane Querfeld; Jasmine Zain; Steven T. Rosen; Primary Cutaneous T-Cell Lymphomas: Mycosis Fungoides and Sezary Syndrome. Infectious Complications in Cancer Patients 2018, 176, 225-248, 10.1007/978-3-319-99716-2_11.
- Marco Ardigó; Giovanni Borroni; Luca Muscardin; Helmut Kerl; Lorenzo Cerroni; Hypopigmented mycosis fungoides in Caucasian patients: a clinicopathologic study of 7 cases.. Journal of the American Academy of Dermatology 2003, 49, 264-270, 10.1067/s0190-9622(03)00907-1.
- Ivan V. Litvinov; Anna Shtreis; Kenneth Kobayashi; Steven Glassman; Matthew Tsang; Anders Woetmann; Denis Sasseville; Niels Ødum; Madeleine Duvic; Investigating potential exogenous tumor initiating and promoting factors for Cutaneous T-Cell Lymphomas (CTCL), a rare skin malignancy. OncoImmunology 2016, 5, e1175799, 10.1080/2162402X.2016.1175799.
- Fernando Gallardo; Juan Sandoval; Angel Díaz-Lagares; Ricard Garcia; Teresa D'altri; Jessica González; Victor Alegre; Octavio Servitje; Ana B. Crujeiras; Ólafur-Andri Stefánsson; et al.B. EspinetMaria-Inmaculada HernándezBeatriz BellosilloManel EstellerRamón M. PujolAnna BigasLluis Espinosa Notch1 Pathway Activation Results from the Epigenetic Abrogation of Notch-Related MicroRNAs in Mycosis Fungoides. Journal of Investigative Dermatology 2015, 135, 3144-3152, 10.1038/jid.2015.328.
- Johannes Lundin Brockdorff; Anders Woetmann; Tomas Mustelin; Keld Kaltoft; Qian Zhang; Mariusz A Wasik; Carsten Röpke; Niels Ødum; SHP2 REGULATES IL-2 INDUCED MAPK ACTIVATION, BUT NOT Stat3 OR Stat5 TYROSINE PHOSPHORYLATION, IN CUTANEOUS T CELL LYMPHOMA CELLS. Cytokine 2002, 20, 141-147, 10.1006/cyto.2002.1986.
- Feras M. Ghazawi; Elena Netchiporouk; Elham Rahme; Matthew Tsang; Linda Moreau; Steven Glassman; Nathalie Provost; Martin Gilbert; Sara-Elizabeth Jean; Kevin Pehr; et al.D. SassevilleIvan V. Litvinov Comprehensive analysis of cutaneous T-cell lymphoma (CTCL) incidence and mortality in Canada reveals changing trends and geographic clustering for this malignancy. Cancer 2017, 123, 3550-3567, 10.1002/cncr.30758.
- Feras M. Ghazawi; Elena Netchiporouk; Elham Rahme; Matthew Tsang; Linda Moreau; Steven Glassman; Nathalie Provost; Martin Gilbert; Sara-Elizabeth Jean; Osama Roshdy; et al.Kevin PehrDenis SassevilleIvan V. Litvinov Distribution and Clustering of Cutaneous T-Cell Lymphoma (CTCL) Cases in Canada During 1992 to 2010. Journal of Cutaneous Medicine and Surgery 2017, 22, 154-165, 10.1177/1203475417745825.
- Ellen J. Kim; Stephen Hess; Stephen K. Richardson; Sara Newton; Louise C. Showe; Bernice M. Benoit; Ravi Ubriani; Carmela C. Vittorio; Jacqueline M. Junkins-Hopkins; Maria Wysocka; et al.Alain H. Rook Immunopathogenesis and therapy of cutaneous T cell lymphoma. Journal of Clinical Investigation 2007, 117, 836-836, 10.1172/jci24826c1.
- Thomas S. Kupper; Robert C. Fuhlbrigge; Immune surveillance in the skin: mechanisms and clinical consequences. Nature Reviews Immunology 2004, 4, 211-222, 10.1038/nri1310.
- Michael Girardi; Peter W. Heald; Lynn D. Wilson; The Pathogenesis of Mycosis Fungoides. New England Journal of Medicine 2004, 350, 1978-1988, 10.1056/nejmra032810.
- Thorbjørn Krejsgaard; Andreas Willerslev-Olsen; Lise M. Lindahl; Charlotte Menné Bonefeld; Sergei B. Koralov; Carsten Geisler; Mariusz A. Wasik; Robert Gniadecki; Mogens Kilian; Lars Iversen; et al.Anders WoetmannNiels Odum Staphylococcal enterotoxins stimulate lymphoma-associated immune dysregulation. Blood 2014, 124, 761-770, 10.1182/blood-2014-01-551184.
- Gavin P. Dunn; Lloyd J. Old; Robert D. Schreiber; The Immunobiology of Cancer Immunosurveillance and Immunoediting. Immunity 2004, 21, 137-148, 10.1016/j.immuni.2004.07.017.
- Doaa Shalabi; Anna Bistline; Onder Alpdogan; Saritha Kartan; Anjali Mishra; Pierluigi Porcu; Neda Nikbakht; Immune evasion and current immunotherapy strategies in mycosis fungoides (MF) and Sézary syndrome (SS). Chinese Clinical Oncology 2019, 8, 11-11, 10.21037/cco.2019.01.01.
- Henry K Wong; Anjali Mishra; Timothy Hake; Pierluigi Porcu; Evolving Insights in the Pathogenesis and Therapy of Cutaneous T-cell lymphoma (Mycosis Fungoides and Sezary Syndrome). British Journal of Haematology 2011, 155, 150-166, 10.1111/j.1365-2141.2011.08852.x.
- H. Seif El Nasr; Olfat G. Shaker; M.M.T. Fawzi; G. El‐Hanafi; Basic fibroblast growth factor and tumour necrosis factor alpha in vitiligo and other hypopigmented disorders: suggestive possible therapeutic targets. Journal of the European Academy of Dermatology and Venereology 2011, 27, 103-108, 10.1111/j.1468-3083.2011.04368.x.
- Randa Youssef; Doaa Mahgoub; Ola A. Zeid; Dalia M. Abdel-Halim; Marwa El-Hawary; Marwa F. Hussein; Mary Attia Morcos; Dalia M. AboelFadl; Heba Abdelkader; Yosra Abdel-Galeil; et al.Mona Abdel-Halim Hypopigmented Interface T-Cell Dyscrasia and Hypopigmented Mycosis Fungoides. The American Journal of Dermatopathology 2018, 40, 727-735, 10.1097/dad.0000000000001187.
- Gary S. Chuang; Daniel I. Wasserman; H. Randolph Byers; Marie-France Demierre; Hypopigmented T-cell dyscrasia evolving to hypopigmented mycosis fungoides during etanercept therapy. Journal of the American Academy of Dermatology 2008, 59, S121-S122, 10.1016/j.jaad.2008.06.042.
- Richard T Hoppe; L.Jeffrey Medeiros; Roger A Warnke; Gary S Wood; CD8-positive tumor-infiltrating lymphocytes influence the long-term survival of patients with mycosis fungoides. Journal of the American Academy of Dermatology 1995, 32, 448-453, 10.1016/0190-9622(95)90067-5.
- Mei Nasu-Tababuchi; Taku Fujimura; Aya Kakizaki; Kosuke Shido; Naokazu Hatchome; Yoshiyuki Kusakari; Setsuya Aiba; Press Enter Key For Correspondence Information; Hypopigmented mycosis fungoides: An immunological investigation of tumor-infiltrating T cells. Dermatologica Sinica 2016, 34, 96-98, 10.1016/j.dsi.2015.08.006.
- Zeba N Singh; Maria S Tretiakova; Christopher R Shea; Vesna Petronic-Rosic; Decreased CD117 expression in hypopigmented mycosis fungoides correlates with hypomelanosis: lessons learned from vitiligo. Modern Pathology 2006, 19, 1255-1260, 10.1038/modpathol.3800644.
- Anna Wańkowicz-Kalińska; René M J G J Van Den Wijngaard; Bert J Tigges; Wiete Westerhof; Graham Ogg; Vincenzo Cerundolo; Walter J. Storkus; Pranab K. Das; Immunopolarization of CD4+ and CD8+ T cells to Type-1-like is associated with melanocyte loss in human vitiligo.. Laboratory Investigation 2003, 83, 683-695, 10.1097/01.LAB.0000069521.42488.1B.
- Kelly G Paulson; Miranda C Lahman; Aude G Chapuis; Isaac Brownell; Immunotherapy for skin cancer. International Immunology 2019, 31, 465-475, 10.1093/intimm/dxz012.
- Maiana Larsabal; Aurélie Marti; Clément Jacquemin; Jérôme Rambert; Denis Thiolat; Léa Dousset; Alain Taieb; Caroline Dutriaux; Sorilla Prey; K Boniface; et al.J. Seneschal Vitiligo-like lesions occurring in patients receiving anti-programmed cell death–1 therapies are clinically and biologically distinct from vitiligo. Journal of the American Academy of Dermatology 2017, 76, 863-870, 10.1016/j.jaad.2016.10.044.
- K.D. Yazganoğlu; Z. Topkarci; N. Büyükbabani; C. Baykal; Childhood mycosis fungoides: a report of 20 cases from Turkey. Journal of the European Academy of Dermatology and Venereology 2011, 27, 295-300, 10.1111/j.1468-3083.2011.04383.x.
- E. Mary Wain; Guy E. Orchard; Sean J. Whittaker; Margaret F. Spittle M.Sc.; Robin Russell-Jones; Outcome in 34 patients with juvenile-onset mycosis fungoides. Cancer 2003, 98, 2282-2290, 10.1002/cncr.11780.
- A.B. Cervini; Adriana Natalia Torres Huamani; C. Sanchez-La-Rosa; L. Galluzzo; V. Solernou; J. Digiorge; P. Rubio; Micosis fungoide. Experiencia en un hospital pediátrico. Actas Dermo-Sifiliográficas 2017, 108, 564-570, 10.1016/j.ad.2017.01.008.
- Evelyna Derhovanessian; Rafael Solana; Anis Larbi; Graham Pawelec; Immunity, ageing and cancer. Immunity & Ageing 2008, 5, 11-11, 10.1186/1742-4933-5-11.
- Shamir Geller; Emily Lebowitz; Melissa P. Pulitzer; Steven M. Horwitz; Alison J. Moskowitz; Steve Dusza; Patricia L. Myskowski; Outcomes and prognostic factors in African American and black patients with mycosis fungoides/Sézary syndrome: Retrospective analysis of 157 patients from a referral cancer center. Journal of the American Academy of Dermatology 2019, 83, online, 10.1016/j.jaad.2019.08.073.
- Fabricio C. Furlan; Bruna A. Pereira; Mirian Nacagami Sotto; José Antonio Sanches Jr; Hypopigmented Mycosis Fungoides versus Mycosis Fungoides with Concomitant Hypopigmented Lesions: Same Disease or Different Variants of Mycosis Fungoides?. Dermatology 2013, 229, 271-274, 10.1159/000363319.