Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Glen Borchert and Version 3 by Lindsay Dong.
RNA fragments derived from full-length small nucleolar RNAs (snoRNAs) have been shown to be specifically excised and functional. These sno-derived RNAs (sdRNAs) have been implicated as gene regulators in a multitude of cancers, controlling a variety of genes post-transcriptionally via association with the RNA-induced silencing complex (RISC).
- cancer
- gene regulation
- small nucleolar RNA (snoRNA)
- small nucleolar derived RNA (sdRNA)
- microRNA (miRNA)
- RNA
- genetics
1. snoRNA Structure and Function
One class of ncRNA found to give rise to functional ndRNAs is the snoRNA. SnoRNAs constitute a class of ncRNA localized to the nucleolus that can be further divided into two main subgroups: C/D box and H/ACA box snoRNA [1][3]. C/D box snoRNA are structurally distinguished by the presence of a 5′ box C motif (RUGAUGA, R = purine) and its C’ box partner, as well as a 3′ box D motif (CUGA) with its D’ box partner. Self-complementarity drives the formation of the C/D box snoRNA stem structure that in turn binds accessory proteins NOP56, NOP58, 15.5 K, and fibrillarin to form the functional sno-ribonucleoprotein (snoRNP) (Figure 1 and Figure 2). Situated in the nucleolus, antisense domains located within the C/D box snoRNA serve as guides for the snoRNP to bind target ribosomal RNAs (rRNAs). The methyltransferase fibrillarin is then responsible for carrying out 2′-O-ribose-methylation of the rRNA [2][3][4][4,5,6]. H/ACA box snoRNA contain a 3′ ACA box (ACANNN, N = any nt) and two hairpins linked by an H box (ANANNA, N = any nt). The H/ACA box snoRNP complex is formed by association with NHP2, NOP10, GAR1, and dyskerin proteins (Figure 1 and Figure 3). The H/ACA snoRNA hairpins guide the snoRNP to target rRNAs where dyskerin carries out pseudouridylation [5][6][7][7,8,9]. Erroneous expression and/or activity of either of these classes of snoRNA have been extensively linked to tumorigenesis. However, reports have implied that several proteins comprising the snoRNP are required for the biogenesis of distinct species of sdRNA [8][10].

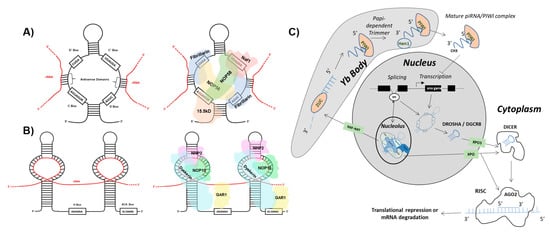
Figure 1. sdRNAs arise from full-length snoRNAs. (A) C/D box snoRNA structure and accessory proteins. (Left) The C/D box snoRNA consists of a 5′ C box (RUGAUGA) motif, a 3′ D box (CUGA) motif, and the C’ and D’ boxes located internally. Antisense domains identify target rRNAs (red) via complementarity. (Right) The C/D box snoRNP complex consists of NOP56, NOP58, 15.5 K, and fibrillarin proteins [2][3][4][4,5,6]. (B) H/ACA box snoRNA structure and accessory proteins. (Left) The H/ACA box snoRNA consists of a 3′ ACA box (ACANNN, N = any nt) and two hairpins that target rRNA (red) linked by an H box (ANANNA, N = any nt). (Right) The H/ACA box snoRNP complex consists of NHP2, NOP10, GAR1, and dyskerin proteins [5][6][7][7,8,9]. (C) sdRNA biogenesis and function. Biogenesis of Argonaute 2 (AGO2)-associating sno-derived RNAs (sdRNAs) Full length small-nucleolar RNAs (snoRNAs) are generated either as products of transcription or splicing [9][10][11][12][11,12,13,14]. snoRNAs produced by transcription can give rise to microRNA-like sdRNAs which are specifically excised from parent snoRNA transcripts by employment of the classical microRNA (miRNA) processing pathway. This occurs by processing of parent snoRNAs into smaller transcripts by the microprocessor complex which consists of Drosha Ribonuclease III (DROSHA) and DiGeorge syndrome critical region 8 (DGCR8). The intermediate snoRNA then undergoes cytoplasmic exportation via exportin 5 (XPO5). Following this, the smaller cytoplasmic snoRNA is processed by Dicer RNase III endonuclease (DICER) to generate the mature sdRNA which associates with AGO2, leading to the formation of the RNA-induced Silencing Complex (RISC). Similar to miRNAs, these sdRNAs function in post-transcriptional gene suppression by antisense binding to target mRNA transcripts within RISC [13][15]. That said, snoRNAs produced by splicing can also enter the classical miRNA processing pathway. Spliced snoRNAs, however, can bypass processing from DROSHA/DGCR8 and/or DICER as a result of trafficking to the nucleolus and subsequent processing by the fibrillarin complex followed by cytoplasmic export via a transporter belonging to the Exportin (XPO) family of proteins [8][10]. Biogenesis of PIWI-associated RNA (piRNA) like sdRNAs Spliced snoRNAs which arrive at the nucleolus for fibrillarin processing can be trafficked into Yb bodies via Nuclear RNA Export Factor (NXF1)/ Nuclear Transport Factor 2 Like Export Factor 1 (NXT1) where the 3′ end is cleaved by Zucchini (ZUC) and subsequently degraded. The remaining transcript is processed further within the Yb body by the papi-dependent trimmer. Following this, HEN1 double-stranded RNA binding protein binds at the 3′ end of the transcript where it adds a methyl group, generating a mature piRNA/PIWI complex which is exported to the cytoplasm. This piRNA/PIWI complex can then be shuttled back into the nucleus where it functions to inhibit transcription [14][15][16,17].
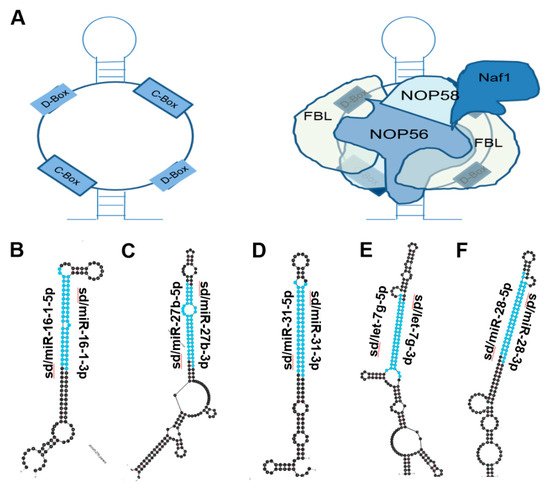
Figure 2. miRNAs derived from C/D Box snoRNA-like transcripts that bind fibrillarin. (A) Cartoon illustration of C/D Box snoRNA (left) and schematic of C/D Box snoRNA bound to fibrillarin complex, giving rise to snoRNP (right). Fibrillarin complex consists of NOP56, NOP58, NAF1 and Fibrillarin [16][18]. (B–F) Most thermodynamically stable secondary structures of C/D Box snoRNA-like transcripts described to bind fibrillarin complex [17][19] were obtained from mfold [18][20]. Mature miRNA sequences embedded in these transcripts are highlighted in blue. (B) miR16-1 (ENSG00000208006) is downregulated in chronic lymphocytic leukemia, gastric, NSCLC, Osteosarcoma, and breast cancer and is embedded within a C/D Box snoRNA-like transcript (GRCh38: chr 13:50048958-50049077:-1) known to bind fibrillarin [19][20][21][22][23][1,21,22,23,24]. (C) miR-27b (ENSG00000207864) is downregulated in prostate, lung, and bladder cancer and is located within a C/D Box snoRNA-like transcript (GRCh38: chr 9:95085436-95085592:1) known to bind fibrillarin [24][25][26][25,26,27]. (D) miR-31 (ENSG00000199177) is upregulated in colorectal, HNSCC, and lung cancer but is downregulated in glioblastoma, melanoma, and prostate cancer. miR31 is also located within a C/D Box snoRNA-like transcript (GRCh38: chr 9:21512102-21512221:-1) known to bind fibrillarin [27][28][29][30][31][32][28,29,30,31,32,33]. (E) miR-let7g (ENSG00000199150) has been shown to be downregulated in NSCLC, colorectal and ovarian cancers and is also embedded within a C/D Box snoRNA-like transcript (GRCh38: chr 3:52268239-52268408:-1) known to bind fibrillarin [33][34][35][34,35,36]. (F) miR-28 (ENSG00000207651) has been shown to be downregulated in B-cell lymphoma, prostate and breast cancer and is located within a C/D Box snoRNA-like transcript (GRCh38: chr 3:188688746-188688887:1) known to bind fibrillarin [36][37][38][37,38,39].
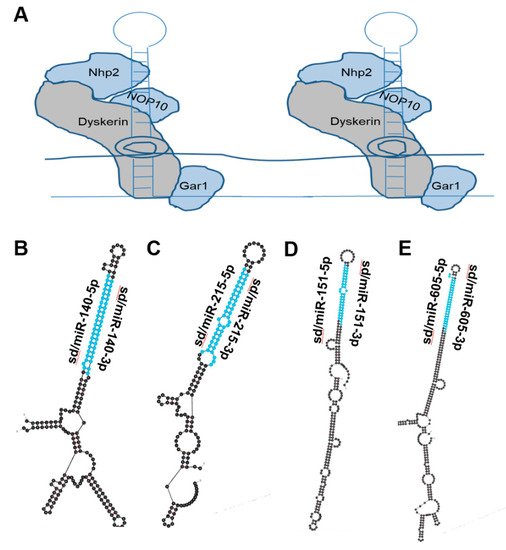
Figure 3. miRNAs derived from Box H/ACA snoRNA-like transcripts that bind dyskerin. (A) Schematic of box H/ACA snoRNA bound to dyskerin complex, giving rise to snoRNP. Dyskerin complex includes Dyskerin, NHP2, NOP10, and GAR1 [16][18]. (B–E) Most thermodynamically stable secondary structures of box H/ACA snoRNA-like transcripts known to bind dyskerin complex [39][40] were generated by mfold [18][20]. Mature miRNA sequences derived from these transcripts are highlighted in blue. (B) Box H/ACA snoRNA-like transcript (GRCh38: chr 16:69933072-69933264:1) was identified to bind dyskerin and encompasses miR-140 (ENSG00000208017) which functions as a tumor-suppressing RNA and is downregulated in prostate cancer [40][41]. (C) Box H/ACA snoRNA-like transcript (GRCh38: chr1:220117845-220118007:-1) was identified to bind dyskerin and surrounds miR-215 (ENSG00000207590) which is known to function as a tumor suppressor and is downregulated in ovarian, colorectal, prostate and lung cancer [41][42][42,43]. (D) Box H/ACA snoRNA-like transcript (GRCh38: chr8:140732552-140732807:-1) was determined to bind dyskerin and encapsulates miR-151 (ENSG00000254324) which is recognized as a tumor-suppressing RNA and is downregulated in prostate cancer [43][44][45][44,45,46]. (E) Box H/ACA snoRNA-like transcript (GRCh38: chr 10:51299393-51299708:1) was determined to bind dyskerin and surrounds miR-605 (ENSG00000207813) which is described to act as a tumor-suppressing RNA in melanoma, colorectal, breast, lung and prostate cancer [46][47][48][49][50][47,48,49,50,51].
2. sdRNAs in Cancer
2.1. sd/miR-664
In 2019 Lv et al. published a paper focused on elucidating the tumor-suppressive role of sd/miR-664 in cervical cancer. Comparing sd/miR-664 expression in cervical cancer patient samples and control tissue revealed that the sdRNA is expressed at lower levels in cancer. Using the Si-Ha cell line as a model of cervical cancer, sd/miR-664 expression was found to enhance apoptosis and reduce cell viability and migration. In a Si-Ha mouse xenograft model, treatment with an sd/miR-664 mimic drastically reduced tumor volume and weight while enhancing tumor apoptosis. The binding target was predicted to be c-Kit using online target prediction software, and this was confirmed via the Renilla luciferase assay. Sd/miR-664 expression was negatively correlated with c-Kit expression, which further solidified the proto-oncogene c-Kit as a target of this tumor-suppressive sdRNA in cervical cancer [51][57].
To assess the role of sd/miR-664 in cutaneous squamous cell carcinoma (cSCC), Li et al. quantified the sdRNA’s expression by in situ hybridization in patient samples as well as cSCC cell lines [52][58]. Both analyses revealed an increase in sd/miR-664 in cSCC compared to control. Phenotypic assays showed increased cSCC viability, colony formation, invasion, and migration when sd/miR-664 levels were elevated in cell lines. Additionally, a murine cSCC xenograft model treated with sd/miR-664 mimic exhibited increased tumor volume compared to control. The 3′-UTR of IRF2 was predicted and then confirmed to be a binding target of sd/miR-664. Taken together, sd/miR-664 functions as an onco-miR in cSCC by downregulating IRF2 expression. While the role of IRF2 has not been established in cSCC, IRF2 has been identified as a tumor suppressor in lung cancer and gastric cancer and, paradoxically, as an oncogene in testicular embryonal carcinoma [53][54][55][78,79,80]. This adds a layer of complexity to sd/miR-664, showing that its overall impact on tumor progression can be tissue context-dependent.
2.2. Sd/miR-1291
Building on their 2016 publication where they found sd/miR-1291 to be downregulated in pancreatic cancer patient tissues, Tu et al. published an article in 2020 to further investigate this mechanism of action in pancreatic cancer cells [56][57][59,60]. They found that increasing sd/miR-1291 reduced ASS1 levels in the ASS1-abundant L3.3 pancreatic cancer cell line and sensitized them to arginine deprivation in vitro. In addition, the glucose transporter GLUT1 mRNA has previously been shown to be a direct target of sd/miR-1291 in renal cell carcinoma (RCC), and Tu et al. confirmed this to be the case in pancreatic cancer cells as well with a resulting decrease in glycolytic capacity [58][61]. Further, sd/miR-1291 treatment sensitized pancreatic cancer cell lines to cisplatin. Taken together, sd/miR-1291 has a clear role as a modulator of cell metabolism to bring about tumor suppression in pancreatic cancer.2.3. Sd/miR-3651
A 2020 publication aimed at identifying novel contributors to colorectal cancer (CRC) found that miR-3651, which arises from SNORA84, was overexpressed in 34/40 CRC patient tumors [59][65]. Model cell lines of CRC were observed to have ~3-fold overexpression of sd/miR-3651. Phenotypic assays revealed that sd/miR-3651 expression was positively correlated with CRC cell proliferation, and that reduction of the sdRNA enhanced apoptosis via deactivation of PI3K/AKT and MAPK/ERK signaling. Target prediction identified the 3′-UTR of tumor suppressor TBX1 as a sd/miR-3651 target, which was confirmed via the luciferase assay. Since TBX1 is a transcription factor that has an established role as a regulator of PI3K/AKT and MAPK/ERK pathways, these results show a coherent mechanism of action by which sd/miR-3651 inactivates TBX1 and thereby activates pro-growth mitogenic pathways to function as an oncogenic sdRNA.2.4. sd/miR-768
In 2012, Su et al. published a study in which they interrogated gastric cancer patient samples for miRNA expression. They found that sd/miR-768-5p, which arises from SNORD71 (Figure 4), was significantly downregulated in cancer samples [60][68]. A 2013 study by Blenkiron et al. identified sd/miR-768-5p as a YB-1 binding partner via CO-IP in MCF7 cells [61][67]. The authors proposed potential explanations for the YB-1/sdRNA interaction including YB-1 functioning as a ncRNA-sponge to prevent ncRNA-mediated transcriptional repression as well as YB-1 potentially playing a role in ncRNA biogenesis.
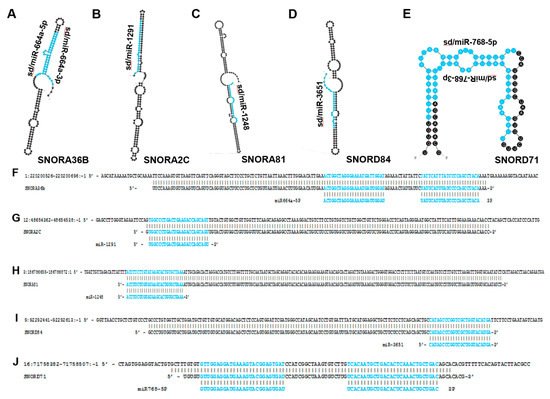
Figure 4. miRNAs that arise from snoRNA transcripts. The most thermodynamically stable secondary structures of snoRNA transcripts were generated by mfold [18][20]. Annotated miRNA sequences embedded in these transcripts are highlighted in blue (A–E). (A) miR-664a is embedded within the SNORA36B transcript and has been shown to function as a tumor-suppressing RNA in HCC and some types of cervical cancer while also functioning as a tumor-promoting RNA in cervical squamous cell carcinoma [62][56]. (B) miR-1291 is located within the SNORA2C transcript and is characterized as a tumor-suppressing RNA in pancreatic, prostate, breast and renal cell carcinoma [56][57][58][63][64][59,60,61,62,63]. (C) miR-1248 is derived from SNORA81 and has been determined to be upregulated in prostate cancer [65][64]. (D) miR-3651 is embedded within SNORA84 and is upregulated in colorectal cancer but downregulated in esophageal cancer [59][66][65,66]. (E) miR-768 resides within the SNORD71 transcript and has been shown to be downregulated in lung, breast, ovary, liver, parotid gland, thyroid gland cancers and melanoma [60][61][67][67,68,69]. (F–J) Mature miRNA sequences (highlighted in blue) were aligned to their corresponding snoRNA precursor transcripts and snoRNAs were aligned to their respective genomic regions. (F) Alignment between human genome (GRCh38: chr1:220200526-220200696:-1) (top), SNORA36B (ENSG00000222370) (middle), and miR664a (ENSG00000281696) (bottom). (G) Alignment between human genome (GRCh38: chr12:48654362-48654538:-1) (top), SNORA2C (ENSG00000221491) (middle) and miR-1291 (ENSG00000281842) (bottom). (H) Alignment between human genome (GRCh38: chr 3:186786655-186786872:1) (top), SNORA81 (ENSG00000221420) (middle), and miR-1248 (ENSG00000283958) (bottom). (I) Alignment between human genome (GRCh38: chr 9:92292441-92292613:-1) (top) SNORA84 (ENSG00000239183) (middle) and miR-3651 (ENSG00000281156) (bottom). (J) Alignment between human genome (GRCh38: chr 16:71758382-71758507:-1) (top), SNORD71 (ENSG00000223224) (middle) and miR-768 (ENSG00000223224) (bottom).
2.5. sd/miR-1248
There is a clear need for additional reliable biomarkers to aid care providers in their decision on which treatment to apply to prostate cancer patients following radical prostatectomy. Aimed at meeting this need, Pudova et al. published a paper in 2020 focused on identifying differentially expressed miRNAs between prostate cancer patient tumors with or without lymphatic dissemination [65][64]. Among the nine miRNAs found to be significantly upregulated in prostate cancer with lymphatic dissemination, sd/miR-1248 was identified which arises completely from SNORA81.
2.6. hsa-sno-HBII-296B and hsa-sno-HBII-85-29
In 2015, Müller et al. performed small RNA sequencing on six PDAC tissue samples and five normal pancreas tissue samples to assess the expression of ncRNAs not typically found via microarray analysis [68][70]. They considered 45 noncoding RNAs identified as significantly down-regulated in PDAC, of which there were fourteen sdRNAs and a single sno-derived piRNA. The most downregulated sdRNA in PDAC, as determined by log2 fold change, was hsa-sno-HBII-85-29. Four sdRNAs of a total 78 ncRNAs were significantly upregulated in PDAC. The most upregulated sdRNA in PDAC, as determined by log2 fold change, was hsa-sno-HBII-296B. Subsequent analyses were focused on a miRNA identified in Hthe study, however, It i this work constitutes the only publication that characterizes differentially expressed sdRNAs focused specifically in PDAC. PDAC is a particularly difficult cancer to detect and treat, with a reported 5-year survival rate of just 11% [69][82].
