Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Shichang Chen and Version 2 by Amina Yu.
Zeolites have been widely employed in fields of petroleum refining, fine chemicals and environmental protection, but their syntheses are always performed in the presence of organic templates, which have many drawbacks such as high cost and polluted wastes. The seed-directed route opens a new door for synthesizing zeolites with Al-rich features, which are being simple, low-cost and environmentally friendly. In addition, it also has many advantages including accelerating the crystallization rate, increasing the degree of crystallinity and avoiding the generation of impurity phases and controlling the crystal size. The seed-directed synthesis of zeolites is divided into the homonuclear and heteronuclear growth.
- zeolite
- green synthesis
- seed-directed
- utility
1. Homonuclear Growth
Zeolite products have the same structures with the targeted zeolite seed added in the starting gel, which is called a homonuclear growth of seed-directed synthesis. Currently, it has been reported that more than 20 zeolites could be successfully synthesized using this route, such as *BEA [1][2][3][36,37,38], MTT [4][39], CHA [5][6][7][40,41,42], MWW [8][9][43,44], SZR [10][45], MSE [11][46], KFI [12][13][47,48] and so on. Considering the space limitation of thereinis review, it would mainly introduce the zeolites that have been widely used in the industry including *BEA, MTT, CHA and MWW.
1.1. *BEA
Beta zeolite has a three-dimensional structure with a 12-membered ring, which shows excellent performance in the fields of petroleum refining and fine chemicals due to its good thermal stability, unique hydrophobicity and excellent catalytic performance [14][76]. The conventional synthesis of Beta zeolite is usually directed by tetraethylammonium hydroxide (TEAOH) as an organic template. As a result, to obtain the opened micropores, the organic template needs to be removed by high-temperature calcination, which not only causes great economic loss and energy consumption, but also releases a large amount of greenhouse gases and harmful gases when the organic template is decomposed. Therefore, the organotemplate-free synthesis of Beta zeolite is highly desirable.
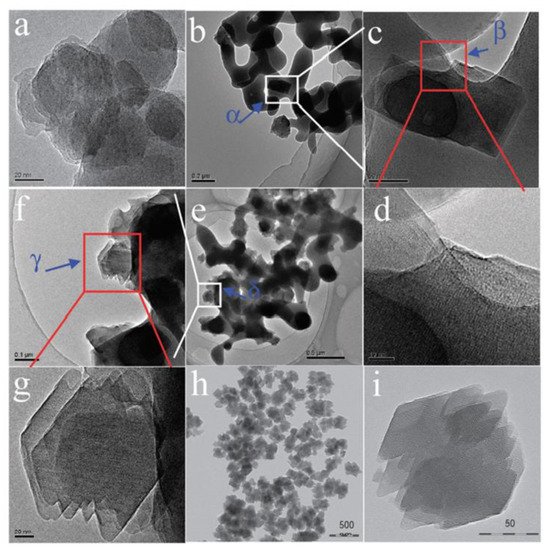
Xie et al. [1][36] reported for the first time that Beta zeolite with high crystallinity (named as Beta-OTF) could be successfully obtained by adding about 10% calcined Beta zeolite as the seed into the starting gel at 140 °C for 18.5 h in the absence of any organic template. The N2 adsorption curve of the Beta-OTF demonstrated that the zeolite already had opened micropores, thus completely avoiding the calcination process and ensuring high crystallinity. In addition, the crystallization mechanism of Beta zeolite synthesized from the seed-directed route was studied by transmission electron microscopy (TEM), X-ray diffraction (XRD) and scanning electron microscopy (SEM), which displayed that the growth process of Beta-OTF followed the “core-shell” mechanism, as shown in Figure 1. In particular, the yield of Beta-OTF from seed-directed synthesis is low (about 30–40%) [2][37]. Subsequently, Yokoi et al. [3][38] displayed that an Al-rich suspension precursor was used for synthesizing Beta zeolite, which could greatly increase the product yield (about 80%). Notably, Beta zeolite synthesized from a seed-directed route is extremely Al-rich (the Si/Al ratio is only about 4), which is much lower than that of Beta zeolite synthesized by using an organic template (Si/Al ratio over 12) [15][16][17][77,78,79]. In addition, the Al-rich Beta zeolite has very stable four-coordinated Al species and good thermal and hydrothermal stability, which provide a new path for the development of high-efficiency zeolite catalysts with a highly acidic density in the future.
1.2. MTT
ZSM-23 zeolite with a one-dimensional 10-membered ring [18][80] exhibits an excellent performance in a series of catalytic reactions, such as methanol to hydrocarbon [19][20][21][81,82,83] and isomerization [4][22][39,84]. Unfortunately, organic templates were necessary in its synthesis, such as isopropylamine [23][85], pyrrolidine [24][86], N,N-dimethylformamide [24][86], diquat-7 [25][87], and so onetc.
To avoid the use of the above organic templates, Wu et al. [4][39] developed a method for synthesizing ZSM-23 zeolite by a seed-directed route (designated as ZJM-6). They studied, in detail, the effects of various factors such as the amount of seeds, the Si/Al ratio and H2O/SiO2 on the synthesis of ZSM-23 zeolite. The experimental results showed that the Si/Al ratio of ZJM-6 zeolite was about 20, which was far lower than that of the conventional ZSM-23 zeolite synthesized with pyrrolidine as an organic template (the Si/Al ratio was about 32–62). It is noteworthy that the crystallization of ZJM-6 takes only 5 h to obtain a targeted product with high crystallinity, which greatly shortens the crystallization time. Furthermore, due to the presence of more acidic sites in Al-rich ZJM-6 than conventional ZSM-23, the conversion of ZJM-6 (10.4%) was higher than that (4.1–9.4%) of conventional ZSM-23 zeolite in the catalytic isomerization of m-xylene to p-xylene. Therefore, it confirmed that the Al-rich feature of the ZSM-23 zeolite synthesized from the seed-directed route enhanced the catalytic activity of the reaction. Very interestingly, the as-synthesized ZJM-6 zeolite product could be used as zeolite seeds again to prepare ZJM-6-2 zeolite in the next run, which provides a green method for synthesizing ZSM-23 zeolite that completely avoids the use of organic templates.
1.3. CHA
CHA zeolite has a three-dimensional eight-membered ring pore structure [26][88]. SSZ-13 zeolite, as a typical representative one, has been widely used in methanol-to-olefin (MTO) [27][28][89,90] and the selective catalytic reduction of NO with ammonia (NH3-SCR) [29][30][91,92]. In 1985, Zones et al. [31][93] firstly synthesized SSZ-13 zeolite using an expensive and toxic organic template of N,N,N-trimethyl-1-adamantammonium hydroxide (TMAdaOH) under hydrothermal conditions, which limited its further application. Subsequently, Martín et al. [32][94] synthesized SSZ-13 zeolite using the cheap tetraethylammonium hydroxide (TEAOH) as an organic template, which greatly reduced the preparation cost. However, the use of an organic template is not environmentally friendly. Therefore, it is highly desirable to synthesize SSZ-13 zeolite in the absence of any organic templates.
In 2014, Takashi et al. [5][40] firstly synthesized SSZ-13 zeolite with an Si/Al ratio of 4 to 5 by a seed-directed route under an organotemplate-free condition. The key to successful synthesis was to add the zeolite seeds and introduce K+ or Cs+ into the synthetic system. At the same time, it was found that the replacement of K+ with Cs+ could increase the Si/Al ratio of SSZ-13 zeolite and the addition of B3+ in the initial gel could increase the Si/Al ratio of the product as high as 6.1.
In 2018, Wang et al. [6][41] successfully synthesized the micro-spherical hierarchical porous SSZ-13 zeolite with abundant mesopores via a seed-directed route using aluminum isopropoxide as an aluminum source. In the MTO reaction, the SSZ-13 zeolite showed the methanol conversion was 100% and the ethylene and propylene selectivity were over 90% plus the longer lifetime than that of the conventional SSZ-13 zeolite. The seed-directed synthesis of SSZ-13 zeolite not only significantly reduced the production cost and environmental pollution, but also shortened the crystallization time. However, the crystal size of SSZ-13 zeolite obtained by this method was 3–6 μm.
However, the micro-scale crystal size of the SSZ-13 zeolite affects their performance in gas adsorption [17][79] and catalysis [33][95] due to its diffusion limitations of the micropore. In 2020, Debost et al. [7][42] reported the direct synthesis of nanoscale SSZ-13 zeolite in a colloidal suspension containing a mixture of inorganic cations (Na+, K+, and Cs+) in the absence of an organic template. As a result, the obtained product showed the crystal size was approximately only 42 nm (thickness, along the c-axis) × 189 nm (width, in the ab-plane). The nanosized SSZ-13 zeolite improved its pore accessibility and thus exhibited an excellent CO2 adsorption capacity.
1.4. MWW
MWW zeolite owns the two-dimensional sinusoidal 10-membered ring network channels (0.49 nm × 0.59 nm) in the inner layer and 12-membered ring super-cages between the layers (0.71 nm × 1.82 nm) [34][96], mainly including MCM-22, MCM-49, SSZ-25, MCM-36, ITQ-2, MCM-56, and so onetc. Due to its unique pore structure and acidic characteristics, it exhibits an excellent performance in the alkylation of benzene with ethylene and propylene [35][97], the dehydrogenation skeletal isomerization of n-butane [36][98] and the aromatization of n-butane [37][99]. The conventional synthesis of MWW zeolites is not sustainable by using toxic and costly hexamethyleneimine (HMI) as an organic template [38][100].
Kamimura et al. [8][43] reported the first seed-directed synthesis of MWW zeolite. In this synthesis, the uncalcined MCM-22 zeolite was synthesized by the conventional method used as with zeolite seeds, which was added into a sodium-aluminosilicate gel system without the addition of an organic template preheated at 120 °C for 5 h in advance. The MCM-22 zeolite with high crystallinity can be obtained under static condition at 160 °C for 4 d. In the crystallization process, before the spontaneous nucleation of MOR zeolite, the more MWW zeolite seeds that were added, the larger the growth surface area provided could be, thereby obtaining MCM-22 zeolite with high quality. More recently, Xie et al. [9][44] used ultrasonic aging technology for the first time to synthesize MCM-49 zeolite by a seed-assisted route without the addition of an organic template. During the crystallization process, the ultrasonic treatment not only maintained the integrity of the zeolite seeds, but also promoted the entry of the aluminosilicate species into the solid phase and thus facilitated the uniformity of the system, which was helpful for the crystallization of MCM-49 zeolite. Theis above study develops a novel route for the efficient, eco-friendly and facile seed-directed synthesis of MCM-49 zeolite.
2. Heteronuclear Growth
Zeolite products have different structures but partly the same composite building units with the targeted zeolite seeds added in the starting gel, which is called the heteronuclear growth of seed-directed synthesis. In recent years, the synthesis of FER [39][73] and NES [40][75] has been reported typically.
2.1. FER
FER zeolite has a two-dimensional pore structure in which an 8-membered ring (0.48 × 0.35 nm) and 10-membered ring (0.54 nm × 0.42 nm) are interlaced with each other [17][79]. Due to its unique micropore system, it displays an excellent performance in catalytic reactions such as 1-butene skeletal isomerization [41][101], methanol or ethanol dehydration [42][43][102,103] and dimethyl ether carbonylation [44][45][104,105]. However, the synthesis of FER zeolite requires the use of organic templates normally, such as cetyltrimethylammonium bromide [46][106], pyrrolidine [47][107], pyridine [48][108] and piperidine [49][109]. Undoubtedly, the use of these organic templates in synthesis increases costs and environmental concerns.
Originally, Weitkamp et al. [50][110] synthesized FER zeolite with good crystallinity by the addition of homonuclear FER zeolite seeds into the organotemplate-free system, but the product Si/Al ratio was low (6.6–7.8), which could not satisfy the application requirements. Later, the researchers found that FER zeolite with a high Si/Al ratio can be prepared by adding zeolite seeds with a different topological structure from FER, which was regarded as a breakthrough in the preparation of high-silica FER zeolite [39][51][73,74]. For example, Zhang et al. [39][73] demonstrated the successful synthesis of high-silica FER zeolite (Si/Al ratio as high as 14.5) by using RUB-37 (CDO structure) zeolite with similar secondary structural units as the zeolite seeds in the absence of an organic template.
2.2. NES
NES zeolite owns 10-membered-ring straight channels and 12-membered-ring sinusoidal channels [52][111], which would be synthesized by using expensive decamethonium as an organic template [53][112].
Iyoki et al. [40][75] successfully prepared NES aluminosilicate zeolite without the addition of an organic template for the first time. The key to the successful synthesis was the use of EUO zeolite seeds with the partly same composite building units as the targeted NES zeolite product. In addition, the targeted NES zeolite product was affected by the initial gel composition (SiO2/Al2O3 and Na2O/SiO2) during the synthesis and pure NES zeolite could only be obtained in a narrow phase diagram. The micropore characteristics of the as-synthesized NES zeolite products were similar to those synthesized from conventional organic templates.
