Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Mireia Tondo and Version 2 by Catherine Yang.
Alzheimer’s disease (AD) is characterized by the accumulation of extracellular amyloid beta (Aβ) and abnormally hyperphosphorylated intracellular tau filaments in neurons. Cholesterol metabolism has been extensively implicated in the pathogenesis of AD through biological, epidemiological, and genetic studies, with the APOE gene being the most reproducible genetic risk factor for the development of AD. The apolipoprotein E (ApoE) 4 genotype seems to be a disruptive element in high-density lipoprotein (HDL)-like-mediated cholesterol transport through the brain.
- Alzheimer’s disease
- apolipoprotein E
- cholesterol trafficking
- HDL
- central nervous sytstem
- dementia
- cholesterol efflux
1. Transporters of Cholesterol in the Brain
Cholesterol trafficking in the CNS involves several transporters with similar roles to those in the peripheral cells [1][29], reinforcing the relevance of the control of lipid homeostasis in the brain. Identically to peripheral tissues, cholesterol efflux in the brain takes place through aqueous diffusion facilitated by scavenger receptor class B type I (SR-BI) and by active pathways involving the ATP-binding cassette (ABC) transporters A1 and G1 (ABCA1 and ABCG1) [2][30]. In contrast to the periphery, where SR-BI is ubiquitous, in the brain, it is only expressed in astrocytes, neurons, and capillary endothelial cells [3][4][31,32], as it is regulated by the sterol regulatory element-binding protein 2 (SREBP-2) transcription factor binding sites [5][6][33,34]. In the CNS, ABC transporters have been found in neurons, astrocytes, and capillary endothelial cells, participating in protein secretion and lipidation [7][8][35,36]. Specifically, ABCA1 acts as the main cholesterol efflux regulatory protein, interacting with mildly lipidated ApoE particles to form small HDL-like lipoproteins containing phospholipids (PL) and unesterified cholesterol (UC) [1][29]. SR-BI and other transporters from the ABC family, such as ABCG1 and ABCG4, interact with already-lipidated forms [9][37], thereby completing the lipidation of small HDL-like particles and generating larger ones via the addition of more cholesterol as well as other lipids [10][38]. Once in the extracellular space, lipoproteins can be further enriched with ApoE [11][12][13][39,40,41].
ABCA1, ABCG1, and ApoE gene transcription can be modulated by the liver X receptors (LXRs) α and β, ligand-activated transcription factors that bind to DNA and form heterodimers with retinoid X receptors (RXRs) to exert their functions [14][15][42,43]. Oxysterols and 9-cis-retinoic acid (RA) are, in turn, endogenous ligands for LXRs and RXRs, respectively [16][17][44,45]. Thus, a high concentration of intracellular cholesterol or its derivatives in astrocytes activates LXR/RXR-mediated transcription for cholesterol transport proteins to facilitate efflux [18][19][46,47]. In this sense, the co-expression of ApoE with ABCA1 by the LXR/RXR system reinforces the important role of ApoE lipidation in the efflux process [20][48]. ABCA1 may also play a critical role in removing excess cholesterol from neurons, which can either be converted into cholesterol esters and kept in the cytoplasm or converted to 24S-hydroxycholesterol (HC) by 24-hydroxylase [21][22][49,50], as discussed below. 24-HC may reach the astrocytes and, through the activation of LXR, inhibit cholesterol synthesis and upregulate ABCA1, ABCG1, and ApoE levels [16][44]. Alternatively, it can be exported from the brain through the BBB [13][23][24][25][41,51,52,53]. Concerning ABCA7, this transporter is highly expressed in the brain, sharing significant homology with ABCA1 [7][35]. However, its transcription is downregulated when intracellular cholesterol concentrations are high [23][51] and upregulated through the SREBP-2 pathway when they are low [26][54]. Its role in cholesterol efflux is less known and seems to be less relevant than those of the other ABC proteins [27][55].
In addition to endogenous LXR/RXR agonists, exogenous ligands have also been described. T0901317 is a synthetic and highly selective agonist for LXRs, with a demonstrated enhancing effect on the ABCA1 and ABCG1 transporters and cholesterol efflux [14][42]. Similarly, ABCA1 activity can also be modulated by cyclic adenosine monophosphate (cAMP) via a protein kinase A (PKA)-dependent pathway. Protein kinase A increases ABCA1 gene transcription and phosphorylates ABCA1 protein, increasing its ability to export cholesterol [28][56].
Regarding cholesterol uptake, mature HDL-like particles deliver cholesterol to brain cells through the interaction of ApoE with specific lipoprotein receptors [29][57], including the low-density lipoprotein (LDL) receptor (LDLR), LDL-receptor-related protein 1 (LRP1), the very low density lipoprotein (VLDL) receptor (VLDLR), and the ApoE receptor (ApoER2), which is mainly expressed in the brain [30][58]. All of them bind ApoE and lipidated ApoE with different degrees of affinity [1][31][29,59]. Characteristically, nascent lipoproteins secreted by astrocytes show a higher affinity for LDLR, whereas CSF ApoE-containing HDL-like particles adhere strongly to LRP1 [32][60]. Despite being present in both neurons and astrocytes, LDLR is most highly expressed in glia, whereas LRP1 is more highly expressed in neurons [33][34][35][36][37][61,62,63,64,65]. Specifically, LRP1 is a critical source of cholesterol for neurite outgrowth, synaptogenesis, and remodeling; however, it may also exert negative feedback to limit the intracellular cholesterol concentration [38][39][66,67]. Regarding VLDLR and ApoER2, they are structurally very similar to the LDLR; however, they mainly bind other ligands involved in neurodevelopment and synaptic functions, such as reelin [40][68]. The information regarding cholesterol transporters and receptors potentially involved in cholesterol processes in the brain is summarized in Table 1.
Table 1.
Cholesterol transporters and receptors potentially involved in cholesterol efflux and uptake processes in the brain.
| Cholesterol Transporters and Receptors | Cellular Expression | Regulation | Main Functions |
|---|---|---|---|
| SR-BI | Astrocytes, neurons, and capillary endothelial cells | SREBP-2 pathway | Cholesterol diffusion to lipidated ApoE forms |
| ABCA1 | Astrocytes, microglia, neurons, and capillary endothelial cells | LXR/RXR heterodimer/PKA-pathway | Cholesterol efflux to poorly lipidated ApoE |
| ABCG1 | Astrocytes, neurons, and capillary endothelial cells | LXR/RXR heterodimer | Cholesterol efflux to lipidated ApoE forms |
| ABCG4 | Astrocytes, microglia, neurons, and capillary endothelial cells | LXR/RXR heterodimer | Cholesterol efflux to lipidated ApoE forms |
| ABCA7 | Astrocytes, neurons, and microglia | SREBP-2 pathway | Less known roles |
| LDLR | Astrocytes, microglia, neurons, and capillary endothelial cells | PCSK9 | Cholesterol uptake regulator |
| LRP1 | Astrocytes, microglia, neurons, and capillary endothelial cells | PCSK9 | Cholesterol uptake regulator |
| VLDLR | Astrocytes, microglia, neurons, and capillary endothelial cells | PCSK9 | Bind ligands for neurodevelopment and synaptic functions |
| ApoER2 | Neurons | PCSK9 | Bind other ligands involved in neurodevelopment and synaptic functions |
ABC: ATP-binding cassette; Apo: apolipoprotein; ApoER2: apoE receptor 2; LDLR: low-density lipoprotein receptor; LRP1: LDL-receptor-related protein 1; LXR: liver X receptor; PCSK9: protein convertase subtilisin/kexin type 9; PKA: protein kinase A; RXR: retinoid X receptor; SR-BI: scavenger receptor class B type I; SREBP-2: sterol regulatory element-binding protein 2; VLDLR: very low density lipoprotein receptor.
With respect to lipid movement through the central and peripheral compartments, the BBB and the blood–cerebrospinal fluid barrier (BCSFB) act as semipermeable membranes, regulating the exchange of solutes between the blood and CNS [41][69]. Therefore, all lipoproteins, including LDL, VLDL, and chylomicrons, are excluded from the brain [42][70]. ApoA-I’s presence in the CSF derives from the blood circulating HDL [43][44][71,72]. The ABCA1 and ABCG1 transporters present on epithelial cells of the BBB can mediate the lipidation of peripheral apolipoproteins after their entry into the CNS [20][45][48,73], facilitating the formation of ApoE/ApoA-I small HDL-like particles [6][34]. Moreover, small HDL plasma particles can enter the brain via SR-BI-mediated uptake and transcytosis [6][34].
In contrast to cholesterol, the oxysterols 24-HC and 27-hydroxycholesterol (27-HC) (oxidized cholesterol metabolites) can cross the BBB and BCSFB at no energetic cost [46][74]. In that sense, 27-HC is synthesized by CYP27A1, representing the major cholesterol metabolite in the circulation. Its expression takes place in most of the organs and tissues [47][75] and can be transported by diffusion from the circulation into the brain [48][76]. Recent works support the idea that the pool of 27-HC contributes to cholesterol-related metabolite uptake in neurons [49][77]. On the other hand, due to the limited capacity of neurons and glial cell to eliminate cholesterol, spare cholesterol must reach the liver for further conversion to bile acids and final excretion. As previously stated, this function can rely on 24-HC, the main available hydrophilic form of cholesterol in the brain, to be transferred through the BBB [14][23][24][25][42,51,52,53]. 24-HC is originally released by neurons through the neuron-specific enzyme CYP46A1 [25][53]. A secondary excretion pathway through the BBB may involve cholesterol efflux mediated by ABCA1 and ABCG1 [6][50][34,78]. The steady-state levels of both oxysterols are tightly regulated in the brain. Therefore, disturbances in their concentrations have been associated with different forms of dementia [51][79]. In that sense, several works have evaluated plasma or CSF oxysterol levels in patients with AD, with evidence of impaired concentrations [52][53][54][80,81,82]. Particularly, 24-OHC has been found to regulate APP via the production of the amyloidogenic fragment [55][83], whereas 27-HC may contribute to amyloid deposition [46][74]. Higher levels of the metabolites 24-HC and 27-HC in the CSF suggest an increase in the cerebral cholesterol load, as observed in AD brains in postmortem examinations [56][84]. Overall, these results suggest a key role of these cholesterol metabolites in AD pathology. However, the relationship of HDL-like lipoproteins with these metabolites in the CNS remains largely unknown.
2. Apolipoprotein E in the CNS in the Context of AD
Excellent reviews focusing almost exclusively on ApoE and AD exist [57][58][59][60][61][62][63][64][65][66][85,86,87,88,89,90,91,92,93,94]. As previously stated, liver-synthesized ApoE cannot cross the BBB [67][95]. Thus, the ApoE in the CNS is synthesized by astrocytes and microglia [68][96]. In stressful situations, neurons can also produce ApoE [69][97]. It is worth noting that despite the fact that some authors refer to ApoE as a relevant AD component on its own, it is indeed a relevant part of the HDL-like lipoprotein structure that transports multiple proteins and lipid species, thereby affecting their CNS metabolism. Accordingly, ApoE is the main lipid carrier in the CNS, with functions that include the transport of lipids (mainly cholesterol) between neurons and glial cells via interactions with transporters such as ABCA1 and ABCG1 [70][98], the regulation of lipid metabolism [71][99], and the enhancement of axonal growth through LDLR interactions [32][60]. ApoE is also essential for the brain homeostasis-regulating processes of Aβ clearance as well as for the inhibition of inflammatory pathways, both functions of great importance in the AD brain, where Aβ oligomers and neuroinflammatory metabolites tend to accumulate [72][100]. Specifically, lipidated ApoE clears Aβ peptides from the brain [73][101]. In that sense, it is a well-established fact that the ApoE lipidation degree is positively associated with its affinity for soluble Aβ, with poorly lipidated ApoE obstructing Aβ clearance and stimulating Aβ deposition [74][75][76][77][25,102,103,104], thus representing a risk factor for AD.
3. HDL-like Lipoprotein Metabolism in the CNS
To understand the HDL-mediated cholesterol efflux and uptake processes through CNS cells in AD, a brief explanation of the biogenesis, remodeling, and delivery of HDL-like particles is provided below. Like plasma, the lipoproteins present in the CSF are composed of cholesterol, phospholipids, and apolipoproteins. Overall, their brain concentration is very low, representing approximately 1–10% of their plasma levels [78][132]. Brain lipoproteins present with a similar size and density to peripheral HDL particles, and therefore they are defined as “HDL-like particles” [78][132]. However, brain lipoproteins present with unique traits, including a wider size range (8–22 nm) and a different apolipoprotein composition, with ApoE being the major protein component [71][99]. In addition to ApoE, glial-derived discoidal HDL has ApoJ, whereas mature spherical CSF HDL has small amounts of ApoA-I. As stated above, ApoA-I is the most important constituent of plasma HDL; however, it is not produced in the brain and is, rather, delivered to the CNS through the BBB [79][133]. Other minoritarian apolipoproteins present in the brain include ApoA-II, ApoA-IV, ApoD, and ApoH [78][132]. Despite not being fully elucidated, the mechanisms involved in CSF lipoprotein synthesis and remodeling are similar to those observed for plasma HDL. It is well-known that astrocytes and microglia are responsible for the synthesis of most lipoproteins found in the brain and CSF [80][134]. Remodeling enzymes and lipid transfer proteins have also been identified in the CNS [81][135]. Lecithin-cholesterol acyltransferase (LCAT) converts UC and phosphatidylcholine to cholesteryl esters [82][136] via ApoE activation [83][137]. In fact, LCAT can be synthesized in the liver and testes as well as in astrocytes [83][137], suggesting its important role in the remodeling and maturation of nascent HDL-like proteins into larger spherical particles [71][99]. It has been found at concentrations representing around 5% of the levels in plasma [71][84][99,138]. Other remodeling enzymes include phospholipid transfer protein (PLTP), which is reported to be at a concentration representing about 15% of the plasma level [85][86][139,140], and cholesteryl ester transfer protein (CETP), which is reported to be present at about 12% of the level found in plasma [86][140] or non-existent [87][141]. Finally, as described above, cholesterol can be delivered to neurons by mature HDL-like lipoproteins through interactions with specific receptors [88][8]. A detailed description of HDL-like-mediated cholesterol trafficking in healthy and AD brains is shown in Figure 1.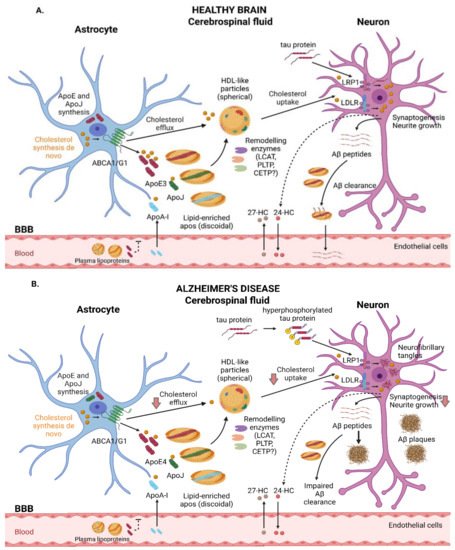
Figure 1. Schematic representation of the main steps involved in cholesterol trafficking in the brain. (A) In healthy subjects, astrocytes are responsible for de novo cholesterol and ApoE synthesis, with ApoE3 being the predominant isoform. Cholesterol efflux from astrocytes occurs, in part, through the ABCA1 and ABCG1 transporters. Lipid-free ApoE and, in smaller amounts, ApoA-I and ApoJ can be further lipidized by remodeling enzymes, resulting in spherical mature HDL-like particles that can interact with membrane receptors such as LRP1 and LDLR, leading to cholesterol uptake by neurons and guaranteeing essential functions such as synaptogenesis and neurite growth. Oxysterols can flux across the BBB. Neurons convert excess cholesterol in 24-HC, which can be eliminated to the bloodstream. In contrast, 27-HC enters the brain, where it promotes various functions. ApoE also contributes to the clearance of Aβ peptides. (B) In AD subjects, the pathological accumulation of hyperphosphorylated tau protein and Aβ plaque deposition may alter physiological functions in the brain. ApoE4, the predominant isoform in AD patients, is poorly lipidated and barely removes Aβ peptides. LRP1 plays a critical role in neuronal tau endocytosis. Recent works suggest alterations regarding cholesterol transport, including reduced HDL-like-mediated cholesterol efflux and impaired cholesterol uptake, leading to cell dysfunction. ABC: ATP-binding cassette; AD: Alzheimer’s disease; Apo: apolipoprotein; Aβ: amyloid beta; BBB: blood–brain barrier; CETP: cholesteryl ester transfer protein; HC: hydroxycholesterol; HDL: high-density lipoprotein; LCAT: lecithin-cholesterol acyltransferase; LDLR: low-density lipoprotein receptor; LRP1: LDLR-related protein 1.
