Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Gong LUO.
Electrocrystallization is a complicated multistep chain reaction involving the diffusion and mass transfer of ions in solution, the removal of solvation shells, electron transfer, the formation of surface adsorbed atoms, the clustering of adsorbed atoms, the generation of crystal nucleus, or embedding into existing lattice. A short historical background to study the nucleation and growth by diffusion-controlled electrocrystallization is provided.
- current transition
- nucleation and growth
- diffusion-controlled
- electrocrystallization
1. Introduction
One of the first topics within the context of electrochemistry is electrochemical metal deposition. With applications in domains such as electrodeposition and solution analysis, electrochemical deposition is a topic of great interest from both a theoretical and practical standpoint. As reviews in ‘Electrochimica Acta’ in 2000 [1] and ‘Journal of Electroanalytical Chemistry’ in 2003 [2] indicate, considerable work has been conducted on investigating the mechanism of such deposition over the last 70 years [3]. Metal electrodeposition occurs at electrode/electrolyte interfaces under the influence of an electric field and includes a number of phase formations. The initial stages in the electrochemical phase formation processes strongly depend on nucleation and growth. Through the study of electrocrystallization, Milchev et al. elaborated the thermodynamics and kinetics related to the electrochemical nucleation and growth of nanoclusters on solid surfaces and integrated the corresponding theoretical and experimental phenomena [4]. The crystal growth during electrodeposition directly determines the structure of deposits. The physical, chemical, electric, and magnetic characteristics of metal deposits such as metal films, modulated multilayers and sandwich structures, and low-dimensional metal systems are determined by nucleation-and-growth processes in electrochemical metal deposition. Future nanotechnology will heavily rely on electrochemically generated nanostructures, particularly for the creation of nanoelectronic devices such as quantum dots and single-electron devices as well as the creation of novel materials with unconventional properties [1]. The atomic structure of the deposit and the surface inhomogeneities of the substrate have a significant impact on the early phases of nucleation and growth processes. Native substrates do not require nucleation, and the growth method is influenced by the substrate’s perfection and the overpotential or supersaturation. The most crucial factors in the overall deposition mechanism on foreign substrates are the metal–substrate interaction, the crystallographic metal–substrate misfit, the overpotential, and the deposition rate. Nucleation is necessary for the majority of electrocrystallization processes.
Through nucleation and growth, electrodeposition takes place. Electrocrystallization is a typical nucleation and growth process in electrochemical metal deposition that has long attracted the interest of scientists and engineers [5,6,7,8,9][5][6][7][8][9]. According to the nucleation-rate rule, nuclei form at active sites on the substrate and expand as more ions from the solution are incorporated. The study of nucleation by electrochemical techniques has several benefits over other ways of investigating heterogeneous nucleation because electrocrystallization and electrochemical reaction occur at the same time. An important aspect of the study of the electrocrystallization mechanism is to establish the functional relationship between the metal-ion reduction current and microcrystallization kinetic process parameters, and to use the current function of electrocrystallization to study the microcrystallization kinetic process. Thus, the microcrystallization-and-growth process of a coating was analyzed, and the crystal-structure information of the electrodeposited coating was obtained including metal ion-mass transfer, electrodeposited crystal-nucleus density, coating grain size, and so on. However, considerable controversy remains over even the most basic principles involved in modeling and the current–time-functions of such systems, as discussed in recent studies [8,10,11,12,13,14][8][10][11][12][13][14].
2. Diffusion-Controlled Electrocrystallization Process
Electrodeposition is a complicated electrochemical process. Positive ions near the cathode obtain electrons and are reduced to metal atoms. Individual metal atoms adsorb onto one another to form metal nuclei and grow gradually. Finally, they can become visible metal deposits, which is called metal electrocrystallization. Electrocrystallization is a complicated multistep chain reaction involving the diffusion and mass transfer of ions in solution, the removal of solvation shells, electron transfer, the formation of surface adsorbed atoms, the clustering of adsorbed atoms, the generation of crystal nucleus, or embedding into existing lattice. The study of the dynamic process of electrocrystallization has always been an important content of electrocrystallization theory and has attracted considerable attention in the field of electrochemistry. The nucleation-and-growth process is the most important research content and has been widely researched [8,9][8][9].
In the early stage, electrodeposition theory focused on the crystal–growth interface structure of electrocrystallization and its effect on the formation of new phases on the electrode surface. Stranski [15] studied the difference of nucleation energy at different sites at the solid–liquid interface, indicating that the new phase was more easily generated at steps or terraces of multiple contact surfaces. According to the theory of electrodeposition microgrowth and the crystallization process, the electrodeposition process can be described as shown in Figure 1.
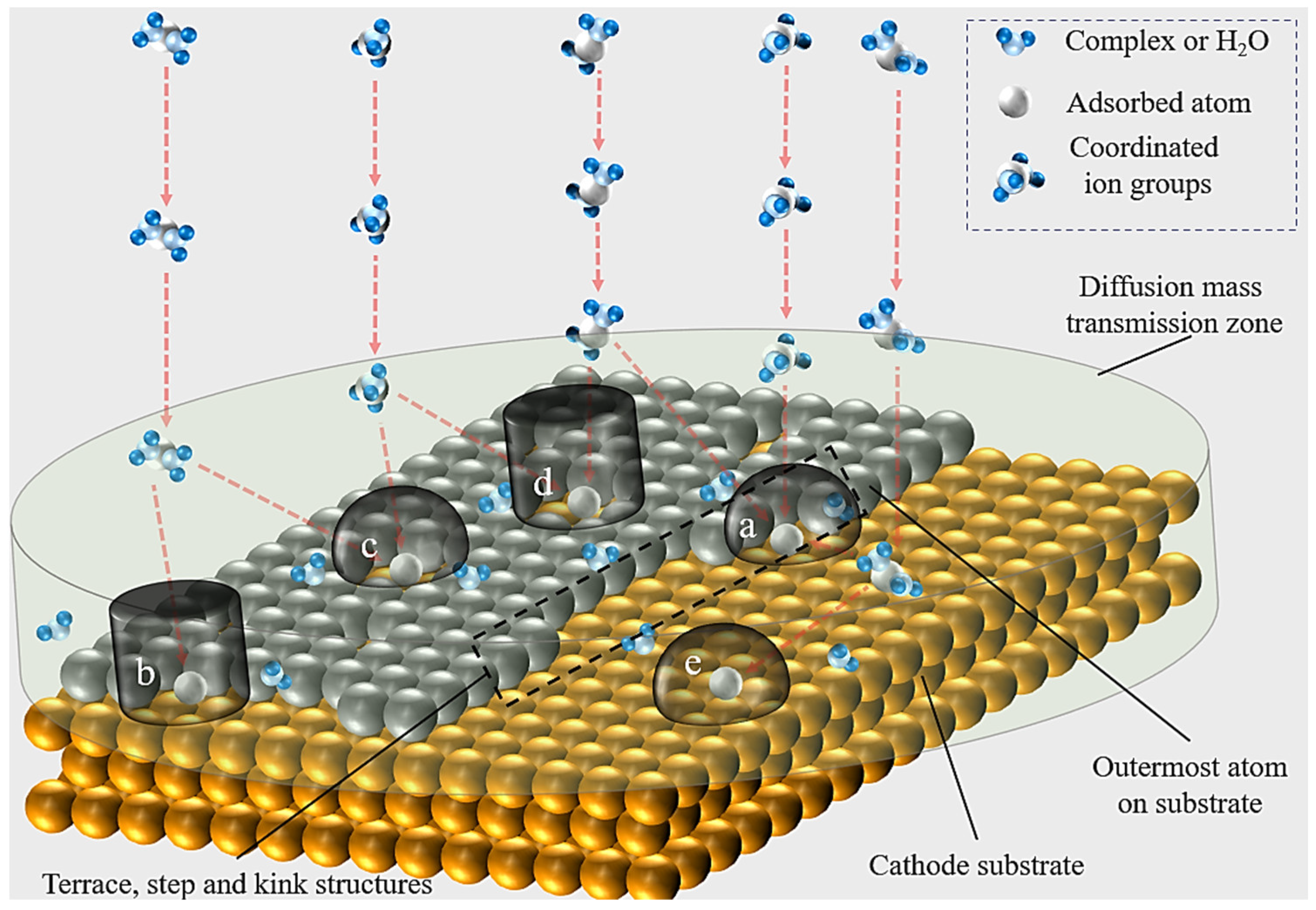
Figure 1. A schematic of the electrocrystallization process. (a,c) Hemispherical diffusion zone on the terrace, step, and kick sites. (b,d) Cylindrical diffusion zone on the terrace, step, and kink sites. (e) Cylindrical or hemispherical diffusion zone on the plane surface. The red dashed arrows represent the ion transport path.
With further research development, considering that electrocrystallization occurs at the solid–liquid interface, the electrochemical nucleation process inevitably produces partially charged particles (i.e., a partially hydrated molecule). Conwav et al. [16] calculated that the charge transfer was more likely to occur in the plane position of the electrode surface. The preferential growth of the lattice at the step or corner was proposed to be accompanied by the surface diffusion of adsorbed particles. Subsequently, Vermilyea and Fleischmann et al. [17,18][17][18] introduced the spiral-dislocation crystal-growth theory into the electrocrystallization theory to explain the spiral-dislocation growth observed on the electrodeposited copper surface [19,20][19][20].
Electrocrystallization is a multistep chain process whose reaction completion is controlled by the slowest step. Accordingly, researchers have studied rate-controlling steps to reflect the entire electrocrystallization process. Theoretically, any step of electrocrystallization can become a rate-controlling step. In fact, nucleation and growth can be broadly classified into two categories: ‘interfacial (or charge) controlled’, in which the nucleus-growth rate is limited by the rapidity with which ions can be incorporated into the new phase, and ‘diffusion controlled’, in which nucleus growth is limited by the rate at which a material is transported through the solution onto the electrode surface. Certain systems also tend toward one or the other due to their complex mechanisms. However, most electrochemical crystallization processes are performed under diffusion control. Furthermore, in the electroplating process controlled by diffusion mass transfer, most metal-electroplating processes are performed by 3D nucleation growth, except for a few metals that show single-layer deposition or two-dimensional nucleation growth such as silver electrodeposition [21,22,23][21][22][23]. This field has also received the greatest concern in the study of the electrocrystallization mechanism, and it is also the main aspect to be summarized in the next section of this paper.
The establishment and research of diffusion-controlled potentiostatic current transient modeling is based on spherical nucleation and growth. The general idea of establishing the model in the study was based on the mass-transfer process of a single-nucleus point. The calculation of current–time transformation on an electrode surface is also based on the current transformation of single-nucleus electrodeposition, which is then extended to the process of multinucleus interaction superposition. The establishment of the coating nucleation-and-growth model controlled by diffusion and mass transfer is usually described as follows. Nuclei are generally accepted to have a hemispherical shape. The radius ‘r’ of a hemispherical nucleus that grows under pure diffusion control is obtained by combining Faraday’s law with the time-independent part of the hemispherical diffusion equation. In other words, the nucleus has such a small size that it can be considered as an ultramicroelectrode. As the radii of the diffusion zones grow and overlap, the electrodeposition current decreases quickly. Many researchers have applied Avrami’s theorem to describe how diffusion zones grow and overlap on the electrode surface.
References
- Budevski, E.; Staikov, G.; Lorenz, W. Electrocrystallization: Nucleation and growth phenomena. Acta 2000, 45, 2559–2574. https://doi.org/10.1016/S0013-4686(00)00353-4.
- Hyde, M.E.; Compton, R.G. A review of the analysis of multiple nucleation with diffusion controlled growth. Electroanal. Chem. 2003, 549, 1–12. https://doi.org/10.1016/s0022-0728(03)00250-x.
- Grujicic, D.; Pesic, B. Electrodeposition of copper: The nucleation mechanisms. Acta 2002, 47, 2901–2912. https://doi.org/10.1016/s0013-4686(02)00161-5.
- Milchev, A. Electrocrystallization: Nucleation and growth of nano-clusters on solid surfaces. J. Electrochem. 2008, 44, 619–645. https://doi.org/10.1134/s1023193508060025.
- Yan, C.; Jiang, L.L.; Yao, Y.X.; Lu, Y.; Huang, J.Q.; Zhang, Q. Nucleation and Growth Mechanism of Anion-Derived Solid Electrolyte Interphase in Rechargeable Batteries. Chem. Int. Ed. 2021, 60, 8521–8525. https://doi.org/10.1002/anie.202100494.
- Xu, J.; Ren, W.; Lian, Z.; Yu, P.; Yu, H. A review: Development of the maskless localized electrochemical deposition technology. J. Adv. Manuf. Technol. 2020, 110, 1731–1757. https://doi.org/10.1007/s00170-020-05799-5.
- Macdonald, D.D. The history of the Point Defect Model for the passive state: A brief review of film growth aspects. Acta 2011, 56, 1761–1772. https://doi.org/10.1016/j.electacta.2010.11.005.
- Abyaneh, M.; Fleischmann, M.; Mehrabi, M. Modelling the growth of a single centre. Electroanal. Chem. 2019, 834, 114–123. https://doi.org/10.1016/j.jelechem.2018.11.030.
- German, S.R.; Edwards, M.A.; Ren, H.; White, H.S. Critical nuclei size, rate, and activation energy of H2 gas nucleation. Am. Chem. Soc. 2018, 140, 4047–4053. https://doi.org/10.1021/jacs.7b13457.
- Abyaneh, M.Y. Modelling diffusion controlled electrocrystallisation processes. Electroanal. Chem. 2006, 586, 196–203. https://doi.org/10.1016/j.jelechem.2005.10.004.
- Isaev, V.A.; Zaykov, Y.P.; Grishenkova, O.V.; Kosov, A.V.; Semerikova, O.L. Analysis of Potentiostatic Current Transients for Multiple Nucleation with Diffusion and Kinetic Controlled Growth. Electrochem. Soc. 2019, 166, D851–D856. https://doi.org/10.1149/2.1061915jes.
- Isaev, V.A.; Grishenkova, O.V.; Zaykov, Y.P. On the theory of 3D multiple nucleation with kinetic controlled growth. Electroanal. Chem. 2018, 818, 265–269. https://doi.org/10.1016/j.jelechem.2018.04.051.
- Yuan, Y.; Luo, G.; Li, N. New in situ description of electrodepositing multiple nucleation processes under galvanostatic stimuli. RSC Adv. 2021, 11, 31526–31532. https://doi.org/10.1039/d1ra04988g.
- Luo, G.; Li, D.; Yuan, G.; Li, N. Potentiostatic Current Transient for Multiple Nucleation: A Limited-Diffusion Process Description. Electrochem. Soc. 2018, 165, D147–D151. https://doi.org/10.1149/2.0711803jes.
- Stranski, I.N. Zur theorie des kristallwachstums. Phys. Chem. 2017, 136, 259–278. https://doi.org/10.1515/zpch-1928-13620.
- Conway, B.; Bockris, J.O. On the calculation of potential energy profile diagrams for processes in electrolytic metal deposition. Acta 1961, 3, 340–366. https://doi.org/10.1016/0013-4686(61)85009-3.
- Fleischmann, M.; Thirsk, H. Anodic electrocrystallization. Acta 1960, 2, 22–49. https://doi.org/10.1016/0013-4686(60)87005-3.
- Vermilyea, D. On the Theory of Electrolytic Crystal Growth. Chem. Phys. 1956, 25, 1254–1263. https://doi.org/10.1063/1.1743189.
- Burton, W.-K.; Cabrera, N.; Frank, F. The growth of crystals and the equilibrium structure of their surfaces. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1951, 243, 299–358. https://doi.org/10.1098/rsta.1951.0006.
- Seiter, H.; Fischer, H.; Albert, L. Elektrochemisch-morphologische studien zur erforschung des mechanismus der elektrokristallisation, fern vom anfangszustand. Acta 1960, 2, 97–120. https://doi.org/10.1016/0013-4686(60)87008-9.
- Budevski, E. Some Fundamental Aspects of Electrocrystallization. Surf. Membr. Sci. 1976, 11, 71–116. ISBN 9780125718110.
- Avrami, M. Kinetics of phase change. I General theory. Chem. Phys. 1939, 7, 1103–1112. https://doi.org/10.1063/1.1750380.
- Rangarajan, S. Electrocrystallisation: Multilayer formation and potentiostatic transients. Electroanal. Chem. Interfacial Electrochem. 1973, 46, 125–129. https://doi.org/10.1016/S0022-0728(73)80184-6.
References
- Budevski, E.; Staikov, G.; Lorenz, W. Electrocrystallization: Nucleation and growth phenomena. Electrochim. Acta 2000, 45, 2559–2574.
- Hyde, M.E.; Compton, R.G. A review of the analysis of multiple nucleation with diffusion controlled growth. J. Electroanal. Chem. 2003, 549, 1–12.
- Grujicic, D.; Pesic, B. Electrodeposition of copper: The nucleation mechanisms. Electrochim. Acta 2002, 47, 2901–2912.
- Milchev, A. Electrocrystallization: Nucleation and growth of nano-clusters on solid surfaces. Russ. J. Electrochem. 2008, 44, 619–645.
- Yan, C.; Jiang, L.L.; Yao, Y.X.; Lu, Y.; Huang, J.Q.; Zhang, Q. Nucleation and Growth Mechanism of Anion-Derived Solid Electrolyte Interphase in Rechargeable Batteries. Angew. Chem. Int. Ed. 2021, 60, 8521–8525.
- Xu, J.; Ren, W.; Lian, Z.; Yu, P.; Yu, H. A review: Development of the maskless localized electrochemical deposition technology. Int. J. Adv. Manuf. Technol. 2020, 110, 1731–1757.
- Macdonald, D.D. The history of the Point Defect Model for the passive state: A brief review of film growth aspects. Electrochim. Acta 2011, 56, 1761–1772.
- Abyaneh, M.; Fleischmann, M.; Mehrabi, M. Modelling the growth of a single centre. J. Electroanal. Chem. 2019, 834, 114–123.
- German, S.R.; Edwards, M.A.; Ren, H.; White, H.S. Critical nuclei size, rate, and activation energy of H2 gas nucleation. J. Am. Chem. Soc. 2018, 140, 4047–4053.
- Abyaneh, M.Y. Modelling diffusion controlled electrocrystallisation processes. J. Electroanal. Chem. 2006, 586, 196–203.
- Isaev, V.A.; Zaykov, Y.P.; Grishenkova, O.V.; Kosov, A.V.; Semerikova, O.L. Analysis of Potentiostatic Current Transients for Multiple Nucleation with Diffusion and Kinetic Controlled Growth. J. Electrochem. Soc. 2019, 166, D851–D856.
- Isaev, V.A.; Grishenkova, O.V.; Zaykov, Y.P. On the theory of 3D multiple nucleation with kinetic controlled growth. J. Electroanal. Chem. 2018, 818, 265–269.
- Yuan, Y.; Luo, G.; Li, N. New in situ description of electrodepositing multiple nucleation processes under galvanostatic stimuli. RSC Adv. 2021, 11, 31526–31532.
- Luo, G.; Li, D.; Yuan, G.; Li, N. Potentiostatic Current Transient for Multiple Nucleation: A Limited-Diffusion Process Description. J. Electrochem. Soc. 2018, 165, D147–D151.
- Stranski, I.N. Zur theorie des kristallwachstums. Z. Phys. Chem. 2017, 136, 259–278.
- Conway, B.; Bockris, J.O. On the calculation of potential energy profile diagrams for processes in electrolytic metal deposition. Electrochim. Acta 1961, 3, 340–366.
- Fleischmann, M.; Thirsk, H. Anodic electrocrystallization. Electrochim. Acta 1960, 2, 22–49.
- Vermilyea, D. On the Theory of Electrolytic Crystal Growth. J. Chem. Phys. 1956, 25, 1254–1263.
- Burton, W.-K.; Cabrera, N.; Frank, F. The growth of crystals and the equilibrium structure of their surfaces. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1951, 243, 299–358.
- Seiter, H.; Fischer, H.; Albert, L. Elektrochemisch-morphologische studien zur erforschung des mechanismus der elektrokristallisation, fern vom anfangszustand. Electrochim. Acta 1960, 2, 97–120.
- Budevski, E. Some Fundamental Aspects of Electrocrystallization. Prog. Surf. Membr. Sci. 1976, 11, 71–116.
- Avrami, M. Kinetics of phase change. I General theory. J. Chem. Phys. 1939, 7, 1103–1112.
- Rangarajan, S. Electrocrystallisation: Multilayer formation and potentiostatic transients. J. Electroanal. Chem. Interfacial Electrochem. 1973, 46, 125–129.
More
