Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Giuseppe Pappalardo and Version 2 by Peter Tang.
The amyloid hypothesis, i.e., the abnormal accumulation of toxic Aβ assemblies in the brain, has been considered the mainstream concept sustaining research in Alzheimer’s Disease (AD). However, the course of cognitive decline and AD development better correlates with tau accumulation rather than amyloid peptide deposition. Moreover, all clinical trials of amyloid-targeting drug candidates have been unsuccessful, implicitly suggesting that the amyloid hypothesis needs significant amendments. Accumulating evidence supports the existence of a series of potentially dangerous relationships between Aβ oligomeric species and tau protein in AD.
- Alzheimer’s Disease
- neurodegeneration
- amyloid
1. Introduction
At the end of 1901, Alois Alzheimer, a German neuropathologist, described the presence of neurofibrillary tangles (NFTs) and “senile plaques” in post-mortem neuronal tissues of a patient that experienced memory failure and gradual mental decline [1]. That pioneering article is considered the first report describing senile dementia, a chronic neurodegenerative condition that will be later commonly identified as Alzheimer’s Disease (AD). AD is known to be the prevalent form of dementia especially in aged people: 60–70% of all cases of dementia are diagnosed with AD [2] and about 32% of people 85 years old and older are affected by AD [3]. Presently, the different types of protein aggregates i.e., extracellular deposits of amyloid β (Aβ) peptide [4] and the intracellular hyperphosphorylated forms of tau protein, NFTs, [5] observed in AD brains represent the two distinctive pathological traits of AD [6]. Accumulating evidence suggests that Aβ plays a significant role in AD while human genetics established the relationship between tau malfunction and neurodegeneration. It is demonstrated that inherited Frontotemporal Dementia (FTD) and parkinsonism, with extensive filamentous tau deposits in the brain in the absence of Aβ deposits, are caused by mutations in the MAPT, the microtubule associated protein tau gene [7]. These pathological mutations involve tau hyperphosphorylation which leads to detachment of the functional protein from the microtubules, with consequent intracellular cumulation [8][9][8,9]. In AD, abnormal tau levels promote protein aggregation as paired helical filaments (PHFs) or straight filaments (SFs) in the cytosol [8][9][8,9].
Although initially Aβ and tau misfolding follows different routes and district of propagation, as the disease progresses, Aβ plaques and NFT are both formed in neocortical regions [10]. Tau levels have been also measured in the extracellular space where the protein can interact with Aβ [11].
As far as the intracellular trafficking of Aβ is concerned, it is quite clear that the peptide may be present in many cytosolic compartments. This is witnessed by observing that extracellular Aβ preparations are commonly used in in vitro AD experimental models [10]. Their toxicity is mediated via receptor recognition and death signaling, as well as intracellular internalization. Extracellular Aβ can be internalized in the cell via endocytic pathways aided by cell surface heparan sulfate or a variety of receptors and transporters such as the formyl peptide receptor-like protein 1 (FPRL1) or the scavenger receptor for advanced glycation end-products (RAGE) [12][13][14][12,13,14]. Interactions with intracellular proteins such as GM1, prefoldin (PFD), and other molecular chaperones redistribute the internalized Aβ through the neuronal bodies where interaction with tau protein may also occur [15][16][15,16].
The hypothesis of an existing crosstalk between tau and Aβ toxic aggregation is further supported by several studies [17]. Many reports have demonstrated that the toxic cross-talk between tau and Aβ aggregation initiates with the disruption of the mitochondrial membrane [18]. Aβ and tau have been found to co-localize in neurons and astrocytes. Particularly, Aβ accumulates primarily in synapses surrounding senile plaques, and synapses from tau-null animals are protected from Aβ damage [19]. On the other hand, a recent study reported that only 0.02% percent of synapses were found positive for both Aβ and tau, an evidence that argues against a direct Aβ/tau interaction at the synapses [20]. Therefore, this view has been questioned by some recent findings revealing that Aβ and tau accumulate in both pre- and postsynaptic terminals [20]. Remarkably, in both human AD and Drosophila models, tau has been found to attach to presynaptic vesicles, impairing neurotransmitter release [21], accordingly it’s becoming evident that Aβ has an impact on presynaptic function [22]. Intracellular Aβ/tau complexes could conceivably accelerate tau hyperphosphorylation and Aβ nucleation [23][24][23,24].
Therefore, tau/Aβ reciprocal interaction appears to influence the aggregation and toxicity of both molecules [25] and most of the above observations reinforce the idea that Aβ aggregated forms (i.e., plaques, oligomers) may provide a microenvironment that promotes tau aggregation and propagation fostering disease progression [26].
Nevertheless, the growing knowledge on the Aβ-tau cross interactions suggests that for a truly effective therapeutic approach to AD to be pursued, next generation drugs should target both Aβ and tau [27]. A possible approach to achieve this goal should also consider the environmental conditions, as well as the molecular partnerships the two Aβ and tau proteins might interact with.
2. Aβ and Tau Proteins: Molecular Structure and Physiological Functions
The high degree of conservation of the Aβ sequence among vertebrates (>90% sequence homology that reaches 95% in mammals), suggests that it must play an important role in the survival of the species. When present in low quantities, soluble Aβ isoforms play a vital physiological role in the CNS, contributing to normal brain function [28]. Due to its hydrophobic interaction with lipidic membranes, vesicles, and transmembrane receptors [29], as well as neurotrophic or neurotoxic effects depending on its concentration, monomeric Aβ homeostasis appears to be crucial in the modulation of synaptic function. In addition to its role in synapsis regulation, the Aβ monomer has neuroprotective properties that are mediated through the activation of several pathways. Aβ monomers have been shown to increase neuronal survival by activating the phosphatidylinositol-3-kinase (PI-3-K) pathway, which appears to be mediated by IGF-1/insulin receptor stimulation [30]. The activation of this route in neurons has been shown to cause functional synaptogenesis, or the development of new synapses [31]. Additional non-toxic yet atypical functions of soluble Aβ have been observed. These include: protective antimicrobial properties [32]; protection against cancer [33]; assisting the brain to recover from traumatic and ischemic injuries by participating to the blood-brain barrier repair; regulation of the synaptic function [34]. The two 40 or 42 residues long Aβ peptides (Aβ40 or Aβ42, respectively) are produced by the concerted proteolysis of the amyloid precursor protein (APP) [4][35][36][4,35,36]. APP processing may occur via two distinct pathways, i.e., non-amyloidogenic and amyloidogenic. The generation of Aβ peptides belongs to the activity of two transmembrane proteolytic enzymes (the β-secretase and the γ-secretase), on the membrane-bound APP. APP can be also processed by a different protease (α-secretase) that cleaves APP between amino acids 16 and 17 of the Aβ peptide, thus blocking Aβ peptides generation [37][38][37,38]. Aβ’s primary structure is composed of a hydrophilic N-terminal region (1–16) alongside a hydrophobic C-terminal domain (17–40/42). Tau is a microtubule-associated protein (MAP) mainly concentrated in the axons of neurons [39]. Six different tau isoforms are present in the brain, each one encompassing from 352 to 441 amino acids residues and including four tubulin-binding domains termed, R1, R2, R3 and R4. In general, tau primary structure can be dived into C-terminal domain, microtubule binding domain, N-terminal projection domain (alternative inserts 0N, 1N or 2N) and Pro-rich region domain (Figure 1). Tau, include 12 histidyl residues as potential metal binding sites [39]. Tau(273–284) and Aβ(25–35), containing both an hydrophobic hexapeptide sequence (VQIINK and GAIIGL, respectively), promote aberrant aggregates (see the section below) [40].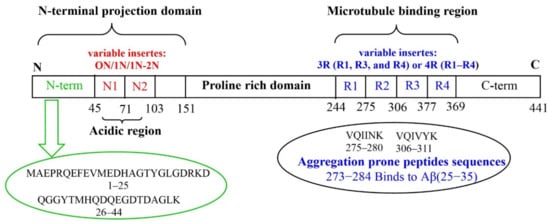
Figure 1.
Tau protein: longest tau isoform (441 amino acids) containing the variable inserts (N1, N2, R1, R2, R3 and R4).
