Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Eugenia Judith Olguín and Version 2 by Catherine Yang.
Microalgae-based biorefineries allow the simultaneous production of microalgae biomass enriched in a particular macromolecule and high-added and low-value products if a proper selection of the microalgae species and the cultivation conditions are adequate for the purpose.
- third-generation biorefineries
- multi-product biorefineries
- circular economy
1. Introduction
Microalgae have been recognized as a very important group of photosynthetic microorganisms for many of their attributes and the roles that they play in nature. Their importance as one of the major groups for CO2 sequestration and for releasing oxygen either in marine environments [1], in freshwater bodies [2], and even in arid and semiarid environments [3], has attracted the attention of several research groups for decades. On the other hand, microalgae have been identified as a very versatile and diverse group of organisms with a very high potential for application in biotechnology [1][4][1,4] due to their high capacity to produce and accumulate various macromolecules (proteins, carbohydrates, and lipids) with several uses in the food and feed industry, and more recently, as a source of biofuels [5][6][5,6]. Furthermore, microalgae are a very important source of several high-added value products such as pigments, PUFAs (poly-unsaturated fatty acids), peptides, exo-polysaccharides, among others, with a clear benefit to human health and nutrition [7][8][7,8] (Figure 1).
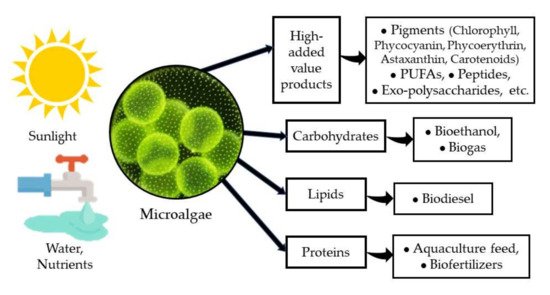
Figure 1. Microalgae and their products.
Despite all of these attributes that indicate that microalgae are a very useful feedstock for biofuels [9], food and feed supplements [10], and high-added value products [11], it has been recognized that several constraints still have to be overcome to make their large-scale production economically feasible, such as low productivity, risk of contamination in outdoor cultures, and high harvesting and downstream processing costs [12]. Thus, one of the strategies to overcome such drawbacks is their production following a “Biorefinery” concept.
A widely used Biorefinery definition is the one launched by the IEA (International Energy Agency) Bioenergy Task 42: “Biorefinery is the sustainable processing of biomass into a spectrum of marketable products (food, feed, materials, chemicals) and energy (fuels, power, heat)” [13], and it has been applied in recent decades to the processing of biomass for production of biofuels and chemical products with the expectation of reduced environmental impact [14][15][14,15]. It has drawn the attention of several research groups following different approaches. With the purpose of optimizing the environmental and economic performance of a biorefinery, a useful tool that combines the economic value and the environmental impact (EVEI) has been developed [16].
On the other hand, microalgae have been used for treating wastewater since many decades ago, when the pioneer group led by Prof. William Oswald initiated their use on a large scale in California [17]. Following this approach, research has been oriented toward the design and establishment of integrated systems in which microalgae were used for treating wastewater and producing valuable biomass [18][19][20][21][18,19,20,21]. Currently, microalgae-based biorefineries have been investigated by several research groups [22][23][24][22,23,24], and in some cases, such a type of biorefinery has integrated the treatment of wastewater and used some of the treated effluents or digestates as a source of nutrients [25][26][27][25,26,27].
It is important to mention that both the integrated systems for treating wastewater with microalgae and the microalgae-based biorefineries treating and using wastewater follow the circular economy concept (Figure 2). In fact, the term circular economy has been described as the efficient utilization of biomass, including waste, for the sustainable production of high-added and low-value products (e.g., food, feed, biomaterials, biofuels, biofertilizers, and biostimulants) with the benefit of lowering the carbon footprint and the valorization of waste materials [28].
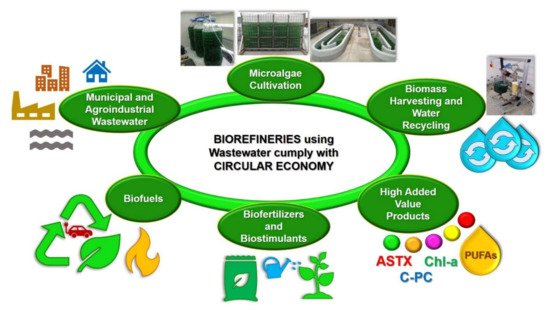
Figure 2.
Microalgae-based biorefineries using and treating wastewater, follow the Circular Economy concept.
2. Photoautotrophic Production of High-Added Value Products
Despite the large potential of several microalgae for producing high-added value products, only a few species are produced at a large scale under photoautotrophic conditions: Arthrospira sp., Chlorella sp., Dunaliella sp., Haematococcus sp., and Nannochloropsis sp. [29][30][31][32][33][29,30,31,32,33].
The production of food supplements or specific bioactive compounds gained interest for large-scale commercialization [34], mainly due to the demand in high-income countries. The genus Arthrospira sp. is the one which is being produced in the largest quantity (tons/year) and in many countries due to its high content of protein and other well-known high added-value compounds [35]. Earthrise Nutraceuticals® started production of this valuable cyanobacteria in California and Hawaii more than 40 years ago. Arthrospira is currently one of the most widely commercially cultivated microalgae in open raceway ponds worldwide, with China and the United States leading the global production, followed by Japan, Taiwan, Thailand, India, and Australia. According to a recent publication from Meticulous Research, the Arthrospira market is expected to record a compounded annual growth rate (CAGR) of 13.2% from 2021 to 2028 to reach USD 968.6 million dollars by 2028. In terms of volume, the Arthrospira market is expected to register a CAGR of 18.1% from 2021 to 2028 to reach 98,768.5 tons by 2028 [36][37][36,37].
The second genus produced ina very large quantity is Chlorella, also in various countries. Dunaliella sp. is being produced either as whole dried biomass or for the direct production of β-carotene. Haematococcus sp. has also been cultivated outdoors for the production of asthaxanthin, a very valuable pigment [35].
On the other hand, the production of pigments is still an attractive field of research, and there are several recent interesting reports dealing with different operational strategies (batch and semi-continuous cultures) and showing variable yield and productivity, depending on each strategy used and the strain of microalgae utilized. It is important to note that new information regarding new strains or new types of processes might lead to new commercial large-scale production worldwide.
3. Mixotrophic Production of High-Added Value and Low-Value Products Using Wastewater
Following the concept of circular economy and sustainable development, microalgae-based biorefineries treating and using wastewater have also been an important field of research in recent decades [5]. The initial trend was to produce biomass for biofuels production [25][26][27][38][25,26,27,52], and more recently, the trend is to produce biomass for biofuels and high-added value products [39][40][41][53,54,55]. Within this context, Arthrospira platensis, Haematococcus pluvialis, Chlorella sorokiniana, Dunaliella sp., and Nannochloropsis limnética, Nannochloropsis gaditana, among others, have been cultivated in municipal wastewater [42][43][44][45][56,57,58,59], industrial wastewater [46][47][48][60,61,62], and agroindustrial wastewater [49][50][51][63,64,65] for the production of some of the most demanded pigments (astaxanthin and β-carotene). There have been estimations that by using this type of mixotrophic culture, the cost of biomass could be less than 5 EUR/kg of biomass [52][53][66,67].4. Production of Microalgae Biomass Enriched in Carbohydrates
The photosynthetic metabolism of microalgae, as well as the metabolism of carbohydrates and lipids, are similar to those of terrestrial plants. However, the photosynthetic efficiency of microalgae is in the range of 6–10%, exceeding the 1–2% that of terrestrial plants [54][85]. Some authors mention that in most microalgae, starch accumulates as a primary source of energy and carbon reserve, while lipids serve as secondary storage [55][86]. Carbohydrate productivity in microalgae is usually higher than the lipid one, as the accumulation of lipids requires intense stress, whereas carbohydrate production is “easily” achieved by photosynthesis through the Calvin cycle and is markedly increased by nitrogen deficiency [56][87]. The contents of lipids, carbohydrates, and proteins depend on the type of microalgae and the environmental and nutritional conditions applied to the culture [57][58][59][88,89,90]. The ability of microalgae to produce energy-rich compounds, such as lipids and carbohydrates, has led them to be considered one of the most important raw materials used in the production of third-generation biofuels [57][88].5. Polysaccharides from Cyanobacteria for Developing New Bioactive Products
Many cyanobacteria are characterized by the presence, outside their outer cell membrane, of additional external structures of a polysaccharidic nature classified, according to their characteristics, as sheaths, capsules, and slimes. The sheath is a well-defined electron-dense thin layer that surrounds single cells, single filaments, or cell groups, reflecting their shape. The capsule is a gelatinous layer that is intimately associated with the cell surface and capable of absorbing large quantities of water. The capsule is characterized by sharp outlines and is structurally coherent to exclude particles. The slime is an amorphous, mucilaginous shroud, only loosely associated with the cell surface and capable of enclosing a large number of cells or filaments that may or may not be also surrounded by capsules. In the three cases, most exopolysaccharide (EPS)-producing cyanobacteria release into the culture medium during the growth or at the end of the linear phase of the growth, and large amounts of water-soluble polymeric material that was named released polysaccharides (RPS) [60][139]. Most of these polymers are characterized by an anionic nature, owing to the presence of uronic acids and/or other charged groups such as the sulphate or ketal-linked pyruvate groups [61][140]. Owing to the peculiar features of the polysaccharides produced by these microorganisms, EPS-producing cyanobacteria have been considered a promising resource of biopolymers of applied interest, and their exploitation to produce macromolecules suitable for specific industrial applications has been proposed in many studies. The specific features that have been considered of particular interest for the exploitation of cyanobacterial EPSs for developing new industrial products for biomedical applications are the following [61][140]:- (i)
-
Most of the polymers so far studied (up to now close to 200) are characterized by the presence in the macromolecule of a large number of different types of monosaccharides (in about 75% of the cases ranging from 6 up to 15), a feature rarely found in the EPS of other microorganisms. This feature makes it possible to have polymers with similar composition but organized in various structures and conformational forms, enlarging the chances of finding new products with useful properties;
- (ii)
-
In most of the polymers are present uronic acids and sulphate groups, which are rare in other bacterial EPS and confer an anionic charge to the polymers. In addition to that, the presence of sulphate groups has been considered relevant for conferring antiviral properties to the polymers [62][141];
- (iii)
-
The presence of peptides and deoxysugars, which confer hydrophobicity of some areas of the polymers;
- (iv)
-
The frequent presence of uncommon sugars, such as acetylated or amine sugars, which are considered possibly involved in polymers’ biological activity.
6. Microalgae-Bioactive Compounds, A Natural Source of Potential Therapeutical and Health Promoting Agents: Insights from Innovative In Vivo Functional Studies
Microalgae are light energy-based biofactories that synthesize high-value bioactive compounds, being the more representative examples with health relevance: proteins, pigments, polyphenols, antioxidants, polyunsaturated fatty acids, vitamins, minerals, sterols, and polysaccharides. These bioactive compounds have multiple health properties as antiviral, anticancer, antioxidant, antidiabetic, antibacterial, antifungal, anti-inflammatory, neuro, cardiorespiratory and hepatoprotective, among others [7][10][78][79][7,10,157,158]. Within this frame, herein, recent functional studies in animal of microalgae-bioactive compounds with their remarkable health-promoting biological activities are summarized. These innovative microalgae bioactivities revisited here support the relevance that algal biomass has gained and are nowadays considered a valuable source of health-promoting agents with potential nutritional, biomedical, and therapeutical applications. Furthermore, microalgae-bioactive compounds are potential health-promoting agents for the prevention and therapeutic application of relevant infectious, chronic, and degenerative diseases such as viral, fungal, bacterial infections, diabetes, metabolic, cardiorespiratory, cancer, inflammatory, neurodegenerative, among other pathologies.Algal Health-Promoting Agents
Microalgae-bioactive compounds have shown very important therapeutical bioactivities for the prevention or treatment of diseases as proven by functional studies in animals:-
Potential skin healing/antifibrotic agents:
-
Promising liver health-promoters:
-
Natural immunostimulators:
-
Non-toxic anticancer agents:
-
Improvement of nutrients deficiency:
-
Prevention of type 2 Diabetes Mellitus development:
-
Natural antioxidant agents:
