Tea and coffee are consumed worldwide and epidemiological and clinical studies have shown their health beneficial effects, including anti-cancer effects. Epigallocatechin gallate (EGCG) and chlorogenic acid (CGA) are the major components of green tea polyphenols and coffee polyphenols, respectively, and believed to be responsible for most of these effects. Although a large number of cell-based and animal experiments have provided convincing evidence to support the anti-cancer effects of green tea, coffee, EGCG, and CGA, human studies are still controversial and some studies have suggested even an increased risk for certain types of cancers such as esophageal and gynecological cancers with green tea consumption and bladder and lung cancers with coffee consumption.
- cancer
- tea
- coffee
- EGCG
1. Definition
Green tea is produced by processing of leaves of the plant
Camellia sinensis
(Theaceae) and is popularly consumed worldwide. Green tea has been shown to have beneficial effects on human health such as anti-cancer, anti-obesity, anti-diabetic, anti-cardiovascular, anti-infectious and anti-neurodegenerative effects
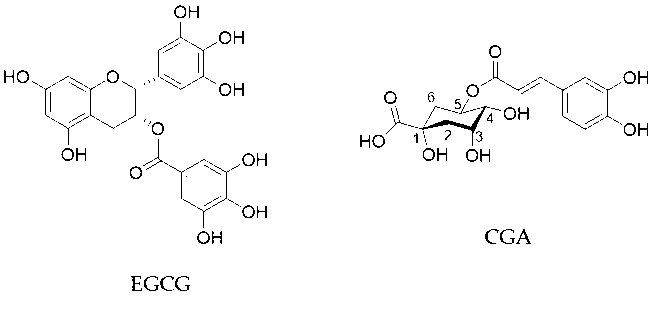
. (−)-Epigallocatechin gallate (EGCG) is the most abundant catechin in green tea and believed to be mostly responsible for these biological effects (Figure 1). A cup of green tea typically brewed from 2.5 g of tea leaves contains 240–320 mg of catechins, of which EGCG accounts for 60–65%
[3]
.
Black tea is produced also from
C. sinensis
through enzymatic processing (so called fermentation) by intrinsic enzymes and microorganisms during which catechins can be polymerized to give catechin derivatives such as theaflavins and theasinensins
[4]
. Black tea has been shown to have physiological effects similar to those of green tea with lesser effects as compared with green tea due to its lower content of EGCG.
Coffee is also consumed worldwide and has various health effects. It contains about 2000 different chemicals and the major polyphenols are chlorogenic acid (CGA, Figure 1) and its derivatives which amount to about 3% w/w of roasted coffee powder
. A single cup of coffee may contain 20–675 mg of CGAs
[6]
.
Figure 1. Chemical structures of EGCG and CGA.
2. Anti-Cancer Effects of Green Tea
Several epidemiological studies have shown the anti-cancer effects of consumption of tea. A survey in 2013 conducted by Yang and Hong of prospective cohort and case-controlled studies which had been reported by 2008 revealed that green tea consumption showed risk-reduction in a total of 39 cases of breast, colon, esophagus, kidney/bladder, lung, ovary, pancreas, prostate, stomach cancers, whereas 46 cases showed no risk-reduction [1,7]. In the case of black tea, 28 and 92 cases showed risk-reduction and no risk-reduction, respectively, for these cancers
Several epidemiological studies have shown the anti-cancer effects of consumption of tea. A survey in 2013 conducted by Yang and Hong of prospective cohort and case-controlled studies which had been reported by 2008 revealed that green tea consumption showed risk-reduction in a total of 39 cases of breast, colon, esophagus, kidney/bladder, lung, ovary, pancreas, prostate, stomach cancers, whereas 46 cases showed no risk-reduction [1][7]. In the case of black tea, 28 and 92 cases showed risk-reduction and no risk-reduction, respectively, for these cancers
[7]
. These findings suggest that green and black teas have a preventive effect in some types of cancer.
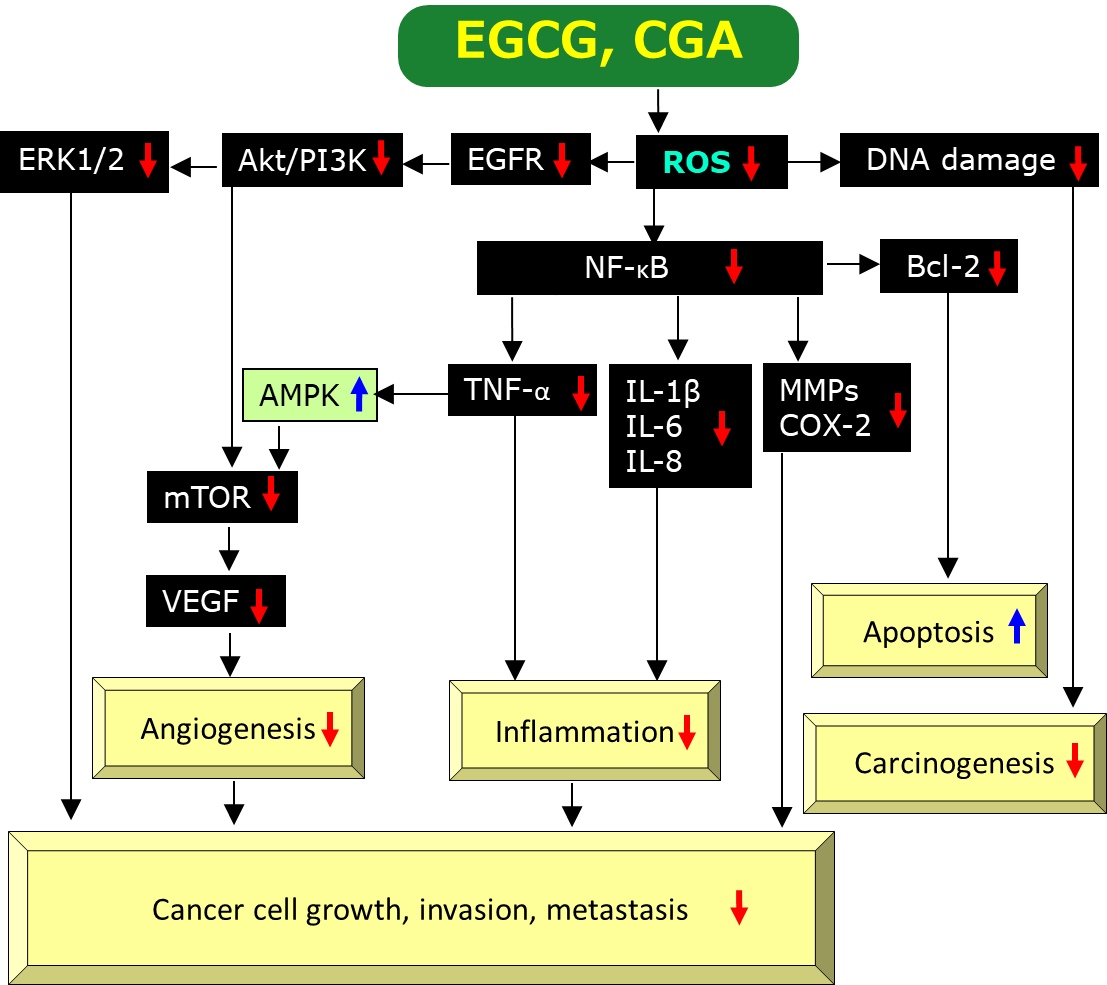
EGCG is a prominent anti-oxidant and quenches reactive oxygen species (ROS), which facilitate oxidative DNA damage, mutagenesis, and tumor promotion, leading to anti-cancer effects
[8]
. EGCG can exhibit anti-oxidant activity through several mechanisms including catalytic metal chelation, hydrogen atom transfer, and electron transfer. Chemically, the anti-oxidant activity of EGCG can be interpreted by the existence of the polyhydroxyl structure and the gallate group which play key roles to scavenge free radicals and by the presence of phenolic groups with sensitivity to be oxidized, resulting in generation of a quinone
.
Figure 2. A possible mechanism by which EGCG and CGA exert anti-cancer effects via scavenging/downregulation of ROS. Red↓and blue↑marks represent downregulation/suppression and upregulation/stimulation, respectively.
Chronic inflammation is thought to have an important role on the onset and progression of human cancer by modulating the tumor microenvironment
[11]
. A number of studies have provided evidence EGCG’s anti-inflammatory effects. These studies found that EGCG can inhibit activation of transcription factors such as nuclear factor-κB (NF-κB), activating protein-1, MyD88-dependent signaling pathway, Toll-interleukin-1 receptor domain-containing adaptor inducing interferon-β-dependent signaling pathways of Toll-like receptors, and expressions of inflammatory genes including cyclooxygenase (COX), nitric oxide synthase, and tumor necrosis factor-α (TNF-α)
.
Angiogenesis is the process characterized by the development of new blood vessels from the pre-existing vessels, which supply a tumor with oxygen and nutrients to allow optimal growth. Anti-angiogenesis is thought to be one of the most promising methods of cancer treatment
[15]
.
Cancer cells can adopt to the hypoxic microenvironment by expressing hypoxia-inducible factors-1 (HIF-1) and thereby increasing the levels of its downstream target vascular endothelial growth factor (VEGF), which promotes tumor growth, angiogenesis, and metastasis
. EGCG was shown to decrease the protein expression of HIF-1α and VEGF proteins in gastric cancer SGC7901 cells under hypoxia induced by cobalt chloride
[18]
.
Induction of apoptosis or programmed cell death is one of the most important mechanisms for EGCG to exert anti-cancer effects. Several studies have provided evidence for the induction of apoptosis by EGCG and its mechanism of action. ROS can stimulate gene expression of B-cell lymphoma-2 (Bcl-2) via activation of NF-κB and therefore, EGCG’s scavenging activity of ROS is expected to downregulate the anti-apoptotic protein Bcl-2 (Figure 2), leading to apoptotic cell death of cancer cells (Figure 2).
3. Anti-Cancer Effects of Coffee
A clinical trial with 10 participants found that consumption of 1L unfiltered coffee/day over 5 days resulted in a weak induction of glutathione-S-transferases (GSTs) and 3-fold increase in induction of placental type GST in blood, although other clinical markers for organ damage such as creatinine, aminotransferases, and alkaline phosphatase were not altered
[19]
. The finding suggests that coffee’s induction of placental type GST may lead to protection from chemical carcinogenesis.
In a controlled intervention trial with a cross-over design with 38 participants, consumption of 800 mL coffee daily over 5 days demonstrated the decrease by 12.3% in the extent of DNA-migration attributable to formation of oxidized purines, although other biochemical parameters such as the total anti-oxidant levels in plasma, glutathione concentrations in blood, and the activities of superoxide dismutase and glutathione peroxidase in lymphocytes were not markedly altered. The result indicates that coffee consumption prevents endogenous formation of oxidative DNA-damage in human
[20]
.
Recent evidence has also suggested that coffee drinking may have health benefits on some types of cancer. A review by an International Agency for Research on Cancer working group conducted in 2016 on a large number of epidemiological and experimental studies on anti-cancer effects of coffee found an inverse association for liver and endometrial cancers
[21]
.
In addition, a recent meta-analysis of observational studies on associations between coffee intake and 26 different cancers including 364,749 cancer cases provided evidence to show that coffee intake is inversely associated with endometrial cancer, liver cancer, melanoma, oral cancer, and oral/pharyngeal cancer
[22]
. Additional evidence was also obtained to suggest the reduced risk of cancers of the mouth, pharynx and larynx, and skin cancer. Coffee consumption may also be inversely associated with breast, colon, colorectal, esophageal and nonmelanoma skin cancers.
4. Comparison of Anti-Cancer Effects of Tea and Coffee in Simultaneous Human Studies
Table 1 shows a brief comparison of anti-cancer effects of tea and coffee in simultaneous studies reported since 2018 based on the Medline data base. Several investigations revealed that tea and coffee may have different effects in some cancer types. It is noticeable that coffee may increase a risk in certain types of cancer (bladder cancer, lung cancer, and childhood leukemia) in line with the finding from aforementioned studies which examined effects of either tea or coffee, individually
[22]
.
The reason for the difference is not known at present. As pointed out by Milne et al.
[23]
, the fact that both tea and coffee contain numerous different compounds, are prepared by various methods, and have differences in bioavailability makes it difficult to determine the factor(s) involved in the difference.
Table 1. Comparison of anticancer effects in humans between tea and coffee.
|
Cancer Type |
Tea/Green Tea/Black Tea * |
Coffee/Caffeinated Coffee/Decaffeinated Coffee * |
Type of Epidemiological Study [Reference] |
|
Bladder |
↓ |
+/− |
Cohort study[24] |
|
Bladder |
+/− |
↑ |
Meta-analysis of cohort study and case-control study [25] |
|
Brain |
↓ |
↓ |
Meta-analysis of cohort study and case-control study [26] |
|
Breast |
+/− |
+/− |
Cohort study [27] |
|
Colorectal |
+/− |
+/− |
Cohort study [28] |
|
Colorectal |
↓ |
+/− |
Case-control study [29] |
|
Endometrial |
+/− |
↓ |
Case-control study [27] |
|
Glioma |
↓ |
+/− |
Cohort study [30] |
|
Glioma |
↓ |
↓ |
Case-control study [31] |
|
Leukemia, acute myeloid |
+/− |
+/− |
Cohort study [32] |
|
Leukemia, childhood acute myeloid |
+/− |
↑ |
Meta-analysis of case-control study [33] |
|
Leukemia, childhood acute lymphoblastic |
+/− |
↑ |
Meta-analysis of case-control study [23] |
|
Liver |
+/− |
↓ |
Cohort study [34] |
|
Liver |
+/− |
↓ |
Meta-analysis of cohort study and case-control study [35] |
|
Lung |
↓ |
↑ |
Cohort study [36] |
|
Lymphoma, non-Hodgikin’s |
↓ |
+/− |
Meta-analysis of cohort study and case-control study [37] |
|
Melanoma, cutaneous |
+/− |
↓ |
Meta-analysis of cohort study [38] |
|
Ovarian |
+/− |
+/− |
Cohort study [27] |
|
Prostate |
+/− |
+/− |
Cohort study [39] |
|
Renal cell carcinoma |
+/− |
+/− |
Cohort study [24] |
|
Skin cancer, non-melanoma |
↓ |
↓ |
Cohort study [40] |
|
Stomach |
+/− |
+/− |
Meta-analysis of cohort study and case-control study [41] |
|
Thyroid |
+/− |
+/− |
Cohort [42] |
* Risk decrease, risk increase and no effect are shown by ↓, ↑, and +/−, respectively.
References
- Suzuki, T.; Miyoshi, N.; Hayakawa, S.; Imai, S.; Isemura, M.; Nakamura, Y. Health benefits of tea consumption. In Beverage Impacts on Health and Nutrition; Springer International Publishing: Cham, Switzerland, 2016; pp. 49–67. ISBN 978-3-319-23672-8.
- Hayakawa, S.; Oishi, Y.; Tanabe, H.; Isemura, M.; Suzuki, Y. Tea, Coffee and Health Benefits. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–58. ISBN 978-3-319-78029-0.
- Yang, C.S.; Zhang, J.; Zhang, L.; Huang, J.; Wang, Y. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Mol. Nutr. Food Res. 2016, 60, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Matsuo, Y.; Kouno, I. Biochemical and physicochemical characteristics of green tea polyphenols. In Green Tea Polyphenols; CRC Press: Boca Raton, FL, USA, 2013; pp. 19–38. [Google Scholar]
- Cavalli, L.; Tavani, A. Coffee consumption and its impact on health. In Beverage Impacts on Health and Nutrition; Springer International Publishing: Cham, Switzerland, 2016; pp. 29–47. [Google Scholar]
- Cano-Marquina, A.; Tarín, J.J.; Cano, A. The impact of coffee on health. Maturitas 2013, 75, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Hong, J. Prevention of chronic diseases by tea: Possible mechanisms and human relevance. Annu. Rev. Nutr. 2013, 33, 161–181. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef]
- Shirakami, Y.; Shimizu, M. Possible mechanisms of green tea and its constituents against cancer. Molecules 2018, 23, 2284. [Google Scholar]
- Khan, N.; Mukhtar, H. Tea polyphenols in promotion of human health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef]
- Stark, T.; Livas, L.; Kyprianou, N. Inflammation in prostate cancer progression and therapeutic targeting. Transl. Androl. Urol. 2015, 4, 455–463. [Google Scholar]
- Hayakawa, S.; Saito, K.; Miyoshi, N.; Ohishi, T.; Oishi, Y.; Miyoshi, M.; Nakamura, Y. Anti-cancer effects of green tea by either anti- or pro- oxidative mechanisms. Asian Pac. J. Cancer Prev. 2016, 17, 1649–1654. [Google Scholar] [CrossRef]
- Ohishi, T.; Goto, S.; Monira, P.; Isemura, M.; Nakamura, Y. Anti-inflammatory action of green tea. Antiinflamm. Antiallergy Agents Med. Chem. 2016, 15, 74–90. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Kao, C.-L.; Liu, C.-M. The cancer prevention, anti-inflammatory and anti-oxidation of bioactive phytochemicals targeting the TLR4 Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 2729. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, Q. Therapeutic targets of multiple angiogenic factors for the treatment of cancer and metastasis. Adv. Cancer Res. 2007, 97, 203–224. [Google Scholar] [PubMed]
- Bos, R.; Zhong, H.; Hanrahan, C.F.; Mommers, E.C.; Semenza, G.L.; Pinedo, H.M.; Abeloff, M.D.; Simons, J.W.; van Diest, P.J.; van der Wall, E. Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J. Natl. Cancer Inst. 2001, 93, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Koshikawa, N.; Iyozumi, A.; Gassmann, M.; Takenaga, K. Constitutive upregulation of hypoxia-inducible factor-1alpha mRNA occurring in highly metastatic lung carcinoma cells leads to vascular endothelial growth factor overexpression upon hypoxic exposure. Oncogene 2003, 22, 6717–6724. [Google Scholar] [CrossRef]
- Fu, J.-D.; Yao, J.-J.; Wang, H.; Cui, W.-G.; Leng, J.; Ding, L.-Y.; Fan, K.-Y. Effects of EGCG on proliferation and apoptosis of gastric cancer SGC7901 cells via down-regulation of HIF-1α and VEGF under a hypoxic state. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 155–161. [Google Scholar]
- Steinkellner, H.; Hoelzl, C.; Uhl, M.; Cavin, C.; Haidinger, G.; Gsur, A.; Schmid, R.; Kundi, M.; Bichler, J.; Knasmüller, S. Coffee consumption induces GSTP in plasma and protects lymphocytes against (+/−)-anti-benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide induced DNA-damage: Results of controlled human intervention trials. Mutat. Res. 2005, 591, 264–275. [Google Scholar] [CrossRef]
- Mišík, M.; Hoelzl, C.; Wagner, K.-H.; Cavin, C.; Moser, B.; Kundi, M.; Simic, T.; Elbling, L.; Kager, N.; Ferk, F.; et al. Impact of paper filtered coffee on oxidative DNA-damage: Results of a clinical trial. Mutat. Res. 2010, 692, 42–48. [Google Scholar] [CrossRef]
- Loftfield, E.; Freedman, N.D. Coffee and digestive cancers-what do we know, and where do we go? Br. J. Cancer 2020, 122, 1273–1274. [Google Scholar] [CrossRef]
- Zhao, L.-G.; Li, Z.-Y.; Feng, G.-S.; Ji, X.-W.; Tan, Y.-T.; Li, H.-L.; Gunter, M.J.; Xiang, Y.-B. Coffee drinking and cancer risk: An umbrella review of meta-analyses of observational studies. BMC Cancer 2020, 20, 101. [Google Scholar] [CrossRef]
- Milne, E.; Greenop, K.R.; Petridou, E.; Bailey, H.D.; Orsi, L.; Kang, A.Y.; Baka, M.; Bonaventure, A.; Kourti, M.; Metayer, C.; et al. Maternal consumption of coffee and tea during pregnancy and risk of childhood all: A pooled analysis from the childhood Leukemia International Consortium. Cancer Causes Control 2018, 29, 539–550. [Google Scholar] [CrossRef]
- Hashemian, M.; Sinha, R.; Murphy, G.; Weinstein, S.J.; Liao, L.M.; Freedman, N.D.; Abnet, C.C.; Albanes, D.; Loftfield, E. Coffee and tea drinking and risk of cancer of the urinary tract in male smokers. Ann. Epidemiol. 2019, 34, 33–39. [Google Scholar] [CrossRef]
- Hong, X.; Xu, Q.; Lan, K.; Huang, H.; Zhang, Y.; Chen, S.; Chi, Z.; Lin, J.; Zhou, Y.; Wu, W.; et al. The effect of daily fluid management and beverages consumption on the risk of bladder cancer: A meta-analysis of observational study. Nutr. Cancer 2018, 70, 1217–1227. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Jin, Y.; Guo, J. Association between tea and coffee consumption and brain cancer risk: An updated meta-analysis. World J. Surg. Oncol. 2019, 17, 51. [Google Scholar] [CrossRef]
- Arthur, R.; Kirsh, V.A.; Rohan, T.E. Associations of coffee, tea and caffeine intake with risk of breast, endometrial and ovarian cancer among Canadian women. Cancer Epidemiol. 2018, 56, 75–82. [Google Scholar] [CrossRef]
- Bradbury, K.E.; Murphy, N.; Key, T.J. Diet and colorectal cancer in UK Biobank: A prospective study. Int. J. Epidemiol. 2020, 49, 246–258. [Google Scholar] [CrossRef]
- Quang, L.N.; Hien, N.Q.; Quang, N.T.; Chung, N.T. Active lifestyle patterns reduce the risk of colorectal cancer in the north of Vietnam: A hospital-based case-control study. Cancer Control 2019, 26, 1073274819864666. [Google Scholar] [CrossRef] [PubMed]
- Cote, D.J.; Bever, A.M.; Wilson, K.M.; Smith, T.R.; Smith-Warner, S.A.; Stampfer, M.J. A prospective study of tea and coffee intake and risk of glioma. Int. J. Cancer 2020, 146, 2442–2449. [Google Scholar] [CrossRef] [PubMed]
- Malmir, H.; Shayanfar, M.; Mohammad-Shirazi, M.; Tabibi, H.; Sharifi, G.; Esmaillzadeh, A. Tea and coffee consumption in relation to glioma: A case-control study. Eur. J. Nutr. 2019, 58, 103–111. [Google Scholar] [CrossRef]
- Ugai, T.; Matsuo, K.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Shimazu, T.; Goto, A.; Inoue, M.; Kanda, Y.; Tsugane, S.; et al. Coffee and green tea consumption and subsequent risk of acute myeloid leukemia and myelodysplastic syndromes in Japan. Int. J. Cancer 2018, 142, 1130–1138. [Google Scholar] [CrossRef]
- Karalexi, M.A.; Dessypris, N.; Clavel, J.; Metayer, C.; Erdmann, F.; Orsi, L.; Kang, A.Y.; Schüz, J.; Bonaventure, A.; Greenop, K.R.; et al. Coffee and tea consumption during pregnancy and risk of childhood acute myeloid leukemia: A Childhood Leukemia International Consortium (CLIC) study. Cancer Epidemiol. 2019, 62, 101581. [Google Scholar] [CrossRef]
- Tamura, T.; Wada, K.; Konishi, K.; Goto, Y.; Mizuta, F.; Koda, S.; Hori, A.; Tanabashi, S.; Matsushita, S.; Tokimitsu, N.; et al. Coffee, green tea, and caffeine intake and liver cancer risk: A prospective cohort study. Nutr. Cancer 2018, 70, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Tamakoshi, A.; Sugawara, Y.; Mizoue, T.; Inoue, M.; Sawada, N.; Matsuo, K.; Ito, H.; Naito, M.; Nagata, C.; et al. Coffee, green tea and liver cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 2019, 49, 972–984. [Google Scholar] [CrossRef]
- Seow, W.J.; Koh, W.-P.; Jin, A.; Wang, R.; Yuan, J.-M. Associations between tea and coffee beverage consumption and the risk of lung cancer in the Singaporean Chinese population. Eur. J. Nutr. 2019, 59, 3083–3091. [Google Scholar] [CrossRef]
- Mirtavoos-Mahyari, H.; Salehipour, P.; Parohan, M.; Sadeghi, A. Effects of coffee, black tea and green tea consumption on the risk of non-Hodgkin’s lymphoma: A systematic review and dose-response meta-analysis of observational studies. Nutr. Cancer 2019, 71, 887–897. [Google Scholar] [CrossRef]
- Fortes, C. Are anti-inflammatory foods associated with a protective effect for cutaneous melanoma? Eur. J. Cancer Prev. 2020. [Google Scholar] [CrossRef]
- Sen, A.; Papadimitriou, N.; Lagiou, P.; Perez-Cornago, A.; Travis, R.C.; Key, T.J.; Murphy, N.; Gunter, M.; Freisling, H.; Tzoulaki, I.; et al. Coffee and tea consumption and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2019, 144, 240–250. [Google Scholar] [CrossRef]
- Oh, C.C.; Jin, A.; Yuan, J.-M.; Koh, W.-P. Coffee, tea, caffeine, and risk of nonmelanoma skin cancer in a Chinese population: The Singapore Chinese health study. J. Am. Acad. Dermatol. 2019, 81, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Poorolajal, J.; Moradi, L.; Mohammadi, Y.; Cheraghi, Z.; Gohari-Ensaf, F. Risk factors for stomach cancer: A systematic review and meta-analysis. Epidemiol. Health 2020, 42, e2020004. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Alghamdi, M.A.; Cayssials, V.; Franceschi, S.; Almquist, M.; Hennings, J.; Sandström, M.; Tsilidis, K.K.; Weiderpass, E.; Boutron-Ruault, M.-C.; et al. Coffee and tea drinking in relation to the risk of differentiated thyroid carcinoma: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2019, 58, 3303–3312. [Google Scholar] [CrossRef] [PubMed]