The ability to measure and monitor the concentration of specific chemical and/or gaseous species (i.e., “analytes”) is the main requirement in many fields, including industrial processes, medical applications, and workplace safety management. As a consequence, several kinds of sensors have been developed in the modern era according to some practical guidelines that regard the characteristics of the active (sensing) materials on which the sensor devices are based. These characteristics include the cost-effectiveness of the materials’ manufacturing, the sensitivity to analytes, the material stability, and the possibility of exploiting them for low-cost and portable devices. Consequently, many gas sensors employ well-defined transduction methods, the most popular being the oxidation (or reduction) of the analyte in an electrochemical reactor, optical techniques, and chemiresistive responses to gas adsorption. MIn recent years, many of the efforts devoted to improving these methods have been directed towards the use of certain classes of specific materials.
- gas sensors
- ionic liquids
- metal–organic frameworks
- MOF-based composites
- optical sensors
- chemiresistors
- electrochemical sensors
- oxygen
- hydrogen
- chemical sensing
1. Introduction
| Sensor Type | Examples | Principle of Operation |
|---|---|---|
| Electrochemical | Amperometric, ChemFET |
Analyte molecules are involved in the redox reaction at the working electrode of an electrochemical cell, modulating the electrical current. |
| Analyte | Ionic Liquid | Electrode | Analyte Concentration | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O2 | ||||||||||
| Electrical | Chemoresistors | Adsorbed molecules of the target gas interact with oxygen species adsorbed on the surface of a nanoparticulated semiconductor, modifying its charge depletion regions and its electrical conductivity. | ||||||||
| [C4mpyrr][NTf2] | Clark-type sensor with polycrystalline Pt gauze | 1–20% | [36] | [83] | Gravimetric | Surface acoustic waves, piezoelectric |
A vibration resonance frequency is modified due to the adsorption of the target analyte. The shift in resonance frequency quantifies the analyte concentration. | |||
| [C2mim][NTf2] and [C4mpyrr][NTf2] |
Screen-printed (SP) electrodes | 10–100% and 0.1–5% | [37] | [84] | Thermochemical | Catalytic bead sensors | The target gas is burnt, causing a temperature rise that changes the resistance of the detecting element of the sensor proportional to the concentration of combusted gas. | |||
| Optical | Absorptive Reflective Fluorescence-based |
Adsorbed molecules of the target gas modify in several ways the optical properties of the sensing material (e.g., reflectivity, optical transmission, fluorescence spectrum and/or lifetime, etc.). | ||||||||
2. Ionic Liquids in Amperometric Gas Sensing—Recent Developments
| [N8,2,2,2][NTf2] | ||||||||||||
| Pt MATFE | ||||||||||||
| 10–100% | [ | 38 | ] | [85] | ||||||||
| [C2mim][NTf2] | Pt microdisk and Pt MATFE | 0.1–100% | [39] | [86] | ||||||||
| [MOmim][PF6] | Au microchannel electrode |
5000–25,000 ppm | [40] | [87] | ||||||||
| [Bmim][BF4] | Au interdigitated electrodes | 20–100% | [41] | [88] | ||||||||
| [C4mpyrr][NTf2] | Au on porous PTFE substrate | 5–20% | [42] | [89] | ||||||||
| [C2mim][NTf2] and [C4mim][PF6] |
SP electrodes (graphite) | 0.1–20% and 100% | [43] | [90] | ||||||||
| [C4mpyrr][NTf2] | Au microchannel electrode | 50–400 ppm and 2000–5000 ppm |
[44] | [91] | ||||||||
| [C4mpyrr][NTf2] | Clark-type sensor with polycrystalline Pt gauze | 5–20% | [45] | [92] | ||||||||
| [C4mpyrr][NTf2] | Interdigitated electrodes | 1400–4800 ppm | [46] | [93] | ||||||||
| [C4mim][PF6], [C2mim][PF6] and [C5mim][PF6] | Pt interdigitated electrodes | 0–100% | [47] | [94] | ||||||||
| [C4mim][BF4] | Planar electrodes | 20–100% | [48] | [95] | ||||||||
| [Bmim][BF6] | Pt planar electrodes modified by NiCo2O4/rGO/[Bmim][BF6] composite | 20–100% | [49] | [96] | ||||||||
| [C4mpn][Br] | Pt microelectrodes, 1% Ag-coated chitosan added to the IL | 20–100% | [50] | [97] | ||||||||
| [Bmim][BF4] | SPE, solid polymer electrolyte (PTFE/Carbon nanotubes/IL) | 2.1–12.6% | [51] | [98] | ||||||||
| [Emmim][TFSI] and [Bmim][TFSI] |
Pt electrodes, IL + reduced graphene (rGO) + α-Fe | 2 | O | 3 | electrolyte | 20–100% | [52] | [99] | ||||
| [C2mim][NTf2] | IL membrane on Au-TFE | 20–100% | [53] | [100] | ||||||||
| [C2mim][NTf2] added with Poly[DADMA][NTf2] |
IL/poly(IL) membrane on Au-TFE | 20–100% | [53] | [100] | ||||||||
| O2 | and | NH3 | [C2mim][BF4] and [C4mim][BF4] |
Gel polymer electrolyte (ILs in PVDF) between planar electrodes | 1–20% for O | 2 | ; 1–10 ppm for NH | 3 | [54] | [101] | ||
| O2 | and | H2 | [Bmpy][NTf2] | Planar Pt-Ni alloy electrodes | 500–5000 ppm for O | 2 | ; 500–6250 ppm for H | 2 | [55] | [102] | ||
| H2 | ||||||||||||
| [C4mim][NTf2] and [C4mpyrr][NTf2] |
Clark-type sensor with polycrystalline Pt gauze |
0.05–1.25% | [56] | [103] | ||||||||
| [C4mim]Cl | Pd deposited on carbon gas diffusion electrode | 1–5% | [57] | [104] | ||||||||
| [Bmpy][NTf2] | [Bmpy][NTf2] on Pt/C/Nafion screen-printed electrode | 2000–10,000 ppm | [58] | [105] | ||||||||
| [C2mim][NTf2] | Au microchannel electrodes with electrodeposited Pt nanoparticles | 0.1–10% | [59] | [106] | ||||||||
| NH3 | ||||||||||||
| [C2mim][NTf2] | Pt SPE, TFE, MATFE, and microdisk | 10–100 ppm | [60] | [107] | ||||||||
| [C2mim][NTf2] | SP electrode, thin-film electrode (TFE), microarray thin-film electrode (MATFE), and microdisk. | 10–100 ppm | [61] | [108] | ||||||||
| [C2mim][NTf2] | Pt MATFE | 10–100 ppm | [62] | [109] | ||||||||
| [C2mim][NTf2] | Pt-based MATFE (with different morphologies) | 1–2 ppm LODs (depending on the morphology) | [62] | [109] | ||||||||
| NH3 | and | HCl | [C2mim][NTf2] and [C4mpyrr][NTf2] | Au microchannel electrodes | 20–100 ppm | [63] | [110] | |||||
| VOC | (in air) | [C4mpyrr][NTf2] | Clark-type sensor with polycrystalline Pt gauze | 200–3000 ppm of acetaldehyde | [64] | [111] | ||||||
| CO2 | [Bmpy][NTf2] | Au microchannel electrodes with electrodeposited Cu nanoparticles | 0.14–11% | [65] | [112] | |||||||
| Hexanaldehyde (HA) | [Bmim][OH] | Pt microelectrodes | 2–300 ppm (HA in squalene) | [66] | [113] | |||||||
| C6H6 | and | HCHO | [C2mim][EtSO | 4 | ] | IL and ionogel (IL in poly(N-isopropylacrylamide)) between interdigitated electrodes | 10–50 ppm | [67] | [114] | |||
| SO2 | [C4mpyrr][NTf2] | TFEs and MATFEs | 1–10 ppm | [68] | [115] | |||||||
| H2O (humidity) | [Bmim][DCA] | IL incorporated in gels on interdigitated electrodes | 30–70% RH | [69] | [116] | |||||||
| Ethanol | [Bmim][HSO4] | IL on Au screen-printed electrode | 1–10% | [70] | [117] | |||||||
| NO2 | [Bmim][NTf2] | Solid polymer electrolyte (PVDF + IL) on screen-printed electrodes | 1–10 ppm | [71] | [118] | |||||||
| [Bmim][BF4] | Solid polymer electrolyte (ionic liquid (IL), carbon nanotubes + polyaniline + IL) on SP electrodes | 0–700 ppm | [72] | [119] | ||||||||
| Ethylene (C2H4) | [Bmim][NTf2] | Solid polymer electrolyte (PVDF + IL) on SP electrodes | 100–500 ppm | [73] | [120] | |||||||
3. Metal–Organic-Framework-Based Composites in Gas Sensing—Recent Developments
3.1. General Properties of Metal–Organic Frameworks
Metal–organic frameworks (MOFs) are solid porous materials which, according to the terminology officially adopted by IUPAC in 2013, are classified as a subclass of coordination networks which are a subclass of coordination polymers [76][123]. The MOF network (one-dimensional, two-dimensional, or three-dimensional) arises from the strong coordination bonds between metal nodes (metal ions, metal centers, or metal clusters [77][124]) and the organic linkers. Usually, transition metal ions, especially those of the first row, lanthanides, and some alkaline earth metals are used as metal nodes because of their variable coordination numbers, geometries, and oxidation states [76][78][79][123,125,126]. Organic molecules containing one or more N-donor or O-donor atoms are mostly used as organic linkers, especially carboxylates (either aliphatic or aromatic containing one or more condensed rings), pyridyl (e.g., pyridine, pyrazine and bipyridyl derivatives), cyano compounds, polyamines, and imidazole derivatives; in addition, oxalic acid, phosphonates, sulfonates, and crown ethers are other possible ligands [76][78][123,125]. The main distinctive structural features of MOFs are the high porosity, the large volume of the pores (up to 90% of the crystalline volume or more), the large specific surface area (above 5000 m2 g−1), and the good thermal stability (250–500 °C) due to the presence of strong bonds (e.g., C–C, C–H, C–O, and M–O) [76][78][123,125]. Many of these properties are determined by the mutual interaction between specific metal ions and linkers; as a consequence, MOFs’ characteristics can be tuned by judiciously selecting metal nodes and linkers to have the desired pore size, structure, and functionality for specific applications [80][127]. MOFs’ 3D structure displays cavities and inner surfaces, which are occupied by counterions, solvate molecules and/or guest molecules. The guest species can significantly extend the designed applications of MOFs [80][127]. MOF synthesis is performed mainly in the liquid phase by mixing solutions of the ligand and metal salts under solvothermal/hydrothermal conditions at a high temperature and pressure. Other alternative and consolidated synthetic strategies are based on mechanochemical, electrochemical (for large-scale synthesis), microwave, and sonochemical methods. The most recently proposed methods are: ionothermal synthesis [76][81][123,128], the slow evaporation method [76][123], the diffusion method [76][82][123,129], the use of a microfluidic device [81][128], dry-gel conversion (DGC) [81][128] and microemulsion [76][123]. MOFs are versatile materials which are attracting great interest for application in environmental and biomedical areas as catalysts, as absorbers for toxic gases and metal ions, as materials for electrochemical devices, as drug carriers, as bioimaging agents and also therapeutic agents [83][84][130,131]. Recently, MOFs have been used also as sacrificial templates for the production of metal oxides or metal oxide–carbon hybrids [85][132] with promising morphological and textural properties to be exploited in sensing and electrochemical applications [86][87][133,134]. MOFs also are emerging as novel sensing materials because of their high surface area which enhances detective sensitivity, their specific structural features (open metal sites, tunable pore sizes, etc.) which promote host–guest interactions and selectivity, and flexible porosity which enables the reversible release and uptake of small target molecules [84][88][89][131,135,136]. Presently, the exploitation of MOFs’ potentiality in gas sensing is affected by some limitations [88][90][135,137]: (1) Most pure MOFs are not stable under extreme conditions (high temperature and high humidity levels). (2) The types of gases detectable by MOFs are limited. (3) The inherent electrical conductivity of MOFs is low and this limits their use in the development of electronic sensors. (4) The interaction mechanism between MOFs and the analyte is poorly understood. (5) MOFs are generally produced as powders with a generally low mechanical strength and poor processability. This last limitation is particularly relevant since the sensitivity of gas sensors obtained from powders is low and poses the need for a post-process of MOFs as thin films or membranes. To ensure the potential use of MOFs in sensing applications, most of these concerns must be addressed and, to this aim, the development of new MOF–based materials which have better properties (including processability in harsh conditions) than pure MOFs is gaining a lot of attention [90][91][137,138]. Research efforts have been focused on different strategies, including: (i) post-synthetic modifications [92][139]; (ii) linker change or functionalization; (iii) ion exchange; (iv) active groups grafting; (v) impregnation with suitable active materials; (vi) production of MOF-based hybrids/composites [79][91][92][93][126,138,139,140]. Among the different possibilities, the common adapted solutions are post-synthetic modifications and the production of hybrids/composites integrating MOFs and functional materials. Post-synthetic modifications involve the introduction of desired functional groups into the MOFs after their synthesis [79][92][126,139]. The modification can involve: the heterogeneous exchange of ligands or metal ions by breaking and forming chemical bonds within the original MOF, solvent-assisted ligand exchange, and replacement of the nonbridging ligands and metal nodes [76][92][94][123,139,141]. Mixed-metal MOFs, containing at least two metal ions in their framework, can be prepared under post-synthetic methods and possess new properties and activities due to the presence of the second metal ion. This approach is frequently used to produce MOF-based materials exhibiting improved fluorescent/luminescent properties [92][94][139,141]. By combining MOFs with suitable materials, the functionality and the textural/thermal/magnetic/electric properties can be improved to meet specific requirements. In some cases, the hybrid/composite materials exhibit new properties that are superior to those of the individual components since they combine the advantages of both parent materials. Metal oxides [95][142], polymers [96][143], metal nanoparticles [97][144], silica [98][145], carbon nanotubes [99][146], graphene-related materials (GRMs) [93][100][101][140,147,148] and quantum dots [102][149] have been used for the production of MOF-based hybrids and composites [90][91][137,138]. A wide variety of methods have been applied for the preparation of MOF composites. The in situ growth approach involves the growth of MOF crystals under solvothermal/hydrothermal conditions in the presence of another functional material [91][138]. In this type of synthesis, the MOF structure is built from the precursors around and eventually inside the other composite component. This method has been mainly used for the preparation of MOF composites with carbon-based materials [93][140], metal oxides [103][150] or with metal nanoparticles [97][103][144,150]. During the synthesis, the second material can act also as a templating agent, leading to oriented growth of MOF crystals, and graphene is one such example [104][151]. In the encapsulation method, the MOF composite is formed starting from the second component precursors and the preformed MOF; namely, the second component forms inside the cages of the MOF and at the end of the synthesis, the particles stay stable inside the cages without directly bonding to the MOF structure [91][138]. This is the method primarily used for the production of MOF/polymer and MOF/NPs composites [91][96][138,143]. Solid grinding and impregnation are other strategies to incorporate NPs in MOFs [105][106][152,153]. The electrospinning and solution-blending methods are two possible approaches for the preparation of MOF/polymer membranes [103][150]. MOF composites for biomedical applications have been also produced by coating the MOF structure with silica or a specific polymer with the aim of reducing their cytotoxicity and intrinsic instability under physiological conditions [103][150]. The huge and wide variability in pore size distribution of MOFs allows different guest molecules with different characteristics (size, acid–base behavior, polarization, etc.) to easily access the cavity and interact with the pore surface due to the presence of unsaturated metal sites and Lewis acidic/basic sites [89][107][136,154]. In such a way, specific MOF properties can be influenced, and changes in optical, electrical, and mechanical MOF properties can occur [88][108][135,155]. On this basis, various MOFs have been developed for possible use as chemiresistive, magnetic, ferroelectric, colorimetric, as well as luminescent sensors [88][109][110][135,156,157]; many examples are reviewed in this section. The high porosity of MOFs and the easy reversibility of the interaction with the target guest molecule are valid prerequisites to achieve repeatability, regeneration and robust operability under repeated detection cycles. On the other side, signal transduction is a major challenge for the efficient utilization of MOFs in chemical sensing [89][136]. The sensitivity of MOF-based detection largely depends on the sensing method used for signal transduction. MOFs are generally coupled with such several signal transduction techniques and tools as chemiresistors, interferometry, quartz crystal microbalance (QCM), surface acoustic wave (SAW), and microcantilevers (MCLs). MOF-based thin-film techniques have hence been very recently suggested as a valid advantage for developing next-generation chemical sensors [110][157]. Moreover, recently, conductive MOFs have been synthesized by using proper organic ligands or doping with conductive materials to form hybrids and composites with the aim of generating detectable changes in resistance/capacitance upon guest-molecule exposure [111][158]. An overview of several different sensing mechanisms involving MOFs and the mainly detected gas types is provided in Figure 12 and detailed in the following sections.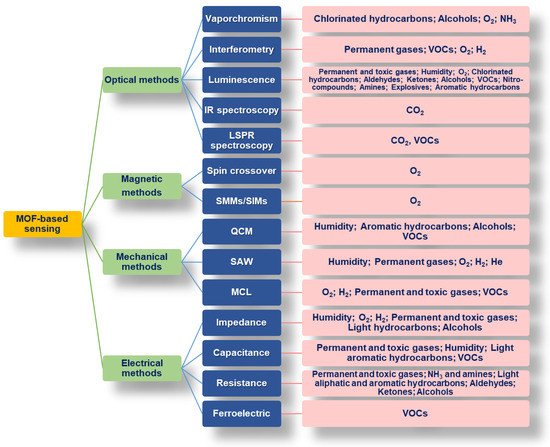
3.2. MOF-Based Sensors Using Gravimetric and Mechanical Methods
The easier approach for gas detection utilizing MOFs is the measurement of changes in MOF mass as the material selectively adsorbs the target analyte. This can be performed on a macroscale, bulk scale, or by using thin films deposited on a mechanical resonator. In this last case, the change in mass of the resonator due to gas adsorption is translated into an electrical signal [107][154]. In these mechanical methods, at first, an analyte is adsorbed into the pores of MOFs grown on electromechanical devices and then the mass changes are converted into an electrical signal through different transduction mechanisms (shifts in frequency or changes in the work function) [107][154]. In this framework, an important aspect is related to the characteristics of the MOF films: a tight contact between the MOF film and the electromechanical-device surface is strictly required to obtain the suitable sensitivity. The main gravimetric and mechanical methods involving MOFs for gas-sensing applications are based on the following electromechanical devices:3.2.1. Quartz Crystal Microbalance (QCM)-Based Sensors
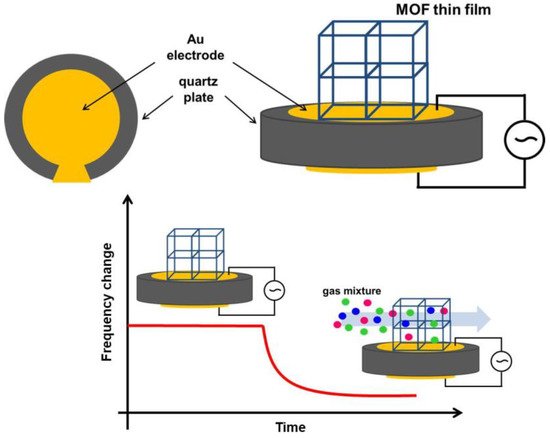
In QCM-based sensing, a thin piezoelectric quartz crystal is the core component of a QCM transducer [112][170]. After being electroplated, the thin slice oscillates when an alternating current (AC) is applied. The mass increases upon the adsorption of analyzed gas molecules, and the resonant frequency decreases (Figure 25) [112][170].